In Vitro Anticancer Activity and Oxidative Stress Biomarkers Status Determined by Usnea barbata (L.) F.H. Wigg. Dry Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Lichen Extracts
2.2. Cell Lines and Cell Culture
2.3. MTT Assay
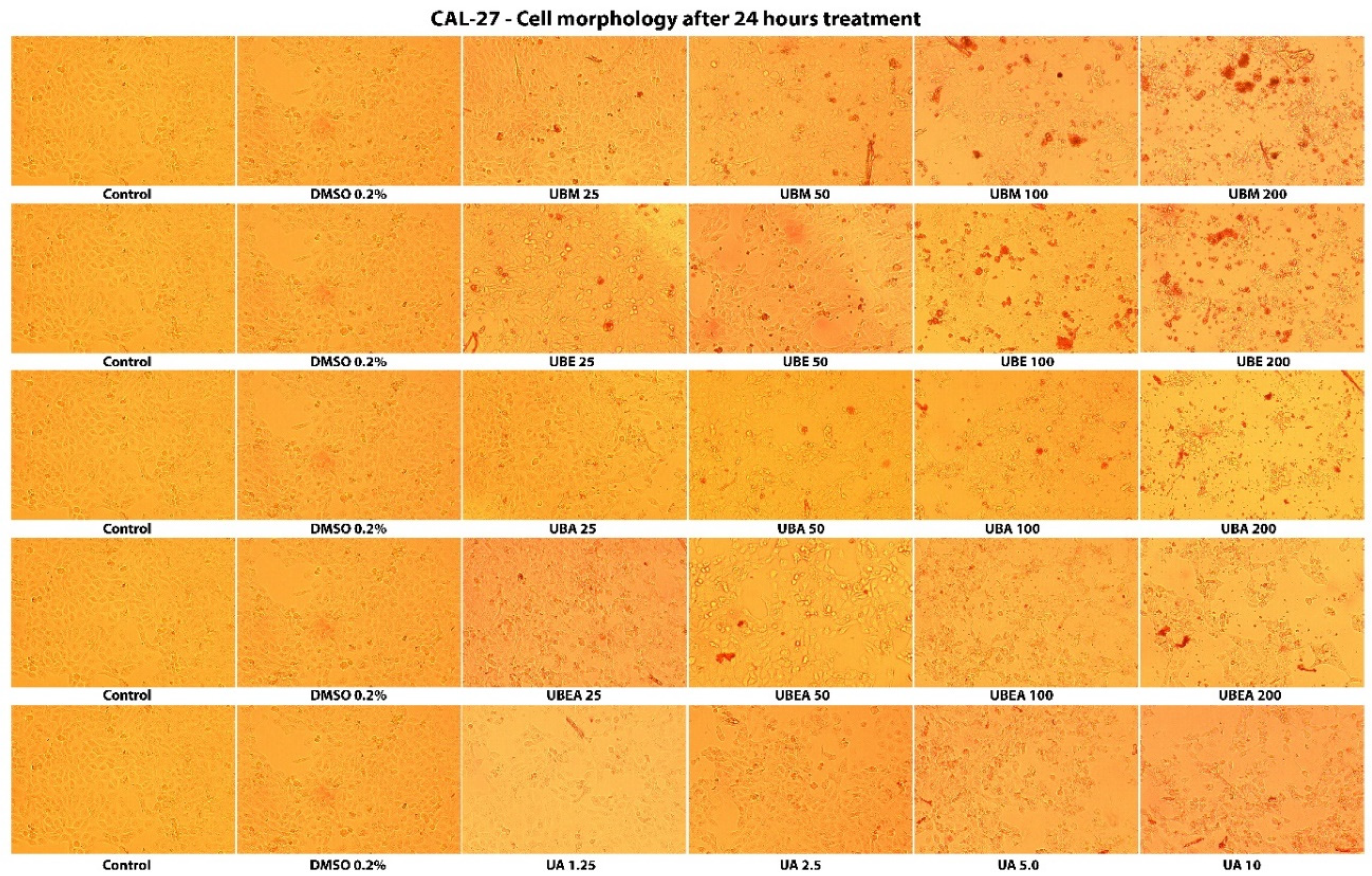
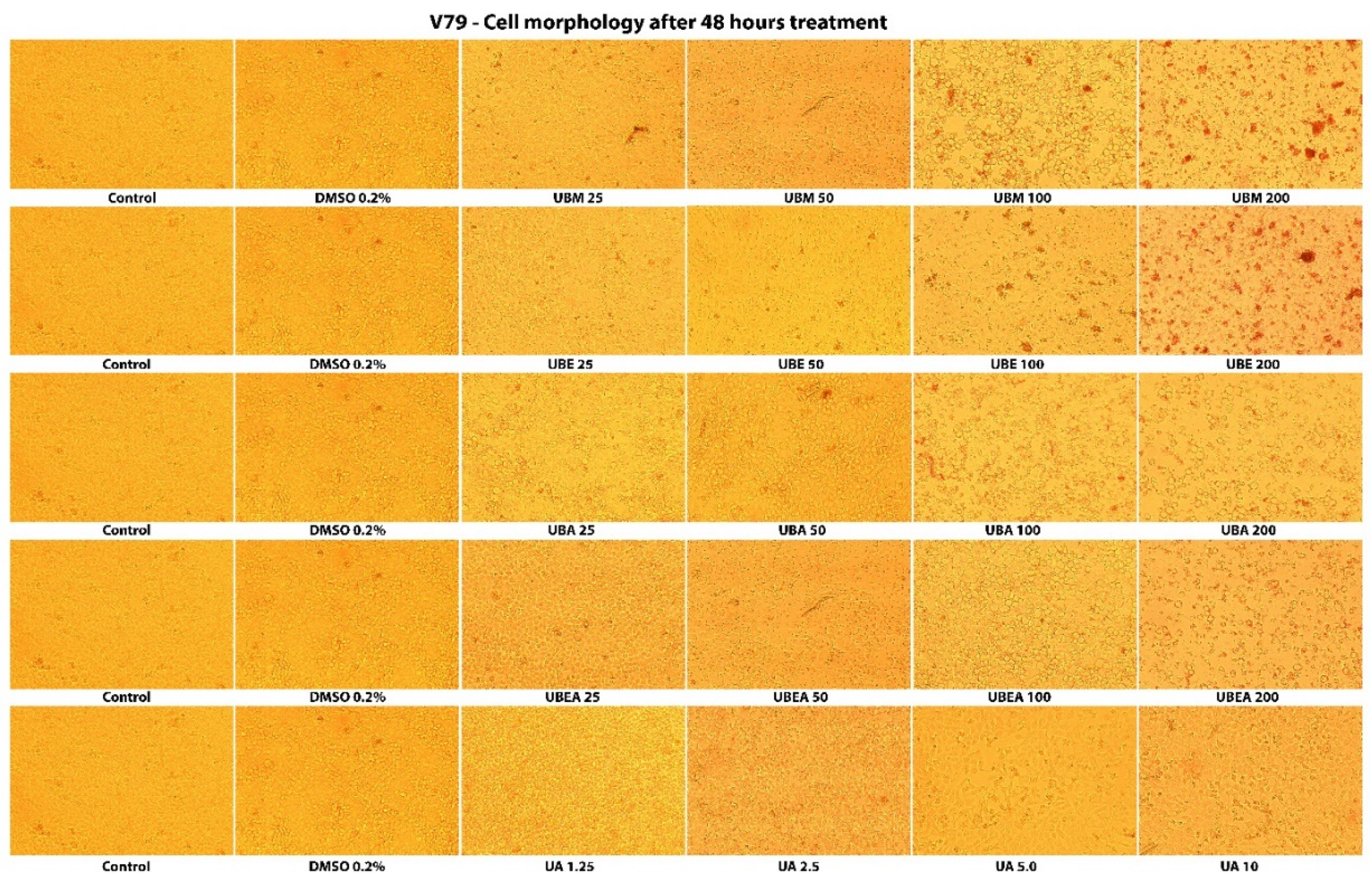
2.4. Cell Morphology Assay
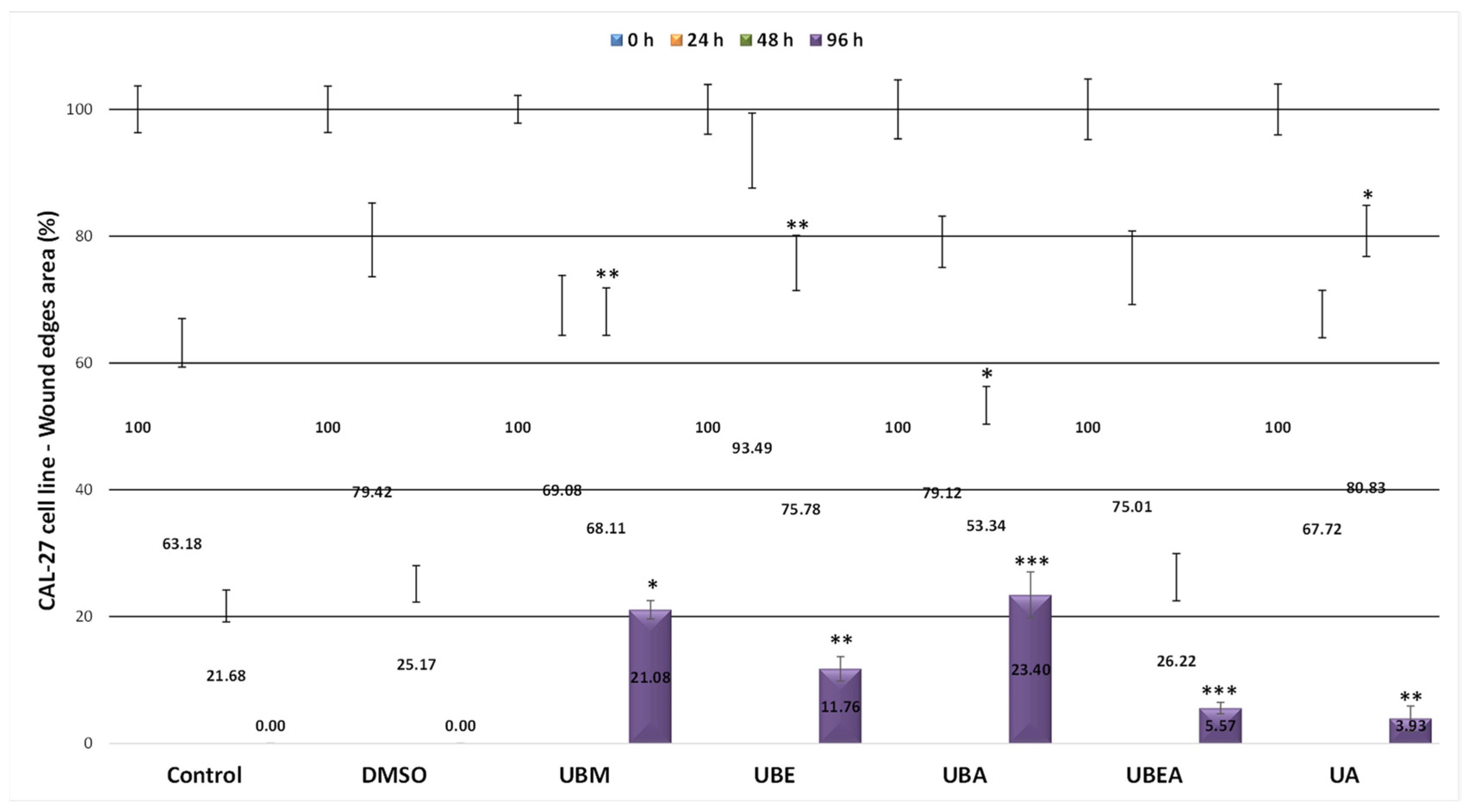
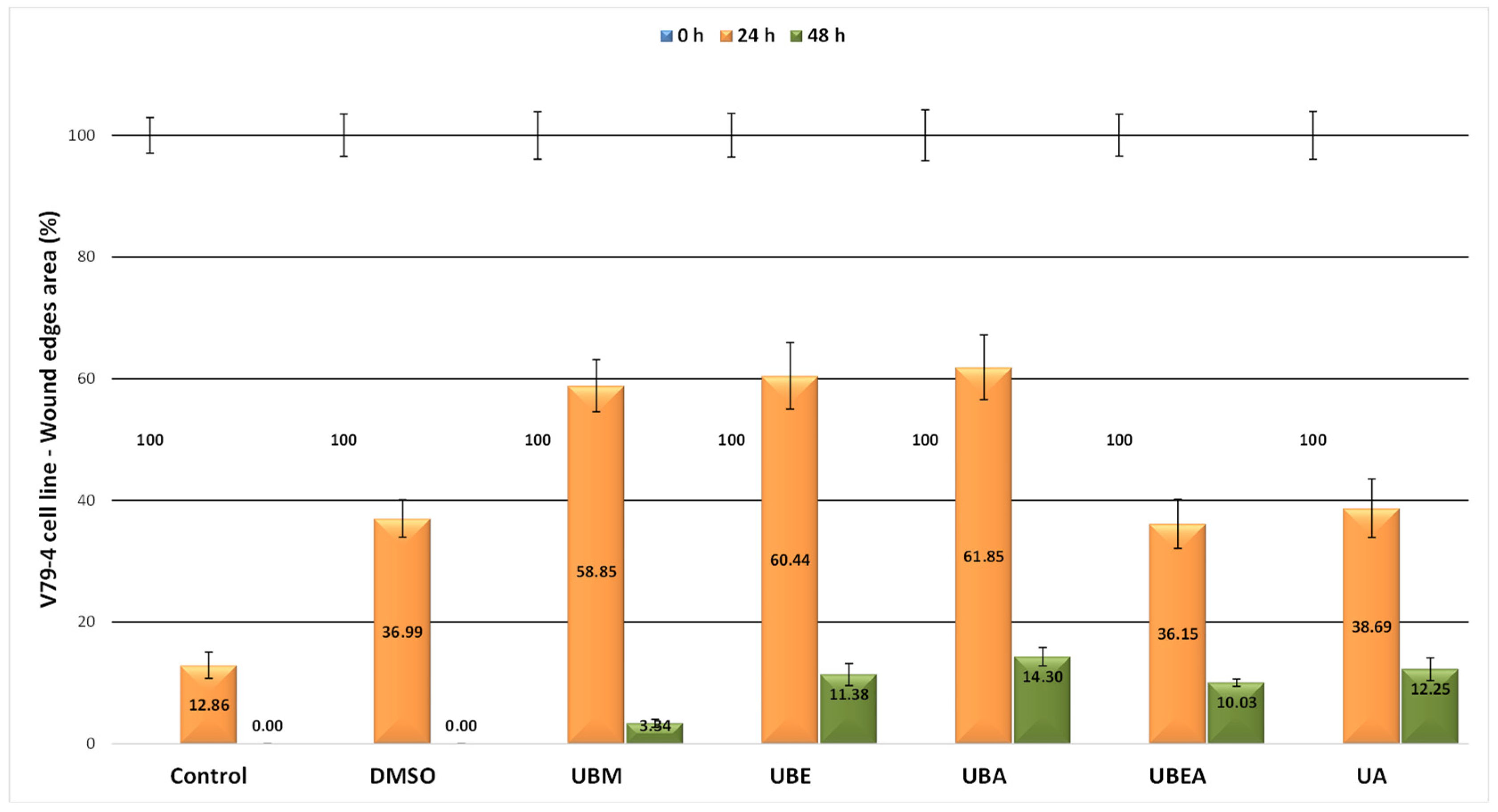
2.5. In Vitro Wound Healing Assay
2.6. Clonogenic Assay
2.7. Antioxidant Enzymes Activity Assay
2.7.1. Determination of Superoxide Dismutase Activity
2.7.2. Determination of Catalase Activity
2.7.3. Determination of Glutathione Peroxidase Activity
2.7.4. Determination of Malondialdehyde Levels
2.8. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Lichen Extracts
| U. barbata Dry Extract | Color | Temperature | Yield% | UA (mg/g) | TPC (mg/g DE) | TC (mg/g DE) |
|---|---|---|---|---|---|---|
| UBE | Light-brown | 75–80 °C | 12.52 | 127.21 | 67.3 | 14.7 |
| UBM | Brown | 65 °C | 11.29 | 137.60 | 70.7 | 9.99 |
| UBA | Yellow-brown | 55–60 °C | 6.36 | 282.78 | 101.09 | 24.4 |
| UBEA | Brown-yellow | 75–80 °C | 6.27 | 376.73 | 42.40 | 3.85 |
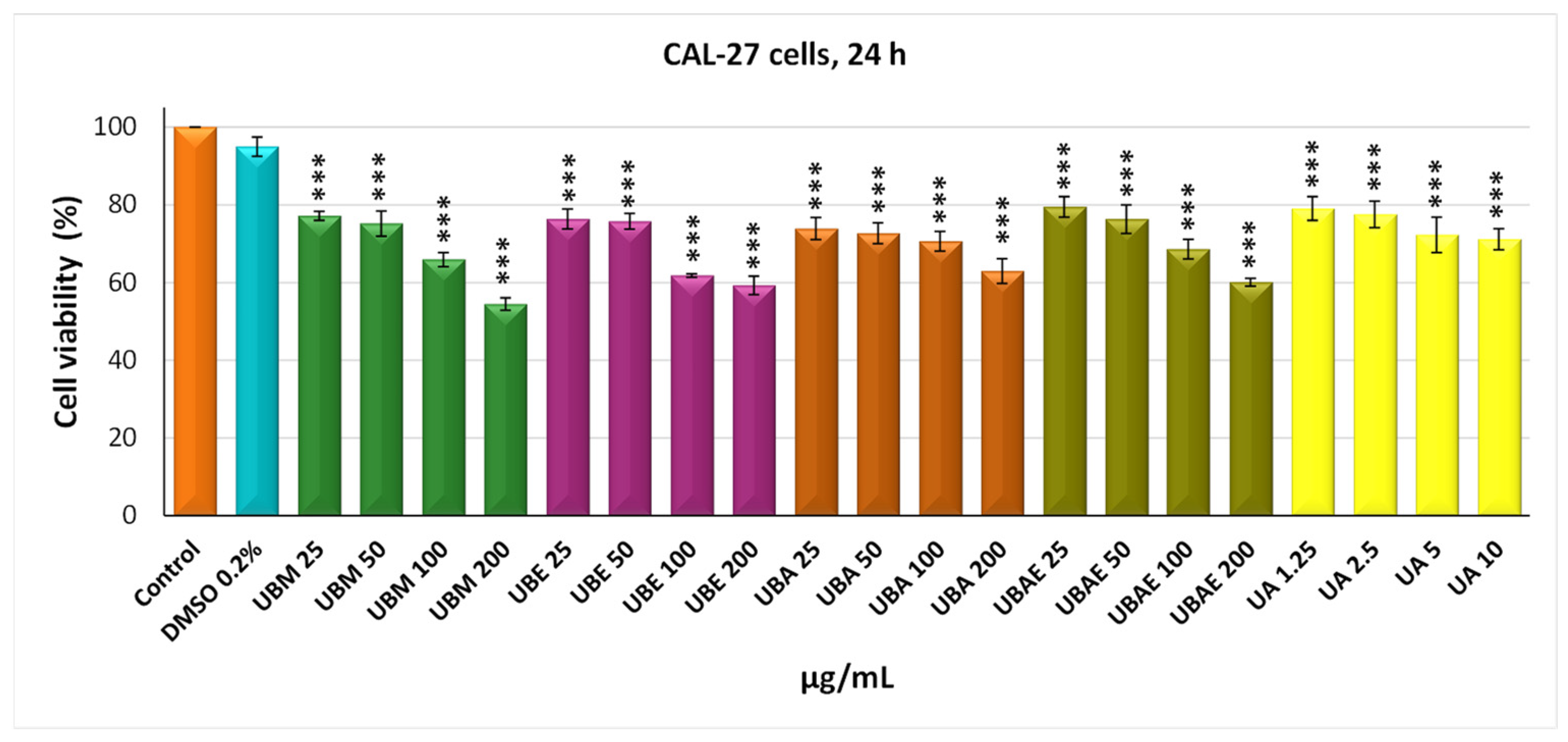
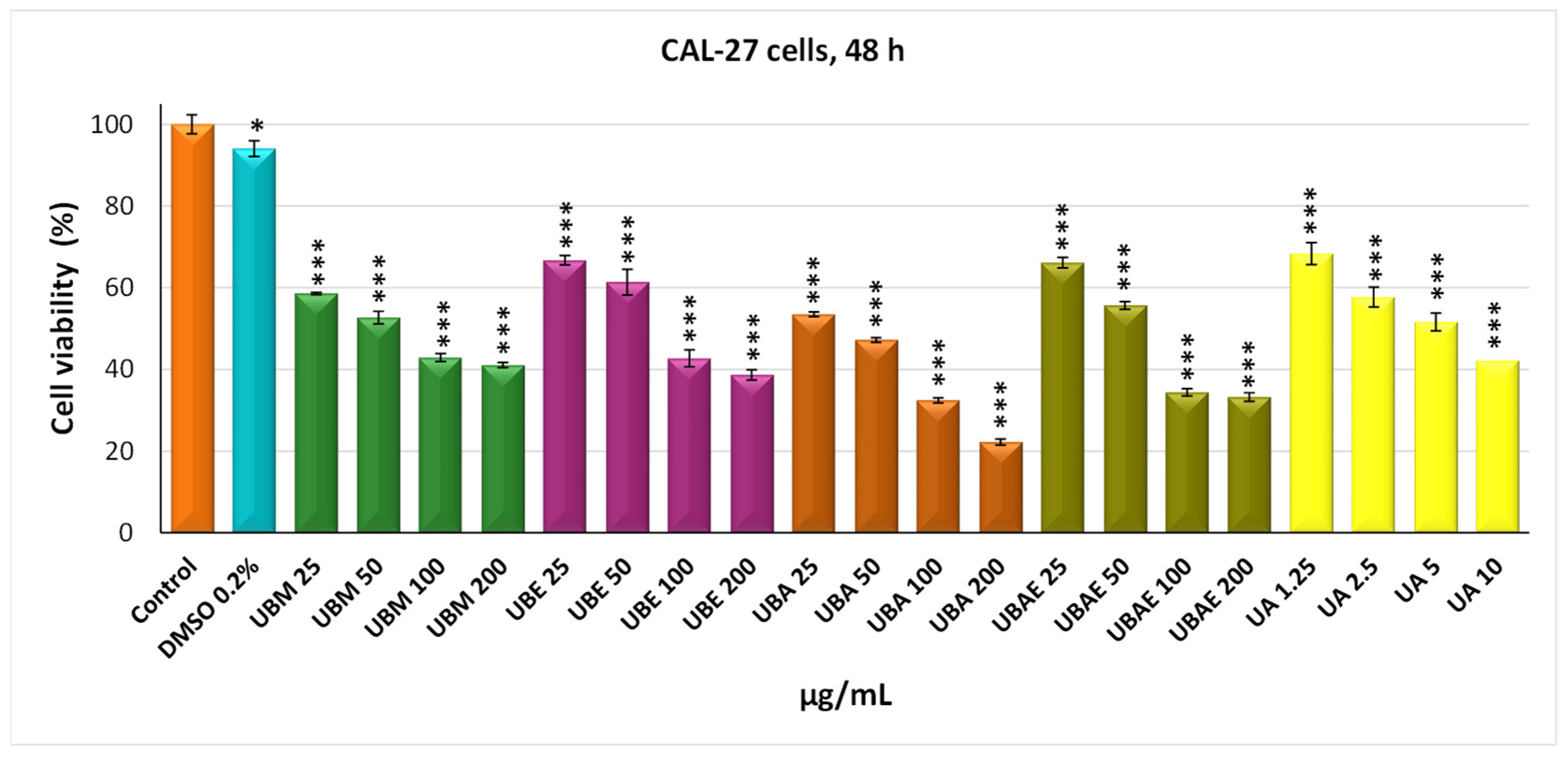
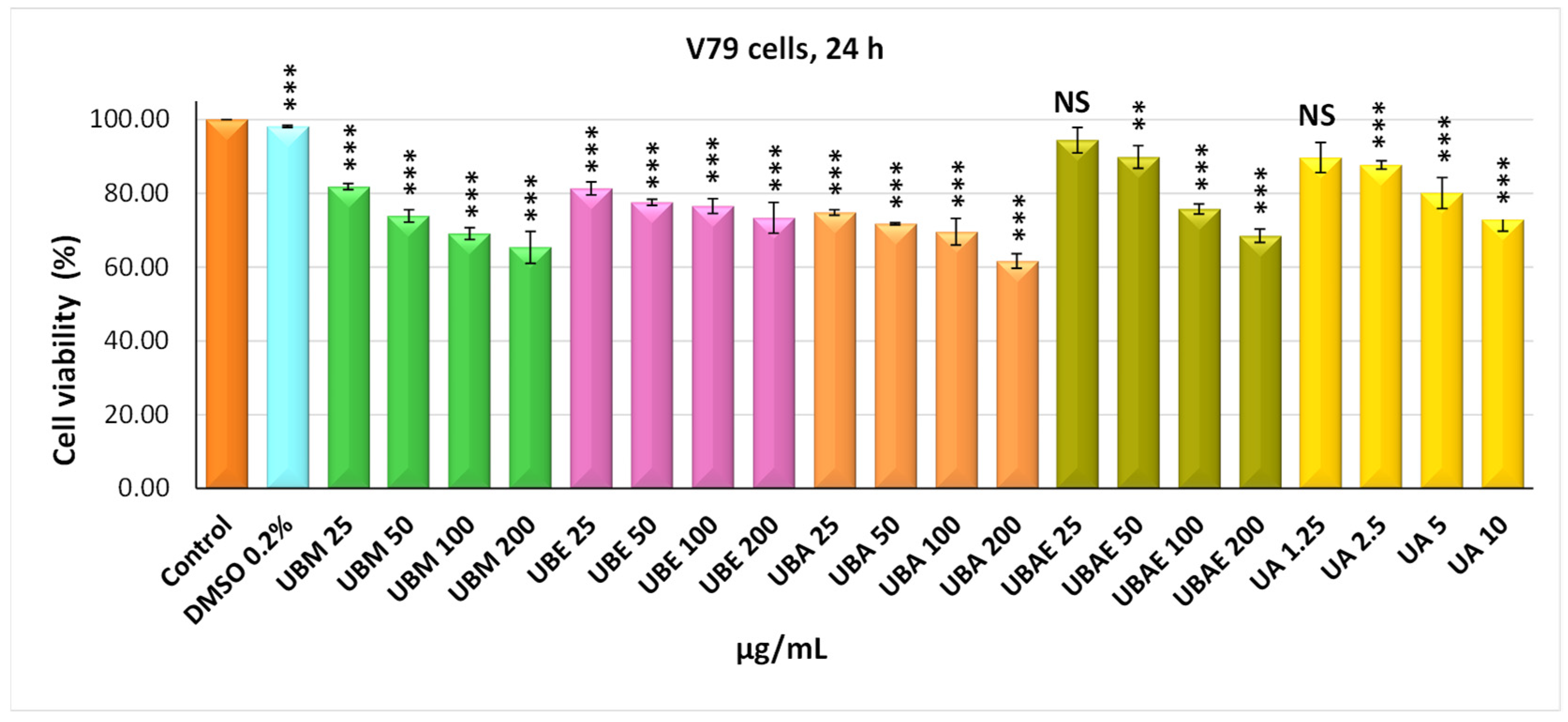
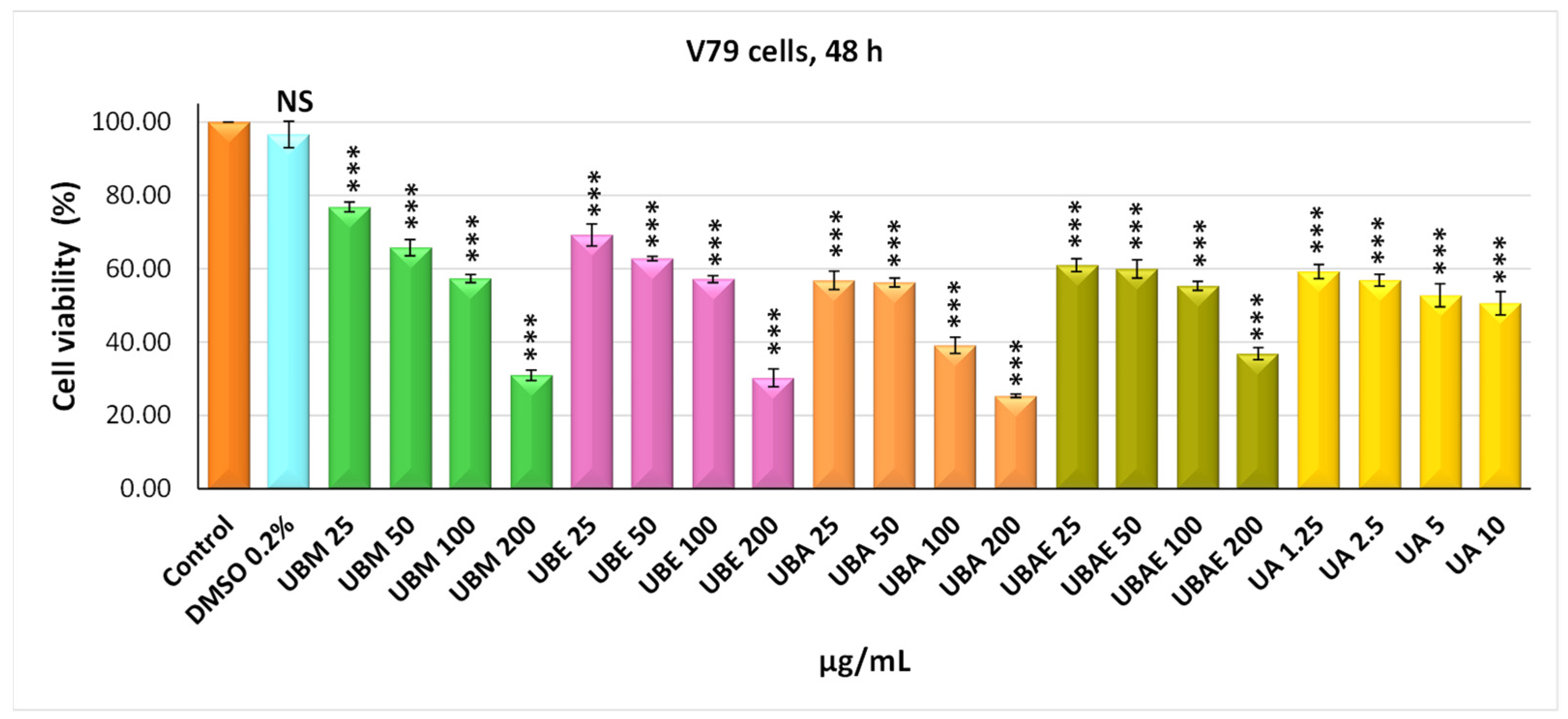
3.2. MTT Assay
3.3. Cell Morphology Assay
3.4. In Vitro Wound Healing Assay
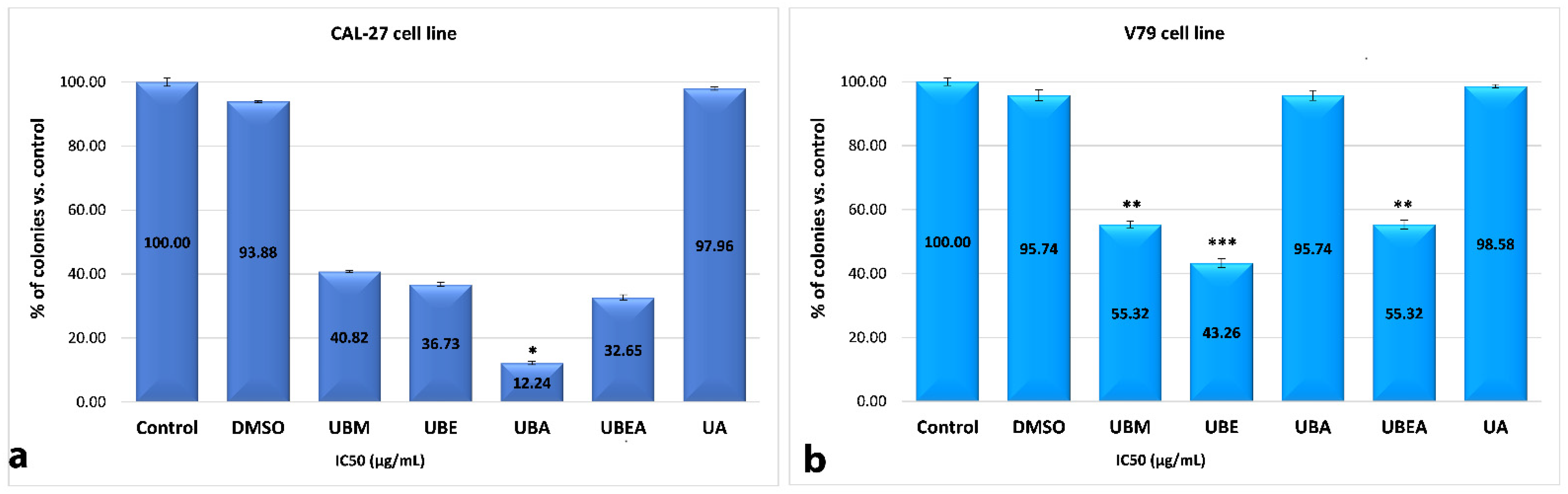
3.5. Clonogenic Assay
3.6. Antioxidant Enzymes Activity Assay
3.6.1. Determination of Superoxide Dismutase Activity
3.6.2. Determination of Catalase Activity
3.6.3. Determination of Glutathione Peroxidase Activity
3.6.4. Determination of Malondialdehyde levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Patafio, F.M.; Brooks, S.C.; Wei, X.; Peng, Y.; Biagi, J.; Booth, C.M. Research output and the public health burden of cancer: Is there any relationship? Curr. Oncol. 2016, 23, 75. [Google Scholar] [CrossRef] [Green Version]
