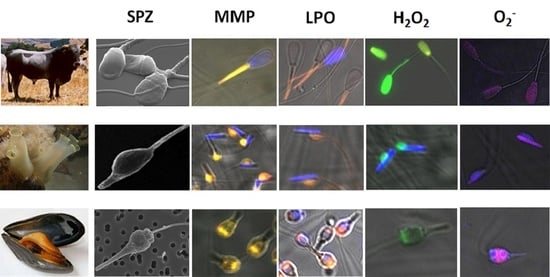
Sperm Motility, Oxidative Status, and Mitochondrial Activity: Exploring Correlation in Different Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sperm Collection and Preparation
2.1.1. Bovine
2.1.2. Mussels
2.1.3. Ascidians
2.1.4. Ethics Approval
2.2. Sperm Motility
2.3. Mitochondrial Membrane Potential Assessment
2.4. Intracellular ROS levels
2.5. Lipid Peroxidation Assessment
2.6. Data Analysis
3. Results
3.1. Data Report
3.2. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inaba, K. Molecular architecture of the sperm flagella: Molecules for motility and signaling. Zool. Sci. 2003, 20, 1043–1056. [Google Scholar] [CrossRef]
- Turner, R.M. Moving to the beat: A review of mammalian sperm motility regulation. Reprod. Fertil. Dev. 2005, 18, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial membrane potential (MMP) regulates sperm motility. Vitr. Cell. Dev. Biol. Anim. 2016, 52, 953–960. [Google Scholar] [CrossRef]
- Barbagallo, F.; Vignera, S.L.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of sperm mitochondrial function: A key organelle for sperm motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [Green Version]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Mitochondria and fertility: The mitochondria critical role on spermatozoa function. JDREAM. J. Interdiscip. Res. Appl. Med. 2017, 1, 21–26. [Google Scholar]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.B. Fluorescent labeling of mitochondria. Methods Cell Biol. 1988, 29, 103–123. [Google Scholar]
- Bezzaouia, A.; Gallo, A.; Silvestre, F.; Tekaya, S.; Tosti, E. Distribution pattern and activity of mitochondria during oocyte growth and maturation in the ascidian Styela plicata. Zygote 2014, 22, 462. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2017, 5, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.C.; Boni, R.; Cuccaro, A.; Tosti, E.; Gallo, A. Sperm motility impairment in free spawning invertebrates under near-future level of ocean acidification: Uncovering the mechanism. Front. Mar. Sci. 2020, 6, 794. [Google Scholar] [CrossRef]
- Gravance, C.; Garner, D.; Baumber, J.; Ball, B. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology 2000, 53, 1691–1703. [Google Scholar] [CrossRef]
- Marchetti, C.; Jouy, N.; Leroy-Martin, B.; Defossez, A.; Formstecher, P.; Marchetti, P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum. Reprod. 2004, 19, 2267–2276. [Google Scholar] [CrossRef] [Green Version]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6 (3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells: Implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997, 411, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Troiano, L.; Granata, A.R.; Cossarizza, A.; Kalashnikova, G.; Bianchi, R.; Pini, G.; Tropea, F.; Carani, C.; Franceschi, C. Mitochondrial membrane potential and DNA stainability in human sperm cells: A flow cytometry analysis with implications for male infertility. Exp. Cell Res. 1998, 241, 384–393. [Google Scholar] [CrossRef]
- Gosalvez, J.; Tvrda, E.; Agarwal, A. Free radical and superoxide reactivity detection in semen quality assessment: Past, present, and future. J. Assist. Reprod. Genet. 2017, 34, 697–707. [Google Scholar] [CrossRef]
- Robinson, K.M.; Janes, M.S.; Pehar, M.; Monette, J.S.; Ross, M.F.; Hagen, T.M.; Murphy, M.P.; Beckman, J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 2006, 103, 15038–15043. [Google Scholar] [CrossRef] [Green Version]
