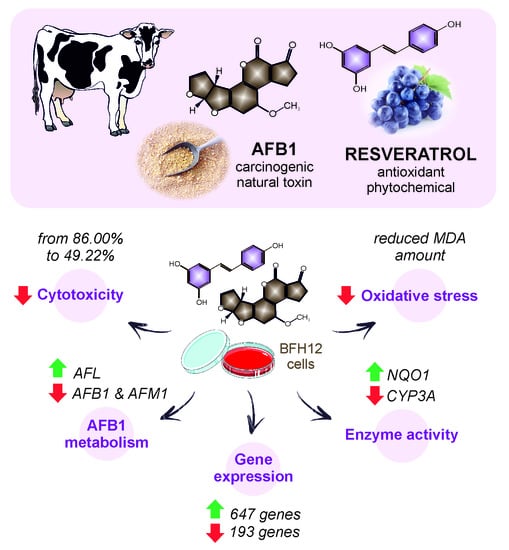
Discovering the Protective Effects of Resveratrol on Aflatoxin B1-Induced Toxicity: A Whole Transcriptomic Study in a Bovine Hepatocyte Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
2.2. Resveratrol Cytotoxicity
2.3. Cells Incubation for Gene Expression Analysis
2.4. Quantitative Real-Time PCR
2.5. RNA-Seq Library Preparation and Sequencing
2.6. Differential Expression Analysis
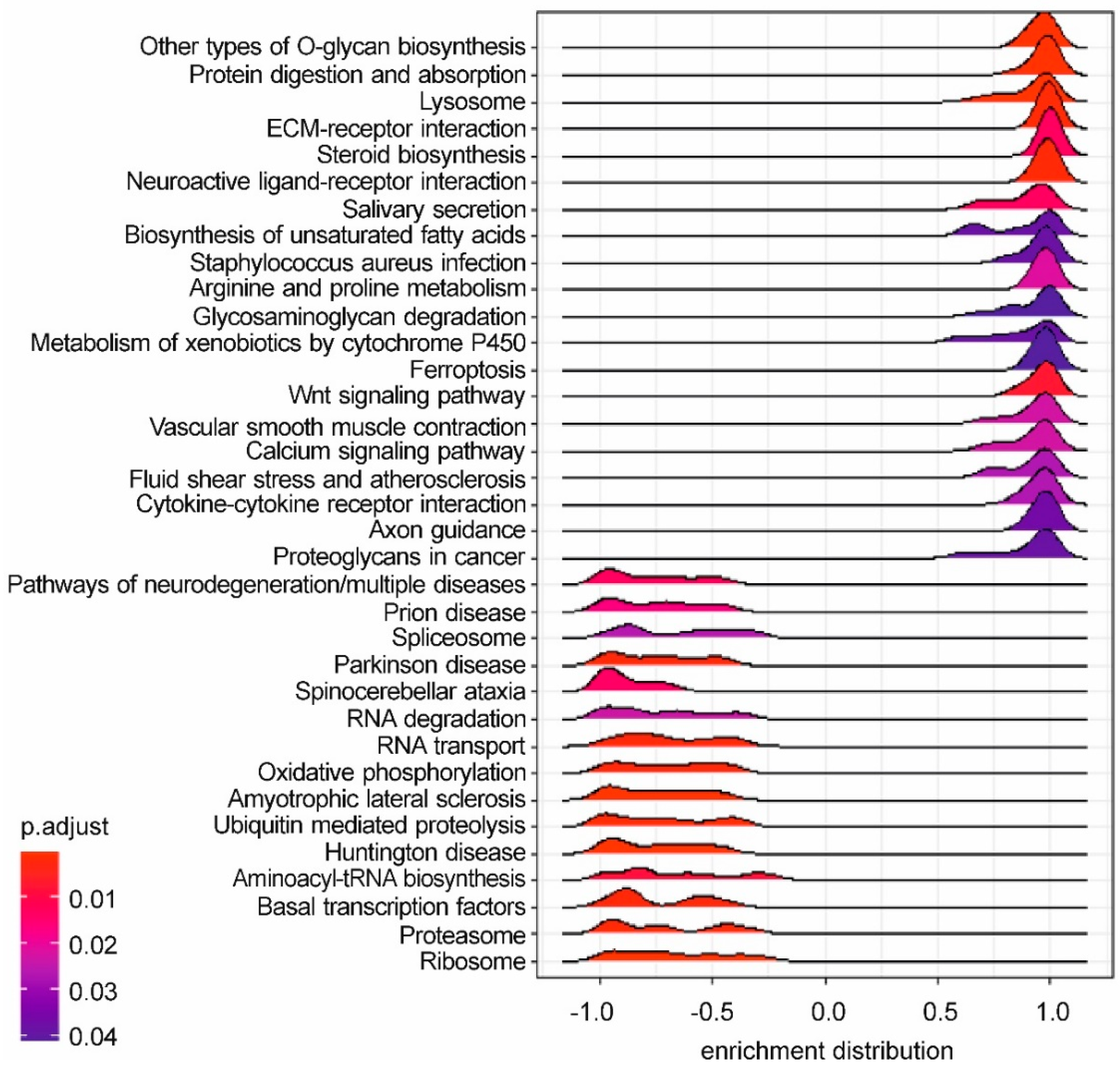
2.7. Functional Enrichment Analysis
2.8. Analytical Investigations
2.9. Oxidative Stress
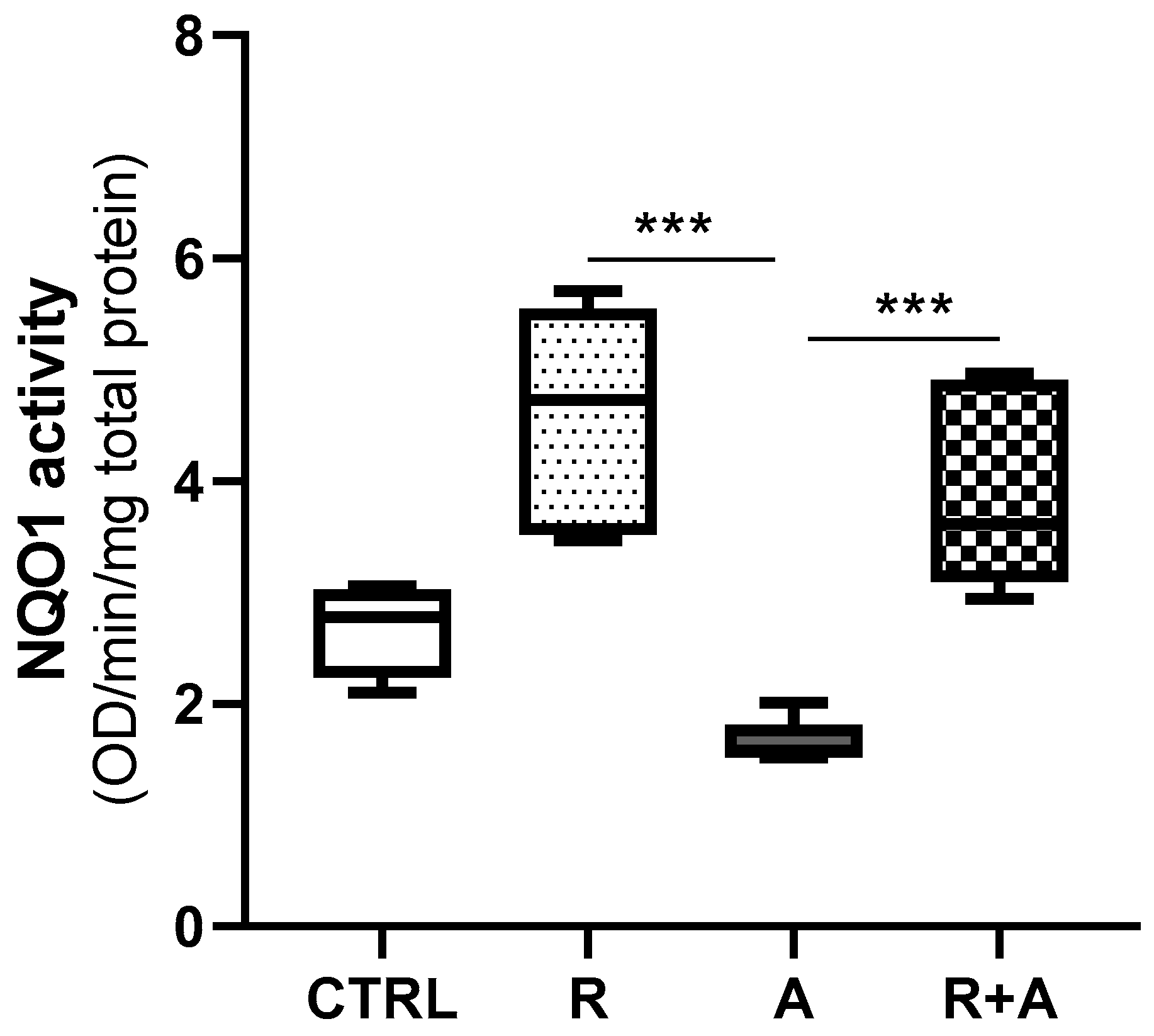
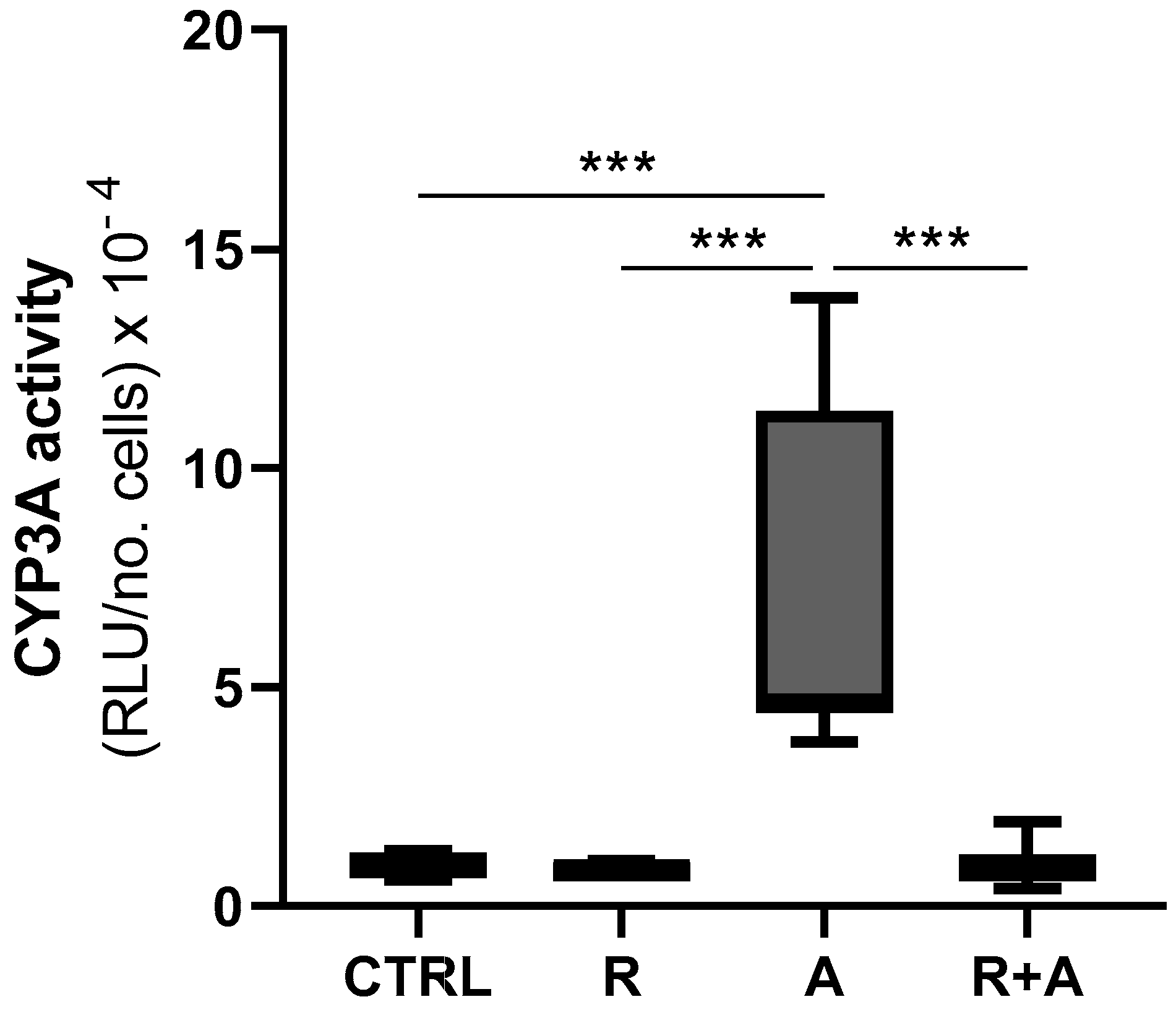
2.10. NQO1 and CYP3A Enzymatic Activity
2.11. Statistical Analysis
3. Results
3.1. Resveratrol Cytotoxicity
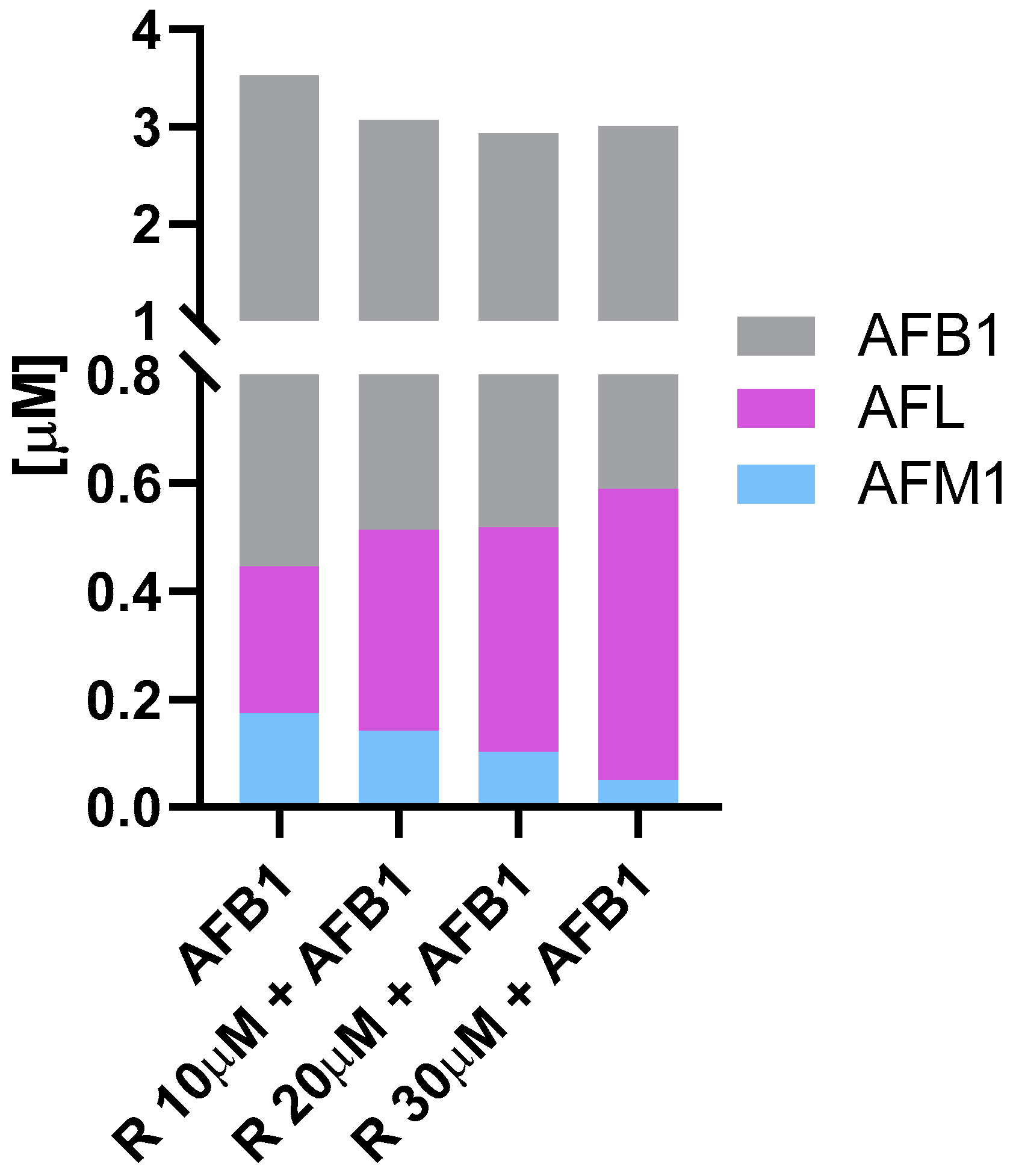
3.2. Aflatoxin B1 Biotransformation in BFH12 Cells
3.3. Target Gene Expression Analysis
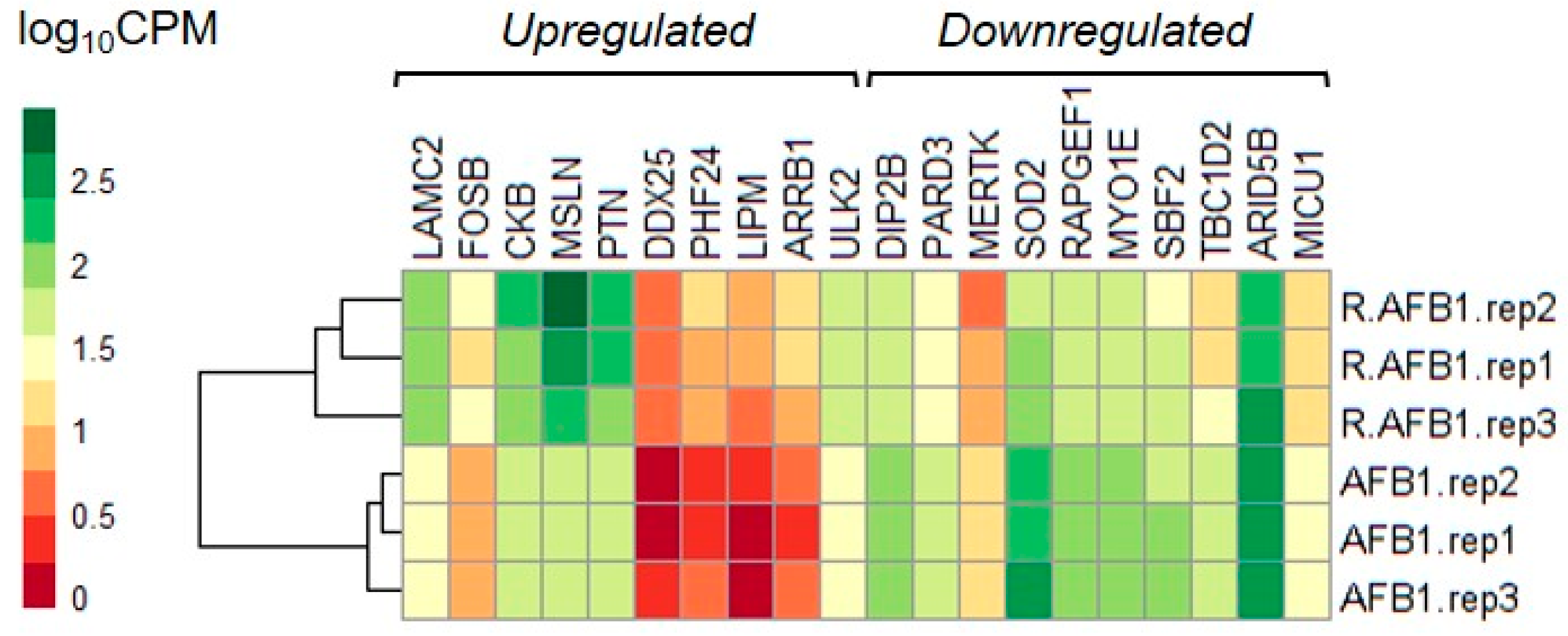
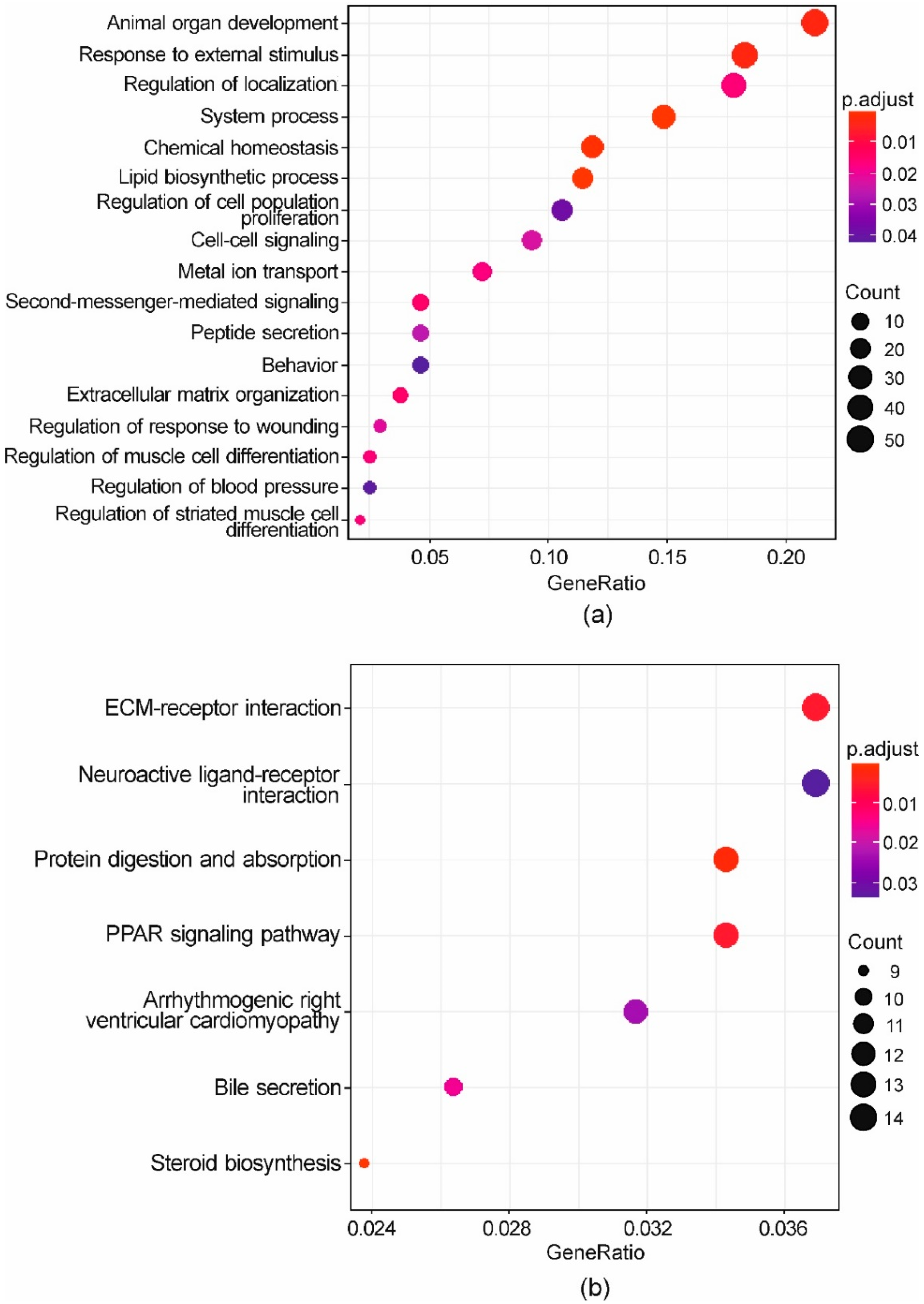
3.4. Whole-Transcriptome Differential Expression Analysis
3.5. Oxidative Stress
3.6. Diaphorase Enzymatic Activity
3.7. Cytochrome P4503A Catalytic Activity
4. Discussion
4.1. Resveratrol Cytotoxicity
4.2. Aflatoxin B1 Biotransformation
4.3. Resveratrol Transcriptional Effects