- Markopoulos, A.K. Current Aspects on Oral Squamous Cell Carcinoma. Open Dent. J. 2012, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.L.; Wu, S.L.; Liao, H.F.; Jiang, C.M.; Huang, R.L.; Chen, Y.Y.; Yang, Y.C.; Chen, Y.J. Induction of apoptosis by three marine algae through generation of reactive oxygen species in human leukemic cell lines. J. Agric. Food Chem. 2005, 53, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Nanni, V.; Canuti, L.; Gismondi, A.; Canini, A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Peng, X.; He, Y.; Gao, Y.; Duan, F.; Chen, J.; Ruan, H. Cytochalasins from an endophytic fungus Phoma multirostrata XJ-2-1 with cell cycle arrest and TRAIL-resistance-overcoming activities. Bioorg. Chem. 2020, 104, 104317. [Google Scholar] [CrossRef] [PubMed]
- Reynertson, K.A.; Charlson, M.E.; Gudas, L.J. Induction of murine embryonic stem cell differentiation by medicinal plant extracts. Exp. Cell Res. 2011, 317, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Chang, W.-T.; Lin, I.-L.; Chen, Y.-F.; Padalwar, N.B.; Cheng, K.-C.; Teng, Y.-N.; Wang, C.-H.; Chiu, C.-C. cancers The Role of Necroptosis in ROS-Mediated Cancer Therapies and Its Promising Applications. Cancers 2020, 12, 2185. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, W.-W.; Zhang, X.; Shi, J.-G.; Jiang, S.; Zheng, L.; Qin, Y.; Liu, B.; Shi, J.-H. TRAF3 promotes ROS production and pyroptosis by targeting ULK1 ubiquitination in macrophages. FASEB J. 2020, 34, 7144–7159. [Google Scholar] [CrossRef] [Green Version]
- Somaida, A.; Tariq, I.; Ambreen, G.; Abdelsalam, A.M.; Ayoub, A.M.; Wojcik, M.; Dzoyem, J.P.; Bakowsky, U. Potent cytotoxicity of four cameroonian plant extracts on different cancer cell lines. Pharmaceuticals 2020, 13, 357. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2020, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Majid, M.Z.; Zaini, Z.M.; Razak, F.A. Apoptosis-Inducing Effect of Three Medicinal Plants on Oral Cancer Cells KB and ORL-48. Sci. World J. 2014, 125353. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef]
