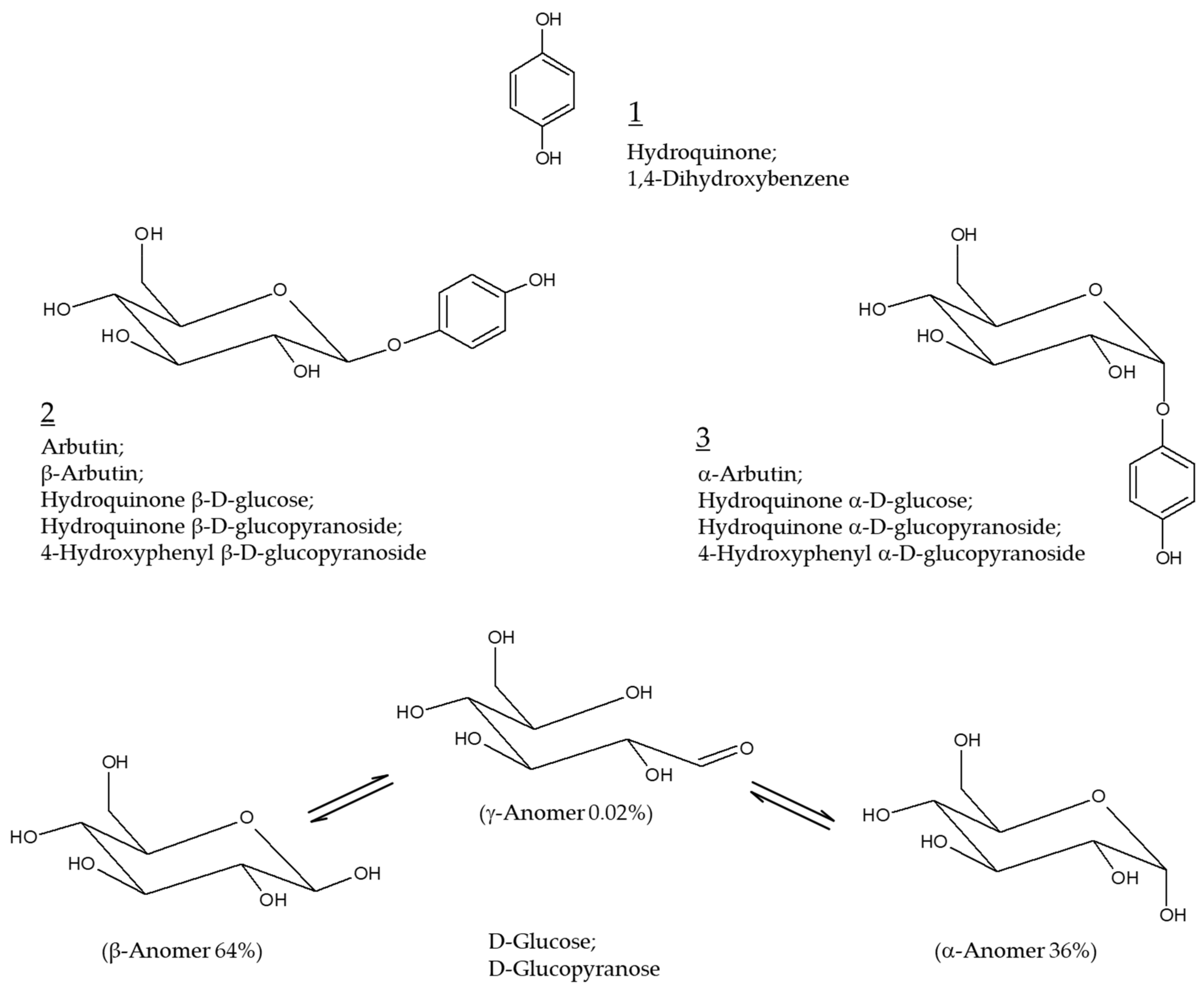
Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties
Abstract
:1. Introduction
2. Modulation of Melanin Synthesis
2.1. Pigmentation and Melanin
2.2. Regulation of Melanin Synthesis
2.3. Melanin Synthesis Pathway
2.4. Artificial Modulation of Melanogenesis
3. Arbutin
3.1. Anti-Melanogenic Effect of Arbutin
3.2. A Possible Production of Hydroquinone from Arbutin
3.3. Pro-Melanogenic Effect of Arbutin
3.4. Preparation of Arbutin
4. α-Arbutin
4.1. Preparation of α-Arbutin
4.2. Anti-Melanogenic Effect of α-Arbutin
5. Other Related Compounds
5.1. Glycosidic Derivatives of Arbutin and α-Arbutin
5.2. Esters of Arbutin
5.3. Calixarbutin
5.4. Deoxyarbutin
6. Formulation and Devise
7. Clinical Evaluation of Skin Depigmenting Efficacy
7.1. Skin Depigmenting Efficacy of Arbutin
7.2. Combination with Other Active Ingredients
7.3. Combination Therapy with a Laser Treatment
8. Antioxidant Properties of Arbutin and α-Arbutin
8.1. Reactive Oxygen Species in Melanin Synthesis
8.2. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)-Mediated Pathway
8.3. Antioxidant Properties of Arbutin and α-Arbutin
9. Discussion
10. Conclusions
Funding
Conflicts of Interest
References
- Saade, D.S.; Maymone, M.B.C.; De La Garza, H.; Secemsky, E.A.; Kennedy, K.F.; Vashi, N.A. Trends in Use of Prescription Skin Lightening Creams. Int. J. Environ. Res. Public Health 2021, 18, 5650. [Google Scholar] [CrossRef] [PubMed]
- Neagu, N.; Conforti, C.; Agozzino, M.; Marangi, G.F.; Morariu, S.H.; Pellacani, G.; Persichetti, P.; Piccolo, D.; Segreto, F.; Zalaudek, I.; et al. Melasma treatment: A systematic review. J. Dermatolog. Treat. 2021, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Jow, T.; Hantash, B.M. Hydroquinone-induced depigmentation: Case report and review of the literature. Dermatitis 2014, 25, e1–e5. [Google Scholar] [CrossRef]
- Ozbey, R.; Okur, M.I. The use of 4% hydroquinone, 0.1% tretinoin, and 0.1% betamethasone creams to prevent hyperpigmentation of split-thickness skin grafts in Long-Evans rats. J. Cosmet. Dermatol. 2020, 19, 2663–2668. [Google Scholar] [CrossRef]
- Jimbow, K.; Obata, H.; Pathak, M.A.; Fitzpatrick, T.B. Mechanism of depigmentation by hydroquinone. J. Investig. Dermatol. 1974, 62, 436–449. [Google Scholar] [CrossRef] [Green Version]
- Kooyers, T.J.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Xu, W.H.; Liang, Q.; Zhang, Y.J.; Zhao, P. Naturally occurring arbutin derivatives and their bioactivities. Chem. Biodivers. 2015, 12, 54–81. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of alpha-arbutin. Phytother. Res. 2021. [Google Scholar] [CrossRef]
- Akiu, S.; Suzuki, Y.; Asahara, T.; Fujinuma, Y.; Fukuda, M. Inhibitory effect of arbutin on melanogenesis-biochemical study using cultured B16 melanoma cells. Nippon Hifuka Gakkai Zasshi 1991, 101, 609–613. [Google Scholar] [PubMed]
- Slominski, A.; Kim, T.K.; Brozyna, A.A.; Janjetovic, Z.; Brooks, D.L.; Schwab, L.P.; Skobowiat, C.; Jozwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1alpha expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Beer, J.Z.; Hearing, V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: Factors influencing the incidence of skin cancer. Arch. Dermatol. Res. 2008, 300 (Suppl. 1), S43–S50. [Google Scholar] [CrossRef]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef]
- Rose, P.T. Pigmentary disorders. Med. Clin. N. Am. 2009, 93, 1225–1239. [Google Scholar] [CrossRef]
- Ganju, P.; Nagpal, S.; Mohammed, M.H.; Nishal Kumar, P.; Pandey, R.; Natarajan, V.T.; Mande, S.S.; Gokhale, R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016, 6, 18761. [Google Scholar] [CrossRef] [Green Version]
- Spritz, R.A.; Andersen, G.H. Genetics of Vitiligo. Dermatol. Clin. 2017, 35, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Iwata, M.; Corn, T.; Iwata, S.; Everett, M.A.; Fuller, B.B. The relationship between tyrosinase activity and skin color in human foreskins. J. Investig. Dermatol. 1990, 95, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinhoff, M.; Stander, S.; Seeliger, S.; Ansel, J.C.; Schmelz, M.; Luger, T. Modern aspects of cutaneous neurogenic inflammation. Arch. Dermatol. 2003, 139, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Hodi, F.S.; Fisher, D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer 2012, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrzesniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.H.; Jin, Z.H. Paracrine regulation of melanogenesis. Br. J. Dermatol. 