4.4. Transcriptional Changes Underlying R Potential as an Anti-AFB1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monbaliu, S.; Van Poucke, C.; Detavernier, C.; Dumoulin, F.; Van De Velde, M.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of Mycotoxins in Feed as Analyzed by a Multi-Mycotoxin LC-MS/MS Method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-Occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [Green Version]
- Monson, M.S.; Settlage, R.E.; McMahon, K.W.; Mendoza, K.M.; Rawal, S.; El-Nezami, H.S.; Coulombe, R.A.; Reed, K.M. Response of the Hepatic Transcriptome to Aflatoxin B1in Domestic Turkey (Meleagris Gallopavo). PLoS ONE 2014. [Google Scholar] [CrossRef] [Green Version]
- Rustemeyer, S.M.; Lamberson, W.R.; Ledoux, D.R.; Wells, K.; Austin, K.J.; Cammack, K.M. Effects of Dietary Aflatoxin on the Hepatic Expression of Apoptosis Genes in Growing Barrows. J. Anim. Sci. 2011. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Seng, X.; Tang, H.; Zhao, S.; Deng, Y.; Jin, R.; Li, Y. Effect of Low Levels of Aflatoxin B1 on Performance, Serum Biochemistry, Hepatocyte Apoptosis and Liver Histopathological Changes of Cherry Valley Ducks. J. Anim. Vet. Adv. 2013, 12, 1126–1130. [Google Scholar] [CrossRef]
- Rawal, S.; Kim, J.E.; Coulombe, R. Aflatoxin B1 in Poultry: Toxicology, Metabolism and Prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guclu, H. Aflatoxin Regulations in a Network of Global Maize Trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B 1 Metabolism: Regulation by Phase I and II Metabolizing Enzymes and Chemoprotective Agents. Mutat. Res. Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Internal Report 14/002; IARC: Lyon, France, 2010; Volume 93, pp. 9–38. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, S.; Kaya, E.; Kisacam, M.A. The Effect on Oxidative Stress of Aflatoxin and Protective Effect of Lycopene on Aflatoxin Damage. In Aflatoxin-Control, Analysis, Detection and Health Risks; Abdulra’uf, L.B., Ed.; InTech: London, UK, 2017. [Google Scholar]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the Occurrence of Aflatoxin M1 in Milk and Dairy Products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of Aflatoxins: Key Enzymes and Interindividual as Well as Interspecies Differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Mycotoxins in Cattle Feeds and Carry-over to Dairy Milk: A Review. Food Addit. Contam. Part A 2008, 25, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Elgioushy, M.M.; Elgaml, S.A.; El-Adl, M.M.; Hegazy, A.M.; Hashish, E.A. Aflatoxicosis in Cattle: Clinical Findings and Biochemical Alterations. Environ. Sci. Pollut. Res. 2020, 27, 35526–35534. [Google Scholar] [CrossRef]
- Council for Agricultural Science and Technology. Mycotoxins: Risks in Plant, Animal, and Human Systems; Task Force Report; Council for Agricultural Science and Technology: Ames, IA, USA, 2003; ISBN 978-1-887383-22-6. [Google Scholar]