- Wang, L.; Qin, X.; Liang, J.; Ge, P. Induction of Pyroptosis: A Promising Strategy for Cancer Treatment. Front. Oncol. 2021, 11, 635774. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Sarcognato, S.; Jong, I.E.M.; Fabris, L.; Cadamuro, M.; Guido, M. Necroptosis in Cholangiocarcinoma. Cells 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- Lou, J.; Zhou, Y.; Feng, Z.; Ma, M.; Yao, Y.; Wang, Y.; Deng, Y.; Wu, Y. Caspase-Independent Regulated Necrosis Pathways as Potential Targets in Cancer Management. Front. Oncol. 2021, 10, 616952. [Google Scholar] [CrossRef]
- Nanni, V.; Di Marco, G.; Sacchetti, G.; Canini, A.; Gismondi, A. Oregano phytocomplex induces programmed cell death in melanoma lines via mitochondria and DNA damage. Foods 2020, 9, 1486. [Google Scholar] [CrossRef]
- Balhamar, S.O.M.S.; Panicker, N.G.; Akhlaq, S.; Qureshi, M.M.; Ahmad, W.; Rehman, N.U.; Ali, L.; Al-Harrasi, A.; Hussain, J.; Mustafa, F. Differential cytotoxic potential of acridocarpus orientalis leaf and stem extracts with the ability to induce multiple cell death pathways. Molecules 2019, 24, 3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, L.; Chakraborty, S.; ArulJothi, K.N.; Mabasa, L.; Sayah, K.; Costa-Lotufo, L.V.; Jardine, A.; Prince, S. Galenia africana plant extract exhibits cytotoxicity in breast cancer cells by inducing multiple programmed cell death pathways. Saudi Pharm. J. 2020, 28, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Pujari, I.; Thomas, A.; Thomas, J.; Jhawar, N.; Guruprasad, K.P.; Rai, P.S.; Satyamoorthy, K.; Babu, V.S. Cytotoxicity and radiosensitizing potency of Moscatilin in cancer cells at low radiation doses of X-ray and UV-C. 3 Biotech 2021, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N.; Hegazy, M.E.F.; Naqishbandi, A.M.; Efferth, T. Induction of apoptosis, autophagy and ferroptosis by Thymus vulgaris and Arctium lappa extract in leukemia and multiple myeloma cell lines. Molecules 2020, 25, 5016. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.S. Cellular senescence: A promising strategy for cancer therapy. BMB Rep. 2019, 52, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirci, D.; Dayanc, B.; Mazi, F.A.; Senturk, S. The Jekyll and Hyde of Cellular Senescence in Cancer. Cells 2021, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Niklas, A.; Uruski, P.; Tykarski, A.; Książek, K. Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells. Cell. Mol. Life Sci. 2020, 77, 213–229. [Google Scholar] [CrossRef] [Green Version]