2018, 178, 632–639. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Garratt, P.J.; Land, E.J.; Pavel, S.; Ramsden, C.A.; Riley, P.A.; Smit, N.P. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997, 272, 26226–26235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Stefania, R.; Aime, S.; Oraevsky, A. Melanin-Based Contrast Agents for Biomedical Optoacoustic Imaging and Theranostic Applications. Int. J. Mol. Sci. 2017, 18, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davy, A.D.; Birch, D.J.S. Evidence for pheomelanin sheet structure. Appl. Phys. Lett. 2018, 113, 263701. [Google Scholar] [CrossRef] [Green Version]
- Grieco, C.; Kohl, F.R.; Hanes, A.T.; Kohler, B. Probing the heterogeneous structure of eumelanin using ultrafast vibrational fingerprinting. Nat. Commun. 2020, 11, 4569. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stab, F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013, 27 (Suppl. S1), 19–23. [Google Scholar] [CrossRef]
- Watanabe, F.; Hashizume, E.; Chan, G.P.; Kamimura, A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: A double-blind and placebo-controlled clinical trial in healthy women. Clin. Cosmet. Investig. Dermatol. 2014, 7, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boo, Y.C.; Jo, D.J.; Oh, C.M.; Lee, S.Y.; Kim, Y.M. The First Human Clinical Trial on the Skin Depigmentation Efficacy of Glycinamide Hydrochloride. Biomedicines 2020, 8, 257. [Google Scholar] [CrossRef]
- Hu, Z.M.; Zhou, Q.; Lei, T.C.; Ding, S.F.; Xu, S.Z. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 2009, 55, 179–184. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J. Soc. Cosmet. Chem. 1991, 42, 361–368. [Google Scholar]
- Lim, Y.J.; Lee, E.H.; Kang, T.H.; Ha, S.K.; Oh, M.S.; Kim, S.M.; Yoon, T.J.; Kang, C.; Park, J.H.; Kim, S.Y. Inhibitory effects of arbutin on melanin biosynthesis of alpha-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch. Pharm. Res. 2009, 32, 367–373. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [PubMed]
- Chakraborty, A.K.; Funasaka, Y.; Komoto, M.; Ichihashi, M. Effect of arbutin on melanogenic proteins in human melanocytes. Pigment Cell Res. 1998, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Kubo, I. Identification of oxidation product of arbutin in mushroom tyrosinase assay system. Bioorg. Med. Chem. Lett. 2003, 13, 2409–2412. [Google Scholar] [CrossRef]
- Hori, I.; Nihei, K.; Kubo, I. Structural criteria for depigmenting mechanism of arbutin. Phytother. Res. 2004, 18, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hasegawa, S.; Yamada, T.; Date, Y.; Mizutani, H.; Nakata, S.; Matsunaga, K.; Akamatsu, H. Analysis of the effects of hydroquinone and arbutin on the differentiation of melanocytes. Biol. Pharm. Bull. 2013, 36, 1722–1730. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.S.; Kim, B.H.; Lee, S.H.; Kwon, H.J.; Bae, H.J.; Kim, S.K.; Park, J.A.; Shim, J.H.; Abd El-Aty, A.M.; Shin, H.C. Simultaneous determination of arbutin and its decomposed product hydroquinone in whitening creams using high-performance liquid chromatography with photodiode array detection: Effect of temperature and pH on decomposition. Int. J. Cosmet. Sci. 2015, 37, 567–573. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, Y.H.; Avula, B.; Wang, M.; Rua, D.; Khan, I.A. Comparative studies on the chemical and enzymatic stability of alpha- and beta-arbutin. Int. J. Cosmet. Sci. 2016, 38, 187–193. [Google Scholar] [CrossRef]
- Bang, S.H.; Han, S.J.; Kim, D.H. Hydrolysis of arbutin to hydroquinone by human skin bacteria and its effect on antioxidant activity. J. Cosmet. Dermatol. 2008, 7, 189–193. [Google Scholar] [CrossRef]
- Chang, N.F.; Chen, Y.S.; Lin, Y.J.; Tai, T.H.; Chen, A.N.; Huang, C.H.; Lin, C.C. Study of Hydroquinone Mediated Cytotoxicity and Hypopigmentation Effects from UVB-Irradiated Arbutin and DeoxyArbutin. Int. J. Mol. Sci. 2017, 18, 969. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M.; Shinoda, I.; Fukuwatari, Y.; Hayasawa, H. Arbutin increases the pigmentation of cultured human melanocytes through mechanisms other than the induction of tyrosinase activity. Pigment Cell Res. 1998, 11, 12–17. [Google Scholar] [CrossRef]
- Rok, J.; Otręba, M.; Buszman, E.; Wrześniok, D. Melanin—From melanocyte to keratinocyte, that is how melanin is transported within the skin. Ann. Acad. Med. Sil. 2012, 66, 60–66. [Google Scholar]