- Marin, D.E.; Taranu, I. Overview on Aflatoxins and Oxidative Stress. Toxin Rev. 2012, 31, 32–43. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Giamouri, E.; Tavrizelou, S.; Zacharioudaki, M.; Danezis, G.; Simitzis, P.E.; Zoidis, E.; Tsiplakou, E.; Pappas, A.C.; Georgiou, C.A.; et al. Impact of Mycotoxins on Animals’ Oxidative Status. Antioxidants 2021, 10, 214. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.O.; Bracarense, A.P.F.L.; Oswald, I.P. Mycotoxins and Oxidative Stress: Where Are We? World Mycotoxin J. 2018, 11, 113–134. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Curcumin Mitigates AFB1-Induced Hepatic Toxicity by Triggering Cattle Antioxidant and Anti-Inflammatory Pathways: A Whole Transcriptomic In Vitro Study. Antioxidants 2020, 9, 1059. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.C.; Chou, C.C.; Liu, J.R.; Cheng, K.C. Protective and Detoxifying Effects Conferred by Dietary Selenium and Curcumin against AFB1-Mediated Toxicity in Livestock: A Review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Muhammad, I.; Yu, H.; Sun, X.; Zhang, X. Detection of Aflatoxin Adducts as Potential Markers and the Role of Curcumin in Alleviating AFB1-Induced Liver Damage in Chickens. Ecotoxicol. Environ. Saf. 2019, 176, 137–145. [Google Scholar] [CrossRef]