- Milczarek, M. The premature senescence in breast cancer treatment strategy. Cancers 2020, 12, 1815. [Google Scholar] [CrossRef]
- Li, L.H.; Wu, P.; Lee, J.Y.; Li, P.R.; Hsieh, W.Y.; Ho, C.C.; Ho, C.L.; Chen, W.J.; Wang, C.C.; Yen, M.Y.; et al. Hinokitiol induces DNA damage and autophagy followed by cell cycle arrest and senescence in gefitinib-resistant lung adenocarcinoma cells. PLoS ONE 2014, 9, e104203. [Google Scholar]
- Zheng, T.; Que, Z.; Jiao, L.; Kang, Y.; Gong, Y.; Yao, J.; Ma, C.; Bi, L.; Dong, Q.; Zhao, X.; et al. Herbal formula YYJD inhibits tumor growth by inducing cell cycle arrest and senescence in lung cancer. Sci. Rep. 2017, 7, 4984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagosklonny, M.V. Cell cycle arrest is not senescence. Aging 2011, 3, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Lee, Y.H.; Sharma, A.R.; Park, J.B.; Jagga, S.; Sharma, G.; Lee, S.S.; Nam, J.S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Dalimunthe, A.; Hasibuan, P.A.Z.; Satria, D. Cell cycle arrest activity of Litsea cubeba lour: Heartwood and fruit extracts against T47D breast cancer cells. Asian J. Pharm. Clin. Res. 2017, 10, 404–406. [Google Scholar] [CrossRef] [Green Version]
- De Souza Grinevicius, V.M.A.; Kviecinski, M.R.; Santos Mota, N.S.R.; Ourique, F.; Porfirio Will Castro, L.S.E.; Andreguetti, R.R.; Gomes Correia, J.F.; Filho, D.W.; Pich, C.T.; Pedrosa, R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016, 189, 139–147. [Google Scholar] [CrossRef]
- Chen, V.; Staub, R.E.; Fong, S.; Tagliaferri, M.; Cohen, I.; Shtivelman, E. Bezielle selectively targets mitochondria of cancer cells to inhibit glycolysis and OXPHOS. PLoS ONE 2012, 7, e0030300. [Google Scholar] [CrossRef] [Green Version]
- Perez, A.T.; Arun, B.; Tripathy, D.; Tagliaferri, M.A.; Shaw, H.S.; Kimmick, G.G.; Cohen, I.; Shtivelman, E.; Caygill, K.A.; Grady, D.; et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res. Treat. 2010, 120, 111–118. [Google Scholar] [CrossRef]