- Wu, X.F.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Tian, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Recent progress on biological production of alpha-arbutin. Appl. Microbiol. Biotechnol. 2018, 102, 8145–8152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, J.; Li, A.; Reetz, M.T. Chemical and Biocatalytic Routes to Arbutin (dagger). Molecules 2019, 24, 3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.Z.; Kayahara, H. Analyses of arbutin and chlorogenic acid, the major phenolic constituents in oriental pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef]
- Tumova, L.; Doleckova, I.; Hendrychova, H.; Kasparova, M. Arbutin Content and Tyrosinase Activity of Bergenia Extracts. Nat. Prod. Commun. 2017, 12, 549–552. [Google Scholar]
- Cho, J.-Y.; Park, K.Y.; Lee, K.H.; Lee, H.J.; Lee, S.-H.; Cho, J.A.; Kim, W.-S.; Shin, S.-C.; Park, K.-H.; Moon, J.-H. Recovery of arbutin in high purity from fruit peels of pear (Pyrus pyrifolia Nakai). Food Sci. Biotechnol. 2011, 20, 801–807. [Google Scholar] [CrossRef]
- Sasaki, C.; Ichitani, M.; Kunimoto, K.K.; Asada, C.; Nakamura, Y. Extraction of arbutin and its comparative content in branches, leaves, stems, and fruits of Japanese pear Pyrus pyrifolia cv. Kousui. Biosci. Biotechnol. Biochem. 2014, 78, 874–877. [Google Scholar] [CrossRef]
- Lee, B.-D.; Eun, J.-B. Optimum extraction conditions for arbutin from Asian pear peel by supercritical fluid extraction (SFE) using Box-Behnken design. J. Med. Plants Res. 2012, 6, 2348–2364. [Google Scholar]
- Jin, Y.H.; Jeon, A.R.; Mah, J.H. Tyrosinase Inhibitory Activity of Soybeans Fermented with Bacillus subtilis Capable of Producing a Phenolic Glycoside, Arbutin. Antioxidants 2020, 9, 1301. [Google Scholar] [CrossRef]
- Shang, Y.Z.; Wei, W.P.; Zhang, P.; Ye, B.C. Engineering Yarrowia lipolytica for Enhanced Production of Arbutin. J. Agric. Food Chem. 2020, 68, 1364–1372. [Google Scholar] [CrossRef]
- Kitao, S.; Sekine, H. alpha-D-Glucosyl Transfer to Phenolic Compounds by Sucrose Phosphorylase from Leuconostoc mesenteroides and Production of alpha-Arbutin. Biosci. Biotechnol. Biochem. 1994, 58, 38–42. [Google Scholar] [CrossRef]
- Nishimura, T.; Kometani, T.; Takii, H.; Terada, Y.; Okada, S. Purification and Some Properties of Alpha-Amylase from Bacillus-Subtilis X-23 That Glucosylates Phenolic-Compounds Such as Hydroquinone. J. Ferment. Bioeng. 1994, 78, 31–36. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Y.T.; Wei, X.M.; Yang, K.D.; Yang, X.K.; Wang, Y.T.; Xu, L.M.; Du, L.Q.; Huang, R.B. Sucrose Isomerase and Its Mutants from Erwinia rhapontici Can Synthesise alpha-Arbutin. Protein Pept. Lett. 2011, 18, 1028–1034. [Google Scholar] [CrossRef]
- Seo, D.H.; Jung, J.H.; Ha, S.J.; Cho, H.K.; Jung, D.H.; Kim, T.J.; Baek, N.I.; Yoo, S.H.; Park, C.S. High-yield enzymatic bioconversion of hydroquinone to alpha-arbutin, a powerful skin lightening agent, by amylosucrase. Appl. Microbiol. Biotechnol. 2012, 94, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Wang, Y.C.; Tian, Y.Q.; Xu, W.; Bai, Y.X.; Zhang, T.; Mu, W.M. Highly efficient biosynthesis of alpha-arbutin from hydroquinone by an amylosucrase from Cellulomonas carboniz. Process Biochem. 2018, 68, 93–99. [Google Scholar] [CrossRef]
- Mathew, S.; Adlercreutz, P. Regioselective glycosylation of hydroquinone to alpha-arbutin by cyclodextrin glucanotransferase from Thermoanaerobacter sp. Biochem. Eng. J. 2013, 79, 187–193. [Google Scholar] [CrossRef]
- Kurosu, J.; Sato, T.; Yoshida, K.; Tsugane, T.; Shimura, S.; Kirimura, K.; Kino, K.; Usami, S. Enzymatic synthesis of alpha-arbutin by alpha-anomer-selective-glucosylation of hydroquinone using lyophilized cells of Xanthomonas campestris WU. J. Biosci. Bioeng. 2002, 93, 328–330. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Liu, L.; Xu, T.; Tan, T.; Wang, F.; Deng, L. Isolation of alpha-arbutin from Xanthomonas CGMCC 1243 fermentation broth by macroporous resin adsorption chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 925, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Ren, Y.; Liu, C.; Liu, R.; Zhang, P.; Wei, Y.; Xu, T.; Wang, F.; Tan, T.; Liu, C. Fermentation scale up for alpha-arbutin production by Xanthomonas BT-112. J. Biotechnol. 2016, 233, 1–5. [Google Scholar] [CrossRef]