- El-Mekkawy, H.I.; Al-Kahtani, M.A.; Shati, A.A.; Alshehri, M.A.; Al-Doaiss, A.A.; Elmansi, A.A.; Ahmed, A.E. Black Tea and Curcumin Synergistically Mitigate the Hepatotoxicity and Nephropathic Changes Induced by Chronic Exposure to Aflatoxin-B1 in Sprague–Dawley Rats. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Petrone, V.; Pontin, K.; Latorre, J.; Beyssac, E.; Hernandez-Velasco, X.; Merino-Guzman, R.; Owens, C.; Hargis, B.; et al. Evaluation of Cellulosic Polymers and Curcumin to Reduce Aflatoxin B1 Toxic Effects on Performance, Biochemical, and Immunological Parameters of Broiler Chickens. Toxins 2019, 11, 121. [Google Scholar] [CrossRef] [Green Version]
- Ghadiri, S.; Spalenza, V.; Dellafiora, L.; Badino, P.; Barbarossa, A.; Dall’Asta, C.; Nebbia, C.; Girolami, F. Modulation of Aflatoxin B1 Cytotoxicity and Aflatoxin M1 Synthesis by Natural Antioxidants in a Bovine Mammary Epithelial Cell Line. Toxicol. Vitr. 2019. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the Efficacy of a Grape Seed Waste in Counteracting the Changes Induced by Aflatoxin B1 Contaminated Diet on Performance, Plasma, Liver and Intestinal Tissues of Pigs after Weaning. Toxicon 2019. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Bulgaru, C.V.; Anghel, C.A.; Pistol, G.C.; Dore, M.I.; Palade, M.L.; Taranu, I. Grape Seed Waste Counteracts Aflatoxin B1 Toxicity in Piglet Mesenteric Lymph Nodes. Toxins 2020, 12, 800. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Hermenean, A.; Bulgaru, C.; Pistol, G.C.; Ciceu, A.; Grosu, I.A.; Marin, D.E. Diet Containing Grape Seed Meal By-Product Counteracts AFB1 Toxicity in Liver of Pig after Weaning. Ecotoxicol. Environ. Saf. 2020, 203, 110899. [Google Scholar] [CrossRef]