- Rugo, H.; Shtivelman, E.; Perez, A.; Vogel, C.; Franco, S.; Tan Chiu, E.; Melisko, M.; Tagliaferri, M.; Cohen, I.; Shoemaker, M.; et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res. Treat. 2007, 105, 17–28. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, Q.; Yang, C.; Yu, P.; et al. Anti-Cancer Activities of Tea Epigallocatechin-3-Gallate in Breast Cancer Patients under Radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef]
- Jasek, K.; Kubatka, P.; Samec, M.; Liskova, A.; Smejkal, K.; Vybohova, D.; Bugos, O.; Biskupska-Bodova, K.; Bielik, T.; Zubor, P.; et al. DNA methylation status in cancer disease: Modulations by plant-derived natural compounds and dietary interventions. Biomolecules 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elix, J.A.; Stocker-Wörgötter, E. Biochemistry and Secondary Metabolites. In Lichen Biology, 2nd ed.; Nash, T.H., Ed.; Cambridge University Press: New York, NY, USA, 2008; pp. 104–133. [Google Scholar]
- Solárová, Z.; Liskova, A.; Samec, M.; Kubatka, P.; Büsselberg, D.; Solár, P. Anticancer potential of lichens’ secondary metabolites. Biomolecules 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Vartia, K.O. Antibiotics in Lichens. In The Lichens; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: New York, NY, USA, 1973; pp. 547–558. [Google Scholar]
- Fahselt, D. Secondary biochemistry of lichens. Symbiosis 1994, 1, 117–165. [Google Scholar]
- Shrestha, G.; Clair, L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Piovano, M.; Russo, A. Potential anticancer activity of lichen secondary metabolite physodic acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Chen, Y.C.; Huang, Y.C.; Lin, W.C.; Lu, C.H. Systemic oxidative stress and cognitive function in Parkinson’s disease with different PWMH or DWMH lesions. BMC Neurol. 2021, 21, 16. [Google Scholar] [CrossRef]