- Wu, P.H.; Nair, G.R.; Chu, I.M.; Wu, W.T. High cell density cultivation of Escherichia coli with surface anchored transglucosidase for use as whole-cell biocatalyst for alpha-arbutin synthesis. J. Ind. Microbiol. Biotechnol. 2008, 35, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Shi, X.X.; Chen, G.R.; Ren, Z.H.; Luo, L.; Yan, J. A new synthesis of alpha-arbutin via Lewis acid catalyzed selective glycosylation of tetra-O-benzyl-alpha-D-glucopyranosyl trichloroacetimidate with hydroquinone. Carbohydr. Res. 2006, 341, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Cepanec, I.; Litvic, M. Simple and efficient synthesis of arbutin. Arkivoc 2008, 2, 19–24. [Google Scholar]
- Funayama, M.; Arakawa, H.; Yamamoto, R.; Nishino, T.; Shin, T.; Murao, S. Effects of alpha- and beta-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci. Biotechnol. Biochem. 1995, 59, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, Y.; Liu, Y.; Chen, Y.; Zhang, P. Dual effects of alpha-arbutin on monophenolase and diphenolase activities of mushroom tyrosinase. PLoS ONE 2014, 9, e109398. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Berna, J.; Rodriguez-Lopez, J.N.; Tudela, J.; Garcia-Canovas, F. Action of tyrosinase on alpha and beta-arbutin: A kinetic study. PLoS ONE 2017, 12, e0177330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Inhibitory effects of alpha-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model. Biol. Pharm. Bull. 2004, 27, 510–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Syntheses of arbutin-alpha-glycosides and a comparison of their inhibitory effects with those of alpha-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 2003, 51, 798–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, K.; Nomura, K.; Nishimura, T.; Kiso, T.; Sugimoto, K.; Kuriki, T. Syntheses of alpha-arbutin-alpha-glycosides and their inhibitory effects on human tyrosinase. J. Biosci. Bioeng. 2005, 99, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.H.; Nam, S.H.; Kang, J.; Kim, Y.M.; Lee, J.H.; Kang, H.K.; Breton, V.; Jun, W.J.; Park, K.D.; Kimura, A.; et al. Enzymatic synthesis and characterization of arbutin glucosides using glucansucrase from Leuconostoc mesenteroides B-1299CB. Appl. Microbiol. Biotechnol. 2007, 77, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Park, K.M.; Choi, K.W.; Jang, M.K.; Kang, H.Y.; Lee, S.H.; Park, K.H.; Cha, J. Inhibitory effects of arbutin-beta-glycosides synthesized from enzymatic transglycosylation for melanogenesis. Biotechnol. Lett. 2008, 30, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Rudeekulthamrong, P.; Kaulpiboon, J. Optimization of amylomaltase for the synthesis of alpha-arbutin derivatives as tyrosinase inhibitors. Carbohydr. Res. 2020, 494, 108078. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, D.; Zhang, Y.; Li, J.; Wu, Z.; Wang, Z.; Wang, D. Investigation of the pro-apoptotic effects of arbutin and its acetylated derivative on murine melanoma cells. Int. J. Mol. Med. 2018, 41, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokiwa, Y.; Kitagawa, M.; Raku, T. Enzymatic synthesis of arbutin undecylenic acid ester and its inhibitory effect on mushroom tyrosinase. Biotechnol. Lett. 2007, 29, 481–486. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Xin, X.; Mo, L.; Zou, Y.; Zhao, G.; Yu, Y.; Chen, K. Antityrosinase Mechanism and Antimelanogenic Effect of Arbutin Esters Synthesis Catalyzed by Whole-Cell Biocatalyst. J. Agric. Food Chem. 2021, 69, 4243–4252. [Google Scholar] [CrossRef]
- Masyita, A.; Salim, E.; Asri, R.M.; Nainu, F.; Hori, A.; Yulianty, R.; Hatta, M.; Rifai, Y.; Kuraishi, T. Molecular modeling and phenoloxidase inhibitory activity of arbutin and arbutin undecylenic acid ester. Biochem. Biophys. Res. Commun. 2021, 547, 75–81. [Google Scholar] [CrossRef]
- Yamashita-Higuchi, Y.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Nakai, T. Grevillosides J-Q, arbutin derivatives from the leaves of Grevillea robusta and their melanogenesis inhibitory activity. Chem. Pharm. Bull. 2014, 62, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffarzadeh, J.; Nasuhi Pur, F. Calixarbutin: A Novel Calixarene-based Potential Water-soluble Anti-tyrosinase Agent with High Anti-melanoma Activity. Iran. J. Pharm. Res. 2020, 19, 236–241. [Google Scholar]
- Boissy, R.E.; Visscher, M.; DeLong, M.A. DeoxyArbutin: A novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp. Dermatol. 2005, 14, 601–608. [Google Scholar] [CrossRef]