- Rawat, D.; Chhonker, S.K.; Naik, R.A.; Koiri, R.K. Modulation of Antioxidant Enzymes, SIRT1 and NF-κB by Resveratrol and Nicotinamide in Alcohol-aflatoxin B1-induced Hepatocellular Carcinoma. J. Biochem. Mol. Toxicol. 2021, 35. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Y.; Yu, H.; Shan, A.; Shen, J.; Zhou, C.; Zhao, Y.; Fang, H.; Wang, X.; Wang, J.; et al. Resveratrol Inhibits Aflatoxin B1-Induced Oxidative Stress and Apoptosis in Bovine Mammary Epithelial Cells and Is Involved the Nrf2 Signaling Pathway. Toxicon 2019, 164, 10–15. [Google Scholar] [CrossRef]
- Sridhar, M.; Suganthi, R.U.; Thammiaha, V. Effect of Dietary Resveratrol in Ameliorating Aflatoxin B1-Induced Changes in Broiler Birds. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1094–1104. [Google Scholar] [CrossRef]
- Feng, M.; Zhong, L.-X.; Zhan, Z.-Y.; Huang, Z.-H.; Xiong, J.-P. Resveratrol Treatment Inhibits Proliferation of and Induces Apoptosis in Human Colon Cancer Cells. Med. Sci. Monit. 2016, 22, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martí-Centelles, R.; Murga, J.; Falomira, E.; Carda, M.; Marco, J.A. Synthesis and Biological Evaluation of Imines Structurally Related to Resveratrol as Dual Inhibitors of VEGF Protein Secretion and HTERT Gene Expression. Nat. Prod. Commun. 