- Yang, Y.; Nguyen, T.T.; Jeong, M.H.; Crişan, F.; Yu, Y.H.; Ha, H.H.; Choi, K.H.; Jeong, H.G.; Jeong, T.C.; Lee, K.Y.; et al. Inhibitory activity of (+)-usnic acid against non-small cell lung cancer cell motility. PLoS ONE 2016, 11, e0146575. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Y.; Park, S.Y.; Nguyen, T.T.; Seo, Y.W.; Lee, K.H.; Lee, J.H.; Kim, K.K.; Hur, J.S.; Kim, H. The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis. Sci. Rep. 2017, 7, 8136. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.H.; Dinh, M.H.; Chi, H.T.; Wang, S.L.; Nguyen, Q.V.; Tran, T.D.; Nguyen, A.D. Antioxidant and cytotoxic activity of lichens collected from Bidoup Nui Ba National Park, Vietnam. Res. Chem. Intermed. 2019, 45, 33–49. [Google Scholar] [CrossRef]
- Taş, İ.; Han, J.; Park, S.Y.; Yang, Y.; Zhou, R.; Gamage, C.D.B.; Van Nguyen, T.; Lee, J.Y.; Choi, Y.J.; Yu, Y.H.; et al. Physciosporin suppresses the proliferation, motility and tumourigenesis of colorectal cancer cells. Phytomedicine 2019, 56, 10–20. [Google Scholar] [CrossRef]
- Bjerke, J.W.; Elvebakk, A.; Domínguez, E.; Dahlback, A. Seasonal trends in usnic acid concentrations of Arctic, alpine and Patagonian populations of the lichen Flavocetraria nivalis. Phytochemistry 2005, 66, 337–344. [Google Scholar] [CrossRef]
- Ingolfsdóttir, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Araújo, A.A.S.; De Melo, M.G.D.; Rabelo, T.K.; Nunes, P.S.; Santos, S.L.; Serafini, M.R.; Santos, M.R.V.; Quintans, L.J.; Gelain, D.P. Review of the biological properties and toxicity of usnic acid. Nat. Prod. Res. 2015, 29, 2167–2180. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 2. effects on higher organisms. Molecular and physicochemical aspects. Russ. J. Bioorg. Chem. 2016, 42, 249–268. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef] [Green Version]
- Prateeksha; Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.N.R.; Nayaka, S.; Joshi, Y.; et al. The genus Usnea: A potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv. 2016, 26, 21672–21696. [Google Scholar]
- Popovici, V.; Bucur, L.; Popescu, A.; Schröder, V.; Costache, T.; Rambu, D.; Cucolea, I.E.; Gîrd, C.E.; Caraiane, A.; Gherghel, D.; et al. Antioxidant and cytotoxic activities of usnea barbata (L.) f.h. wigg. dry extracts in different solvents. Plants 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Sigma Aldrich IR Spectrum Table & Chart|Sigma-Aldrich. IR Spectr. Table Chart 2018, 10, 909.