- Chawla, S.; de Long, M.A.; Visscher, M.O.; Wickett, R.R.; Manga, P.; Boissy, R.E. Mechanism of tyrosinase inhibition by deoxyArbutin and its second-generation derivatives. Br. J. Dermatol. 2008, 159, 1267–1274. [Google Scholar] [CrossRef]
- Anwar, A.I.; Asmarani, Y.; Madjid, A.; Patellongi, I.; Adriani, A.; As’ad, S.; Kurniadi, I. Comparison of 2% deoxyarbutin and 4% hydroquinone as a depigmenting agent in healthy individuals: A double-blind randomized controlled clinical trial. J. Cosmet. Dermatol. 2021. [Google Scholar] [CrossRef]
- SCCS; Degen, G.H. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Opinion on the safety of the use of beta-arbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2015, 73, 866–867. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, Z.H.; Zhang, T.L.; Tu, J.B.; Song, Y.; Hu, X.Y.; Li, G.G. The effect of aloesin on melanocytes in the pigmented skin equivalent model. Zhonghua Zheng Xing Wai Ke Za Zhi 2008, 24, 50–53. [Google Scholar] [PubMed]
- SCCS; Degen, G.H. Opinion of the Scientific Committee on Consumer safety (SCCS)—Opinion on the safety of the use of deoxyarbutin in cosmetic products. Regul. Toxicol. Pharmacol. 2016, 74, 77–78. [Google Scholar]
- Frias, M.A.; Winik, B.; Franzoni, M.B.; Levstein, P.R.; Nicastro, A.; Gennaro, A.M.; Diaz, S.B.; Disalvo, E.A. Lysophosphatidylcholine-arbutin complexes form bilayer-like structures. Biochim. Biophys. Acta 2008, 1778, 1259–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, F.; Cai, H.Y.; Chen, X.; Sun, W.; Shen, W.Y. Structural characterization of inclusion complex of arbutin and hydroxypropyl-beta-cyclodextrin. Trop. J. Pharm. Res. 2016, 15, 2227–2233. [Google Scholar] [CrossRef] [Green Version]
- Wen, A.H.; Choi, M.K.; Kim, D.D. Formulation of liposome for topical delivery of arbutin. Arch. Pharm. Res. 2006, 29, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Xu, K.; Bessarab, D.; Obaje, J.; Xu, C. Arbutin encapsulated micelles improved transdermal delivery and suppression of cellular melanin production. BMC Res. Notes 2016, 9, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Belwal, T.; Liu, S.B.; Duan, Z.H.; Luo, Z.S. Novel multi-phase nano-emulsion preparation for co-loading hydrophilic arbutin and hydrophobic coumaric acid using hydrocolloids. Food Hydrocoll. 2019, 93, 92–101. [Google Scholar] [CrossRef]
- Ayumi, N.S.; Sahudin, S.; Hussain, Z.; Hussain, M.; Abu Samah, N.H. Polymeric nanoparticles for topical delivery of alpha and beta arbutin: Preparation and characterization. Drug Deliv. Transl. Res. 2019, 9, 482–496. [Google Scholar] [CrossRef]
- Park, J.J.; Hwang, S.J.; Kang, Y.S.; Jung, J.; Park, S.; Hong, J.E.; Park, Y.; Lee, H.J. Synthesis of arbutin-gold nanoparticle complexes and their enhanced performance for whitening. Arch. Pharm. Res. 2019, 42, 977–989. [Google Scholar] [CrossRef]
- Liao, A.H.; Ma, W.C.; Wang, C.H.; Yeh, M.K. Penetration depth, concentration and efficiency of transdermal alpha-arbutin delivery after ultrasound treatment with albumin-shelled microbubbles in mice. Drug Deliv. 2016, 23, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP Dissolving Microneedles: A Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech 2019, 21, 25. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P. Fabrication, characterization and comparison of alpha-arbutin loaded dissolving and hydrogel forming microneedles. Int. J. Pharm. 2020, 586, 119508. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, K.J.; Lee, M.W.; Lee, S.Y.; Yun, Y.H.; Shim, W.G.; Yoon, S.D. Preparation and release properties of arbutin imprinted inulin/polyvinyl alcohol biomaterials. Int. J. Biol. Macromol. 2020, 161, 763–770. [Google Scholar] [CrossRef]
- Choi, S.; Park, Y.I.; Lee, S.K.; Kim, J.E.; Chung, M.H. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin. Exp. Dermatol. 2002, 27, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Ertam, I.; Mutlu, B.; Unal, I.; Alper, S.; Kivcak, B.; Ozer, O. Efficiency of ellagic acid and arbutin in melasma: A randomized, prospective, open-label study. J. Dermatol. 2008, 35, 570–574. [Google Scholar] [CrossRef]
- Morag, M.; Nawrot, J.; Siatkowski, I.; Adamski, Z.; Fedorowicz, T.; Dawid-Pac, R.; Urbanska, M.; Nowak, G. A double-blind, placebo-controlled randomized trial of Serratulae quinquefoliae folium, a new source of beta-arbutin, in selected skin hyperpigmentations. J. Cosmet. Dermatol. 2015, 14, 185–190. [Google Scholar] [CrossRef]