2017, 12, 699–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yu, H.; Lin, Q.; Liu, X.; Cheng, Y.; Deng, B. Anti-Inflammatory Effect of Resveratrol Attenuates the Severity of Diabetic Neuropathy by Activating the Nrf2 Pathway. Aging 2021, 13, 10659–10671. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.; Kotzbeck, P. The Impact of Resveratrol on Skin Wound Healing, Scarring, and Aging. Int. Wound J. 2021. [Google Scholar] [CrossRef]
- Bishayee, A.; Politis, T.; Darvesh, A.S. Resveratrol in the Chemoprevention and Treatment of Hepatocellular Carcinoma. Cancer Treat. Rev. 2010, 36, 43–53. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Luo, J.; Yu, B.; Chen, J.; Chen, D. Effects of Resveratrol on Lipid Metabolism in Muscle and Adipose Tissues: A Reevaluation in a Pig Model. J. Funct. Foods 2015, 14, 590–595. [Google Scholar] [CrossRef]
- Al-Hussaini, H.; Kittaneh, R.S.; Kilarkaje, N. Effects of Trans-Resveratrol on Type 1 Diabetes-Induced up-Regulation of Apoptosis and Mitogen-Activated Protein Kinase Signaling in Retinal Pigment Epithelium of Dark Agouti Rats. Eur. J. Pharmacol. 2021, 904, 174167. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Zhao, X.; Chen, X.; Wang, L.; Geng, Z. Effect of Dietary Resveratrol Supplementation on Meat Quality, Muscle Antioxidative Capacity and Mitochondrial Biogenesis of Broilers: Resveratrol on Meat Quality, Muscle Antioxidative Capacity and Mitochondrial Biogenesis. J. Sci. Food Agric. 