- Massarczyk, M.; Rudack, T.; Schlitter, J.; Kuhne, J.; Kötting, C.; Gerwert, K. Local Mode Analysis: Decoding IR Spectra by Visualizing Molecular Details. J. Phys. Chem. B 2017, 121, 3483–3492. [Google Scholar] [CrossRef]
- Winter, A. How to Identify Carbonyls, Alkenes, Alkynes, and Aromatics in the IR Spectrum. Dummies 2020. Available online: https://www.dummies.com/education/science/chemistry/how-to-identify-carbonyls-alkenes-alkynes-and-aromatics-in-the-ir-spectrum/ (accessed on 25 June 2021).
- Mosmann, T. Rapid colorimetric assay for cellular growth and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laville, N.; Aït-Ässa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Pinto, B.I.; Cruz, N.D.; Lujan, O.R.; Propper, C.R.; Kellar, R.S. In Vitro Scratch Assay to Demonstrate Effects of Arsenic on Skin Cell Migration. J. Vis. Exp. 2019, 144, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Rafehi, H.; Orlowski, C.; Georgiadis, G.T.; Ververis, K.; El-Osta, A.; Karagiannis, T.C. Clonogenic assay: Adherent cells. J. Vis. Exp. 2011, 10, 2573. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 7, 389–394. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A. Glutathione peroxidase activity in tissues of vitamin e-deficient mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artenie, V.; Ungureanu, E. Negură Anca Mihaela, Metode de investigare a metabolismului glucidic şi lipidic. Ed. Pim Iaşi 2008, 97–102, 108–110. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fletcher, O. Maths from Scratch for Biologists. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 390. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Carreño, E.A.; Alberto, A.V.P.; de Souza, C.A.M.; de Mello, H.L.; Henriques-Pons, A.; Alves, L.A. Considerations and technical pitfalls in the employment of the MTT assay to evaluate photosensitizers for photodynamic therapy. Appl. Sci. 2021, 11, 2603. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary metabolite profiling of species of the genus usnea by UHPLC-ESI-OT-MS-MS. Molecules 2018, 23, 54. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.S.; Siva, B.; Kumar, K.; Phani Babu, V.S.; Sravanthi, V.; Boustie, J.; Lakshma Nayak, V.; Tiwari, A.K.; Rao, C.H.V.; Sridhar, B.; et al. Comprehensive Analysis of Secondary Metabolites in Usnea longissima (Lichenized Ascomycetes, Parmeliaceae) Using UPLC-ESI-QTOF-MS/MS and Pro-Apoptotic Activity of Barbatic Acid. Molecules 2019, 24, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondratiuk, A.S.; Savchuk, O.M.; Hur, J.S. Optimization of protein extraction for lichen thalli. Mycobiology 2015, 43, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Bézivin, C.; Tomasi, S.; Lohezic-Le Devehat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Rančić, A.; Manojlović, N. Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents. LWT Food Sci. Technol. 2014, 59, 518–525. [Google Scholar] [CrossRef]
- Emsen, B.; Ozdemir, O.; Engin, T.; Togar, B.; Cavusoglu, S.; Turkez, H. Inhibition of growth of U87MG human glioblastoma cells by Usnea longissima ach. Anais Acad. Bras. Cienc. 2019, 91, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Wu, K.H.; Wang, Y.Y.; Farooqi, A.A.; Huang, H.W.; Yuan, S.S.F.; Jian, R.I.; Tsao, L.Y.; Chen, P.A.; Chang, F.R.; et al. Methanol extract of usnea barbata induces cell killing, apoptosis, and dna damage against oral cancer cells through oxidative stress. Antioxidants 2020, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Aoussar, N.; Laasri, F.E.; Bourhia, M.; Manoljovic, N.; Mhand, R.A.; Rhallabi, N.; Ullah, R.; Shahat, A.A.; Noman, O.M.; Nasr, F.A.; et al. Phytochemical analysis, cytotoxic, antioxidant, and antibacterial activities of lichens. Evid. Based Complement. Altern. Med. 2020, 8104538. [Google Scholar] [CrossRef]
- Ari, F.; Aztopal, N.; Oran, S.; Bozdemir, S.; Celikler, S.; Ozturk, S.; Ulukaya, E. Parmelia sulcata Taylor and Usnea filipendula Stirt induce apoptosis-like cell death and DNA damage in cancer cells. Cell Prolif. 2014, 47, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zugic, A.; Jeremic, I.; Isakovic, A.; Arsic, I.; Savic, S.; Tadic, V. Evaluation of anticancer and antioxidant activity of a commercially available CO2 supercritical extract of old man’s beard (Usnea barbata). PLoS ONE 2016, 11, e0146342. [Google Scholar] [CrossRef] [Green Version]
- Tram, N.T.T.; Anh, D.H.; Thuc, H.H.; Tuan, N.T. Investigation of chemical constituents and cytotoxic activity of the lichen Usnea undulata. Vietnam J. Chem. 2020, 58, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, S.; Erkisa, M.; Oran, S.; Ulukaya, E.; Celikler, S.; Ari, F. Lichens exerts an anti-proliferative effect on human breast and lung cancer cells through induction of apoptosis. Drug Chem. Toxicol. 2019, 44, 259–267. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.A.; Schröder, V.; Gherghel, D.; Mihai, C.T.; Caraiane, A.; Badea, F.C.; Vochița, G.; Badea, V. Evaluation of the cytotoxic activity of the Usnea barbata (L.) F. H. Wigg dry extract. Molecules 2020, 8, 1865. [Google Scholar] [CrossRef] [Green Version]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- Sepahvand, A.; Studzińsk-Sroka, E.; Ramak, P.; Karimian, V. Usnea sp.: Antimicrobial potential, bioactive compounds, ethnopharmacological uses and other pharmacological properties; a review article. J. Ethnopharmacol. 2021, 268, 113656. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Li, Y.; Bai, H.; Ma, T.; Song, X.; Zhao, J.; Gao, L. The effects of sodium usnic acid by topical application on skin wound healing in rats. Biomed Pharmacother. 2018, 97, 587–593. [Google Scholar] [CrossRef]
- Burlando, B.; Ranzato, E.; Volante, A.; Appendino, G.; Pollastro, F.; Verotta, L. Antiproliferative effects on tumour cells and promotion of keratinocyte wound healing by different lichen compounds. Planta Med. 2009, 75, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.; Trucchi, B.; Burlando, B.; Ranzato, E.; Martinotti, S.; Akkol, E.K.; Süntar, I.; Keleş, H.; Verotta, L. (+)-Usnic acid enamines with remarkable cicatrizing properties. Bioorg. Med. Chem. 2013, 21, 1834–1843. [Google Scholar] [CrossRef]