- Jin, Y.H.; Lee, S.J.; Chung, M.H.; Park, J.H.; Park, Y.I.; Cho, T.H.; Lee, S.K. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch. Pharm. Res. 1999, 22, 232–236. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, Z.H.; Tu, J.B.; Li, P.; Hu, X.Y. The effects of aloesin and arbutin on cultured melanocytes in a synergetic method. Zhonghua Zheng Xing Wai Ke Za Zhi 2004, 20, 369–371. [Google Scholar]
- Hseu, Y.C.; Cheng, K.C.; Lin, Y.C.; Chen, C.Y.; Chou, H.Y.; Ma, D.L.; Leung, C.H.; Wen, Z.H.; Wang, H.M. Synergistic Effects of Linderanolide B Combined with Arbutin, PTU or Kojic Acid on Tyrosinase Inhibition. Curr. Pharm. Biotechnol. 2015, 16, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Chen, H.J.; Xiang, S.J.; Cao, S.W.; An, B.C.; Ruan, S.F.; Zhang, B.; Weng, L.D.; Zhu, H.X.; Liu, Q. Capsaicin reverses the inhibitory effect of licochalcone A/beta-Arbutin on tyrosinase expression in b16 mouse melanoma cells. Pharmacogn. Mag. 2018, 14, 110–115. [Google Scholar] [PubMed]
- Crocco, E.I.; Veasey, J.V.; Boin, M.F.F.D.; Lellis, R.F.; Alves, R.O. A Novel Cream Formulation Containing Nicotinamide 4%, Arbutin 3%, Bisabolol 1%, and Retinaldehyde 0.05% for Treatment of Epidermal Melasma. Cutis 2015, 96, 337–342. [Google Scholar]
- Anwar, A.I.; Wahab, S.; Widita, W.; Nurdin, A.R.; Budhiani, S.; Seweng, A. Randomized control trial outcomes of tranexamic acid combination serum as a depigmenting agent for the use in healthy individuals. Dermatol. Ther. 2019, 32, e13146. [Google Scholar] [CrossRef] [PubMed]
- Kalasho, B.D.; Minokadeh, A.; Zhang-Nunes, S.; Zoumalan, R.A.; Shemirani, N.L.; Waldman, A.R.; Pletzer, V.; Zoumalan, C.I. Evaluating the Safety and Efficacy of a Topical Formulation Containing Epidermal Growth Factor, Tranexamic Acid, Vitamin C, Arbutin, Niacinamide and Other Ingredients as Hydroquinone 4% Alternatives to Improve Hyperpigmentation: A Prospective, Randomize Controlled Split Face Study. J. Cosmet. Sci. 2020, 71, 263–290. [Google Scholar]
- Polnikorn, N. Treatment of refractory melasma with the MedLite C6 Q-switched Nd:YAG laser and alpha arbutin: A prospective study. J. Cosmet. Laser Ther. 2010, 12, 126–131. [Google Scholar] [CrossRef]
- Abbas, K.; Qadir, M.I.; Anwar, S. The Role of Melanin in Skin Cancer. Crit. Rev. Eukaryot Gene Expr. 2019, 29, 17–24. [Google Scholar] [CrossRef]
- Smit, N.P.M.; Nieuwpoort, F.A.; Marrot, L.; Out, C.; Poorthuis, B.; van Pelt, H.; Meunier, J.R.; Pavel, S. Increased melanogenesis is a risk factor for oxidative DNA damage—Study on cultured melanocytes and atypical nevus cells. Photochem. Photobiol. 2008, 84, 550–555. [Google Scholar] [CrossRef]
- Dumbuya, H.; Hafez, S.Y.; Oancea, E. Cross talk between calcium and ROS regulate the UVA-induced melanin response in human melanocytes. FASEB J. 2020, 34, 11605–11623. [Google Scholar] [CrossRef]
- Suo, D.F.; Zeng, S.W.; Zhang, J.L.; Meng, L.H.; Weng, L.S. PM2.5 induces apoptosis, oxidative stress injury and melanin metabolic disorder in human melanocytes. Exp. Ther. Med. 2020, 19, 3227–3238. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, J.; Quintana, C.; Gonzalez, I.; Hernandez, I.; Rubio, S.; Loro, J.F.; Reiter, R.J.; Estevez, F.; Quintana, J. Melatonin Induces Melanogenesis in Human SK-MEL-1 Melanoma Cells Involving Glycogen Synthase Kinase-3 and Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 4970. [Google Scholar] [CrossRef]
- Chang, S.P.; Huang, H.M.; Shen, S.C.; Lee, W.R.; Chen, Y.C. Nilotinib induction of melanogenesis via reactive oxygen species-dependent JNK activation in B16F0 mouse melanoma cells. Exp. Dermatol. 2018, 27, 1388–1394. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, N.C.; Grossman, D. Role of Melanin in Melanocyte Dysregulation of Reactive Oxygen Species. Biomed. Res. Int. 2013, 2013, 908797. [Google Scholar] [CrossRef] [PubMed]
- Nagapan, T.S.; Lim, W.N.; Basri, D.; Ghazali, A.R. Oral supplementation of L-glutathione prevents ultraviolet B-induced melanogenesis and oxidative stress in BALB/c mice. Exp. Anim. 2019, 68, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjinpathana, N.; Asawanonda, P. Glutathione as an oral whitening agent: A randomized, double-blind, placebo-controlled study. J. Dermatol. Treat. 2012, 23, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gegotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Schafer, M.; Dutsch, S.; Keller, U.A.D.; Navid, F.; Schwarz, A.; Johnson, D.A.; Johnson, J.A.; Werner, S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010, 24, 1045–1058. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.M.; Kim, M.Y.; Sohn, K.C.; Jung, S.Y.; Lee, H.E.; Lim, J.W.; Kim, S.; Lee, Y.H.; Im, M.; Seo, Y.J.; et al. Nrf2 Negatively Regulates Melanogenesis by Modulating PI3K/Akt Signaling. PLoS ONE 2014, 9, e96035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Takebayashi, J.; Ishii, R.; Chen, J.B.; Matsumoto, T.; Ishimi, Y.; Tai, A. Reassessment of antioxidant activity of arbutin: Multifaceted evaluation using five antioxidant assay systems. Free Radic. Res. 2010, 44, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Kohno, M.; Niwano, Y. Alleviation effect of arbutin on oxidative stress generated through tyrosinase reaction with L-tyrosine and L-DOPA. BMC Biochem. 2014, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.H.; Li, P.; Zhao, Q.L.; Piao, J.L.; Jiao, Y.F.; Kadowaki, M.; Kondo, T. Arbutin, an intracellular hydroxyl radical scavenger, protects radiation-induced apoptosis in human lymphoma U937 cells. Apoptosis 2014, 19, 1654–1663. [Google Scholar] [CrossRef]
- Seyfizadeh, N.; Tazehkand, M.Q.; Palideh, A.; Maroufi, N.F.; Hassanzadeh, D.; Rahmati-Yamchi, M.; Elahimanesh, F.; Borzoueisileh, S. Is arbutin an effective antioxidant for the discount of oxidative and nitrosative stress in Hep-G2 cells exposed to tert-butyl hydroperoxide? Bratisl. Med. J. Bratisl. Lek. Listy 2019, 120, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Ebadollahi, S.H.; Pouramir, M.; Zabihi, E.; Golpour, M.; Aghajanpour-Mir, M. The Effect of Arbutin on The Expression of Tumor Suppressor P53, BAX/BCL-2 Ratio and Oxidative Stress Induced by Tert-Butyl Hydroperoxide in Fibroblast and LNcap Cell Lines. Cell J. 2021, 22, 532–541. [Google Scholar]
- Polouliakh, N.; Ludwig, V.; Meguro, A.; Kawagoe, T.; Heeb, O.; Mizuki, N. Alpha-Arbutin Promotes Wound Healing by Lowering ROS and Upregulating Insulin/IGF-1 Pathway in Human Dermal Fibroblast. Front. Physiol. 2020, 11, 586843. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Li, B.; Kan, Y.; Wang, S.; Cao, B.; Huang, Y.; Zheng, X.; Feng, W. Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine 2021, 82, 153466. [Google Scholar] [CrossRef]
- Nalban, N.; Sangaraju, R.; Alavala, S.; Mir, S.M.; Jerald, M.K.; Sistla, R. Arbutin Attenuates Isoproterenol-Induced Cardiac Hypertrophy by Inhibiting TLR-4/NF-kappaB Pathway in Mice. Cardiovasc. Toxicol. 2020, 20, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Safari, H.; Zabihi, E.; Pouramir, M.; Morakabati, P.; Abedian, Z.; Karkhah, A.; Nouri, H.R. Decrease of intracellular ROS by arbutin is associated with apoptosis induction and downregulation of IL-1beta and TNF-alpha in LNCaP; prostate cancer. J. Food Biochem. 2020, 44, e13360. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Ito, A.; Masui, Y.; Ito, M. A case of allergic contact dermatitis caused by arbutin. Contact Dermat. 2015, 72, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Tobita, R.; Tsuboi, R.; Okubo, Y. Contact dermatitis caused by arbutin contained in skin-whitening cosmetics. Contact Dermat. 2016, 75, 187–188. [Google Scholar] [CrossRef] [PubMed]





| Literature | Compounds | Tyrosinase Inhibitory Effects | Enzymes and Substrates Used | |
|---|---|---|---|---|
| Monophenolase Activity | Diphenolase Activity | |||
| [73] | Hydroquinone | 97.2% inhibition at 3 mM | Mushroom tyrosinase; 0.3 mM L-tyrosine | |
| Arbutin | 82.0% inhibition at 3 mM | |||
| α-arbutin | 72.8% inhibition at 3 mM | |||
| [85] | α-arbutin | IC50 = 0.48 mM | B16 mouse tyrosinase; 3.3 mM L-DOPA | |
| Arbutin | IC50 = 4.8 mM | |||
| α-arbutin | No inhibition | Mushroom tyrosinase; 0.83 mM L-DOPA | ||
| Arbutin | IC50 = 8.4 mM | |||
| [87] | α-arbutin | IC50 = 8 mM | IC50 = 8.87 mM | Mushroom tyrosinase; 0.25 mM L-tyrosine plus 0.01 mM L-DOPA for monophenolase activity; 0.5 mM L-DOPA for diphenolase activity |
| Arbutin | IC50 = 0.9 mM | IC50 = 0.7 mM | ||
| Literature | Compounds | Tyrosinase Inhibitory Effects | Enzymes and Substrates Used | ||
|---|---|---|---|---|---|
| Name | Chemical Structure | Monophenolase Activity | Diphenolase Activity | ||
| [89] | α-arbutin | 3 | IC50 = 2.1 mM | Human tyrosinase; 3.3 mM L-DOPA | |
| Arbutin | 2 | IC50 > 30 mM | |||
| 4-Hydroxyphenyl β-maltoside | 7 | IC50 = 5.7 mM | |||
| 4-Hydroxyphenyl β-maltotrioside | 8 | IC50 = 6.1 mM | |||
| [90] | 4-Hydroxyphenyl α-maltoside | 11 | IC50 = 4.9 mM | Human tyrosinase; 3.3 mM L-DOPA | |
| 4-Hydroxyphenyl α-maltotrioside | 12 | IC50 = 13.9 mM | |||