- Borst, J.W.; Visser, N.V.; Kouptsova, O.; Visser, A.J. Oxidation of unsaturated phospholipids in membrane bilayer mixtures is accompanied by membrane fluidity changes. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2000, 1487, 61–73. [Google Scholar] [CrossRef]
- Pap, E.H.; Drummen, G.; Post, J.; Rijken, P.; Wirtz, K. Fluorescent fatty acid to monitor reactive oxygen in single cells. Methods Enzymol. 2000, 319, 603–612. [Google Scholar] [PubMed]
- Losano, J.; Angrimani, D.; Dalmazzo, A.; Rui, B.; Brito, M.; Mendes, C.; Kawai, G.; Vannucchi, C.; Assumpção, M.; Barnabe, V. Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Reprod. Domest. Anim. 2017, 52, 289–297. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J. Androl. 2011, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Kasai, T.; Ogawa, K.; Mizuno, K.; Nagai, S.; Uchida, Y.; Ohta, S.; Fujie, M.; Suzuki, K.; Hirata, S.; Hoshi, K. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J. Androl. 2002, 4, 97–104. [Google Scholar] [PubMed]
- Rezaee-Tazangi, F.; Zeidooni, L.; Rafiee, Z.; Fakhredini, F.; Kalantari, H.; Alidadi, H.; Khorsandi, L. Taurine effects on bisphenol A-induced oxidative stress in the mouse testicular mitochondria and sperm motility. JBRA Assist. Reprod. 2020, 24, 428. [Google Scholar] [CrossRef]
- Wang, X.; Sharma, R.K.; Gupta, A.; George, V.; Thomas Jr, A.J.; Falcone, T.; Agarwal, A. Alterations in mitochondria membrane potential and oxidative stress in infertile men: A prospective observational study. Fertil. Steril. 2003, 80, 844–850. [Google Scholar] [CrossRef]
- Kao, S.-H.; Chao, H.-T.; Chen, H.-W.; Hwang, T.I.; Liao, T.-L.; Wei, Y.-H. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril. 2008, 89, 1183–1190. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular changes induced by oxidative stress that impair human sperm motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Wilson-Leedy, J.G.; Ingermann, R.L. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 2007, 67, 661–672. [Google Scholar] [CrossRef]
- Guo, H.; Gong, Y.; He, B.; Zhao, R. Relationships between mitochondrial DNA content, mitochondrial activity, and boar sperm motility. Theriogenology 2017, 87, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The role of the mitochondrion in sperm function: Is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr. Top. Dev. Biol. 2007, 77, 3–19. [Google Scholar]
- Volpe, S.; Leoci, R.; Aiudi, G.; Lacalandra, G. Relationship between motility and mitochondrial functional status in canine spermatozoa. Reprod. Domest. Anim. 2009, 44, 275–278. [Google Scholar] [CrossRef]
- Binet, M.; Doyle, C.; Williamson, J.; Schlegel, P. Use of JC-1 to assess mitochondrial membrane potential in sea urchin sperm. J. Exp. Mar. Biol. Ecol. 2014, 452, 91–100. [Google Scholar] [CrossRef]
- Gallo, A.; Boni, R.; Buttino, I.; Tosti, E. Spermiotoxicity of nickel nanoparticles in the marine invertebrate Ciona intestinalis (ascidians). Nanotoxicology 2016, 10, 1096–1104. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Manfra, L.; Boni, R.; Rotini, A.; Migliore, L.; Tosti, E. Cytotoxicity and genotoxicity of CuO nanoparticles in sea urchin spermatozoa through oxidative stress. Environ. Int. 2018, 118, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Menezo, Y.; Dale, B.; Coppola, G.; Dattilo, M.; Tosti, E.; Boni, R. Metabolic enhancers supporting 1-carbon cycle affect sperm functionality: An in vitro comparative study. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Tosti, E. Adverse effect of antifouling compounds on the reproductive mechanisms of the ascidian Ciona intestinalis. Mar. Drugs 2013, 11, 3554–3568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlegel, P.; Binet, M.T.; Havenhand, J.N.; Doyle, C.J.; Williamson, J.E. Ocean acidification impacts on sperm mitochondrial membrane potential bring sperm swimming behaviour near its tipping point. J. Exp. Biol. 2015, 218, 1084–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boni, R.; Gallo, A.; Montanino, M.; Macina, A.; Tosti, E. Dynamic changes in the sperm quality of Mytilus galloprovincialis under continuous thermal stress. Mol. Reprod. Dev. 2016, 83, 162–173. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Sharma, R.K.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Alshahrani, S.; Durairajanayagam, D.; Sabanegh, E. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod. Biol. Endocrinol. 2014, 12, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J., Jr.; Agarwal, A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, F.F.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil. Steril. 2000, 73, 459–464. [Google Scholar] [CrossRef]
- Whittington, K.; Harrison, S.C.; Williams, K.M.; Day, J.L.; Mclaughlin, E.A.; Hull, M.G.; Ford, W.C.L. Reactive oxygen species (ROS) production and the outcome of diagnostic tests of sperm function. Int. J. Androl. 1999, 22, 236–242. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The paradoxical relationship between stallion fertility and oxidative stress. Biol. Reprod. 2014, 91, 77. [Google Scholar] [CrossRef]
- Aitken, R.J.; Wingate, J.K.; de Iuliis, G.N.; McLaughlin, E.A. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol. Hum. Reprod. 2007, 13, 203–211. [Google Scholar] [CrossRef] [Green Version]
| Species | Tot Mot (%) | MMP (F0B/F0A) | LPO (F0A/(F0A + F0B)) × 100 | H2O2 (A.U.) | O2− (A.U.) |
|---|---|---|---|---|---|
| Bos taurus | 58.2 ± 26.3 | 4.2 ± 3.7 | 28.0 ± 17.1 | 26.5 ± 10.8 | 32.6 ± 13.8 |
| Ciona robusta | 42.2 ± 16.5 | 8.8 ± 6.6 | 37.7 ± 3.4 | 159.8 ± 46.8 | 97.6 ± 10.8 |
| Mytilus galloprovincialis | 42.9 ± 27.5 | 11.5 ± 6.1 | 28.2 ± 9.7 | 607.5 ± 478.7 | 247.6 ± 98.0 |
| Species | MMP | LPO | H2O2 | O2− | |
|---|---|---|---|---|---|
| Bos taurus | Tot Mot MMP LPO H2O2 | +0.683 *** | −0.890 *** −0.621 ** | −0.742 *** −0.320 +0.721 *** | +0.299 +0.334 −0.253 −0.398 |
| Ciona robusta | Tot Mot MMP LPO H2O2 | +0.272 | −0.660 * −0.720 *** | −0.790 *** −0.701 *** +0.840 *** | −0.693 *** −0.713 *** +0.946 *** +0.830 *** |
| Mytilus galloprovincialis | Tot Mot MMP LPO H2O2 | −0.637** | −0.595 ** +0.483 * | −0.650 ** +0.605 ** +0.288 | −0.608 ** +0.775 ** +0.208 +0.649 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, A.; Esposito, M.C.; Tosti, E.; Boni, R. Sperm Motility, Oxidative Status, and Mitochondrial Activity: Exploring Correlation in Different Species. Antioxidants 2021, 10, 1131. https://doi.org/10.3390/antiox10071131
Gallo A, Esposito MC, Tosti E, Boni R. Sperm Motility, Oxidative Status, and Mitochondrial Activity: Exploring Correlation in Different Species. Antioxidants. 2021; 10(7):1131. https://doi.org/10.3390/antiox10071131
Chicago/Turabian StyleGallo, Alessandra, Maria Consiglia Esposito, Elisabetta Tosti, and Raffaele Boni. 2021. "Sperm Motility, Oxidative Status, and Mitochondrial Activity: Exploring Correlation in Different Species" Antioxidants 10, no. 7: 1131. https://doi.org/10.3390/antiox10071131