- Brzozowska, B.; Gałecki, M.; Tartas, A.; Ginter, J.; Kaźmierczak, U.; Lundholm, L. Freeware tool for analysing numbers and sizes of cell colonies. Radiat. Environ. Biophys. 2019, 58, 109–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solár, P.; Hrčková, G.; Koptašíková, L.; Velebný, S.; Solárová, Z.; Bačkor, M. Murine breast carcinoma 4T1 cells are more sensitive to atranorin than normal epithelial NMuMG cells in vitro: Anticancer and hepatoprotective effects of atranorin in vivo. Chem. Biol. Interact. 2016, 250, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Guzow-Krzemińska, B.; Guzow, K.; Herman-Antosiewicz, A. Usnic Acid Derivatives as Cytotoxic Agents Against Cancer Cells and the Mechanisms of Their Activity. Curr. Pharmacol. Rep. 2019, 5, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Pyrczak-Felczykowska, A.; Narlawar, R.; Pawlik, A.; Guzow-Krzemińska, B.; Artymiuk, D.; Hać, A.; Ryś, K.; Rendina, L.M.; Reekie, T.A.; Herman-Antosiewicz, A.; et al. Synthesis of Usnic Acid Derivatives and Evaluation of Their Antiproliferative Activity against Cancer Cells. J. Nat. Prod. 2019, 82, 1768–1778. [Google Scholar] [CrossRef]
- Glorieux, C.; Sandoval, J.M.; Fattaccioli, A.; Dejeans, N.; Garbe, J.C.; Dieu, M.; Verrax, J.; Renard, P.; Huang, P.; Calderon, P.B. Chromatin remodeling regulates catalase expression during cancer cells adaptation to chronic oxidative stress. Free Radic. Biol. Med. 2016, 99, 436–450. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Piotrowska, H.; Kucińska, M.; Murias, M.; Bylka, W. Cytotoxic activity of physodic acid and acetone extract from Hypogymnia physodes against breast cancer cell lines. Pharm. Biol. 2016, 54, 2480–2485. [Google Scholar] [CrossRef] [Green Version]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell. Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef]
- De Paz, G.; Raggio, J.; Gómez-Serranillos, M.P.; Palomino, O.M.; González-Burgos, E.; Carretero, M.E.; Crespo, A. HPLC isolation of antioxidant constituents from Xanthoparmelia spp. J. Pharm. Biomed. Anal. 2010, 53, 165–171. [Google Scholar] [CrossRef]
- Su, Z.Q.; Mo, Z.Z.; Liao, J.B.; Feng, X.X.; Liang, Y.Z.; Zhang, X.; Liu, Y.H.; Chen, X.Y.; Chen, Z.W.; Su, Z.R.; et al. Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. Int. Immunopharmacol. 2014, 22, 371–378. [Google Scholar] [CrossRef] [PubMed]





| U. barbata Extract | UBA | UBE | UBM | UBEA | ||||
|---|---|---|---|---|---|---|---|---|
| Domain (cm−1) | Frequency | Interpretation | Frequency | Interpretation | Frequency | Interpretation | Frequency | Interpretation |
| 4000–2500 cm−1 single bonds region | 3448 (sharp) | N–H secondary amine in aromatic nucleus | NA | NA | 3575 (sharp) | OH (phenol) | ||
| 3242 (broad) | OH (phenol) | 3230 (broad) | OH (phenol) | 3235 (broad) | OH (phenol) | 3289 (broad) | OH (phenol) | |
| 2918 | C–H (alkane) stretching | 2918 | C–H (alkane) stretching | 2918 | C–H (alkane) stretching | 2918 | C–H (alkane) stretching | |
| 2850 | C–H stretching | 2850 | C–H stretching | 2851 | C–H stretching | 2849 | C–H stretching | |
| 2500–2000 cm−1 triple bonds region | NA | NA | NA | NA | ||||
| 2000–1500 cm−1 double bonds region | 1767 | C=O stretching | NA | NA | 1771 | C=O stretching | ||
| 1721 | C=O (carboxylic acid) | NA | NA | 1736 | (δ-lactone) | |||
| 1691 | C=O | 1691 | C=O | 1692 | C=O | 1693 | C=O | |
| 1627 | Aromatic compound C=C | 1629 | Aromatic compound C=C | 1629 | Aromatic compound C=C | 1628 | Aromatic compound C=C | |
| 1572 | C=O (amides) | NA | NA | NA | ||||
| 1542 | Carboxylic OH | 1537 | Carboxylic OH | 1541 | Carboxylic OH | 1557 | Carboxylic OH | |
| U SOD/mg Protein | U CAT/mg Protein | U GPx/mg Protein | MDA (mM/mg Protein) | |
|---|---|---|---|---|
| Control | 0.432 ± 0.065 | 3.452 ± 0.058 | 0.00116 ± 0.0001 | 11.694 ± 0.309 |
| DMSO 0.2% | 0.803 ± 0.121 * | 3.115 ± 0.143 | 0.00116 ± 0.0000 | 8.194 ± 0.599 ** |
| UBM | 0.788 ± 0.124 | 3.605 ± 0.093 | 0.00136 ± 0.0000 * | 10.302 ± 1.758 |
| UBE | 0.917 ± 0.133 * | 2.292 ± 0.095 *** | 0.00134 ± 0.0000 * | 10.674 ± 2.163 |
| UBA | 0.777 ± 0.217 | 3.119 ± 0.103 * | 0.00123 ± 0.0000 | 8.180 ± 0.602 ** |
| UBEA | 0.807 ± 0.153 | 3.110 ± 0.225 | 0.00133 ± 0.0000 * | 9.518 ± 1.377 |
| UA | 0.890 ± 264 | 3.395 ± 0.216 | 0.00144 ± 0.0000 | 10.205 ± 0.519 |
| H2O2 100 µM | 1.307 ± 0.04 *** | 3.897 ± 0.269 | 0.00146 ± 0.0001 ** | 9.159 ± 0.532 ** |
| U SOD/mg Protein | U CAT/mg Protein | U GPx/mg Protein | MDA (mM/mg Protein) | |
|---|---|---|---|---|
| Control | 0.969 ± 0.081 | 2.9547 ± 0.035 | 0.0015 ± 0.0000 | 10.150 ± 0.492 |
| DMSO 0.2% | 0.774 ± 0.036 | 2.1940 ± 0.305 ** | 0.0017 ± 0.0001 | 9.177 ± 0.837 |
| UBM | 0.876 ± 0.071 | 1.4983 ± 0.059 *** | 0.0020 ± 0.0000 *** | 8.802 ± 0.378 |
| UBE | 0.609 ± 0.050 ** | 1.9495 ± 0.102 *** | 0.0018 ± 0.0000 ** | 7.631 ± 0.065 ** |
| UBA | 0.393 ± 0.060 * | 2.6878 ± 0.185 | 0.0015 ± 0.0001 | 9.555 ± 0.221 |
| UBEA | 0.872 ± 0.108 | 1.4831 ± 0.270 *** | 0.0017 ± 0.0000 | 9.871 ± 0.882 |
| UA | 0.555 ± 0.110 * | 0.4497 ± 0.242 *** | 0.0017 ± 0.0001 | 7.917 ± 0.240 * |
| H2O2 100 µM | 1.017 ± 0.124 | 3.2741 ± 0.341 | 0.0020 ± 0.0001 ** | 9.016 ± 0.876 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, V.; Bucur, L.; Vochita, G.; Gherghel, D.; Mihai, C.T.; Rambu, D.; Calcan, S.I.; Costache, T.; Cucolea, I.E.; Matei, E.; et al. In Vitro Anticancer Activity and Oxidative Stress Biomarkers Status Determined by Usnea barbata (L.) F.H. Wigg. Dry Extracts. Antioxidants 2021, 10, 1141. https://doi.org/10.3390/antiox10071141
Popovici V, Bucur L, Vochita G, Gherghel D, Mihai CT, Rambu D, Calcan SI, Costache T, Cucolea IE, Matei E, et al. In Vitro Anticancer Activity and Oxidative Stress Biomarkers Status Determined by Usnea barbata (L.) F.H. Wigg. Dry Extracts. Antioxidants. 2021; 10(7):1141. https://doi.org/10.3390/antiox10071141
Chicago/Turabian StylePopovici, Violeta, Laura Bucur, Gabriela Vochita, Daniela Gherghel, Cosmin Teodor Mihai, Dan Rambu, Suzana Ioana Calcan, Teodor Costache, Iulia Elena Cucolea, Elena Matei, and et al. 2021. "In Vitro Anticancer Activity and Oxidative Stress Biomarkers Status Determined by Usnea barbata (L.) F.H. Wigg. Dry Extracts" Antioxidants 10, no. 7: 1141. https://doi.org/10.3390/antiox10071141
APA StylePopovici, V., Bucur, L., Vochita, G., Gherghel, D., Mihai, C. T., Rambu, D., Calcan, S. I., Costache, T., Cucolea, I. E., Matei, E., Badea, F. C., Caraiane, A., & Badea, V. (2021). In Vitro Anticancer Activity and Oxidative Stress Biomarkers Status Determined by Usnea barbata (L.) F.H. Wigg. Dry Extracts. Antioxidants, 10(7), 1141. https://doi.org/10.3390/antiox10071141