| [91] | Arbutin | 2 | Ki = 2.8 mM | Mushroom tyrosinase; 3.3 mM DOPA | |
| 4-Hydroxyphenyl β-isomaltoside | 9 | Ki = 3.7 mM | |||
| 4-Hydroxyphenyl β-isomaltotrioside | 10 | Not determined | |||
| [92] | Arbutin | 2 | IC50 = 6 mM | Mushroom tyrosinase; 0.03% L-tyrosine | |
| β-D-Glucopyranosyl-(1→6)-arbutin | 4 | IC50 = 8 mM | |||
| β-D-Glucopyranosyl-(1→4)-arbutin | 5 | IC50 = 10 mM | |||
| β-D-Glucopyranosyl-(1→3)-arbutin | 6 | IC50 = 5 mM | |||
| α-D-Glucopyranosyl-(1→4)-arbutin | 7 | IC50 = 5 mM | |||
| [93] | α-arbutin | 3 | IC50 = 2.1 mM | Human tyrosinase; 3 mM L-DOPA | |
| α-arbutin-α-glucoside | 11 | IC50 = 6.9 mM | |||
| α-arbutin-α-maltoside | 12 | IC50 = 15.6 mM | |||
| α-arbutin-α-maltotrioside | 13 | Not determined | |||
| Literature | Compounds | Tyrosinase Inhibitory Effects | Enzymes and Substrates Used | ||
|---|---|---|---|---|---|
| Name | Chemical Structure | Monophenolase Activity | Diphenolase Activity | ||
| [95] | Arbutin | 2 | IC50 = 3 mM | IC50 = 40 mM | Mushroom tyrosinase; 1 mM catechol or 1 mM phenol |
| Arbutin undecylenic acid ester | 17 | IC50 = 0.4 mM | IC50 = 0.4 mM | ||
| [98] | Grevilloside M | 19 | No inhibition | Mushroom tyrosinase; 2.5 mM L-DOPA | |
| Robustaside D | 20 | No inhibition | |||
| [96] | Arbutin | 2 | 1.72% inhibition at 0.2 mM | Not detected | Mushroom tyrosinase; 2 mM L-tyrosine or 1 mM L-DOPA |
| Arbutin propionate | 15 | 0.86% inhibition at 0.2 mM | Not detected | ||
| Arbutin octylate | 16 | 8.42% inhibition at 0.2 mM | Not detected | ||
| Arbutin undecenoate | 17 | 15.64% inhibition at 0.2 mM | 8.01% inhibition at 0.2 mM | ||
| Arbutin laurate | 18 | Not detected | Not detected | ||
| [97] | Arbutin | 2 | IC50 = 29.4 mM | Silkworm hemolymph polyphenol oxidase; 14.4 mM L-DOPA | |
| Arbutin undecylenic acid ester | 17 | IC50 = 6.36 mM | |||
| Literature | Compounds | Statements | |
|---|---|---|---|
| Name | Chemical Structure | ||
| [103] | Arbutin | 2 | “The SCCS considers the use of β-arbutin to be safe for consumers in cosmetic products in a concentration up to 7% in face creams provided that the contamination of hydroquinone in the cosmetic formulations remain below 1 ppm.” |
| [104] | α-arbutin | 3 | “The SCCS considers the use of α-arbutin safe for consumers in cosmetic products in a concentration up to 2% in face creams and up to 0.5% in body lotions.” |
| [105] | Deoxyarbutin | 22 | “Therefore, the overall conclusion of the SCCS is that the use of deoxyarbutin up to 3% in face creams is not safe.” |
| Literature | Cells | Effects of Arbutin on Cell Viability |
|---|---|---|
| [50,52] | Human melanocytes derived from neonatal Caucasian or Asian neonatal foreskins | Arbutin treatment at 0.01–1.0 mM for 3 d did not reduce cell viability whereas 5 mM treatment reduced cell viability by 26%. |
| [53] | Normal human melanocytes from foreskins of 18- to 40-year-old Japanese males | Cells grew well in the presence of 0.37 mM arbutin for 5 d, but 1.1 mM arbutin was cytotoxic and cells detached from the dish within 48 h. |
| [56] | BRUCE-4 embryonic stem cells of C57BL/6J mouse; Mouse bone marrow-derived stromal ST2 cells | Arbutin treatment for 24 h did not inhibit the proliferation of either cell at 1 mM. |
| [94] | Murine melanoma B16 cells | After 24 h of treatment, up to 3.6 mM arbutin had no significant effect on cell viability. After 48 h, up to 0.7 mM arbutin did not induce significant toxicity. After 72 h, 0.3–5.4 mM arbutin reduced cell viability by 24–45%. Arbutin at 5.4 mM induced apoptosis. |
| [143] | Normal human skin fibroblasts | Treatment with up to 1 mM arbutin for 24 h did not affect cell viability. |
| [151] | Human prostate carcinoma. The LNCaP cell line Human prostate carcinoma LNCaP cells | Treatment with 125–2000 μM for 24, 48, or 72 h did not significantly affect cell viability.Arbutin induced apoptosis at 1000 μM. |
| [147] | Fibroblast cell line from human newborn foreskins; LNCaP cells | Arbutin reduced the viability of these cells at doses above 1000 μM at 24 and 48 h post-exposure. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. https://doi.org/10.3390/antiox10071129
Boo YC. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants. 2021; 10(7):1129. https://doi.org/10.3390/antiox10071129
Chicago/Turabian StyleBoo, Yong Chool. 2021. "Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties" Antioxidants 10, no. 7: 1129. https://doi.org/10.3390/antiox10071129