2018, 98, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Mohebodini, H.; Jazi, V.; Bakhshalinejad, R.; Shabani, A.; Ashayerizadeh, A. Effect of Dietary Resveratrol Supplementation on Growth Performance, Immune Response, Serum Biochemical Indices, Cecal Microflora, and Intestinal Morphology of Broiler Chickens Challenged with Escherichia Coli. Livest. Sci. 2019, 229, 13–21. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, S.; Bai, Y.; Luo, Z.; Li, Z.; Shi, B.; Shan, A. Effects of Dietary Resveratrol Supplementation in Sows on Antioxidative Status, Myofiber Characteristic and Meat Quality of Offspring. Meat Sci. 2020, 167, 108176. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z. Dietary Resveratrol Attenuation of Intestinal Inflammation and Oxidative Damage Is Linked to the Alteration of Gut Microbiota and Butyrate in Piglets Challenged with Deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Wu, Z.; Liu, K.; Jang, C.H.; Kim, H.J.; Kim, J.-S.; Kim, J.-S. Improved Extraction of Resveratrol and Antioxidants from Grape Peel Using Heat and Enzymatic Treatments. J. Sci. Food Agric. 2019, 99, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Luo, M.; Wang, W.; Zhao, C.; Gu, C.; Li, C.; Zu, Y.; Fu, Y.; Guan, Y. Biotransformation of Polydatin to Resveratrol in Polygonum Cuspidatum Roots by Highly Immobilized Edible Aspergillus Niger and Yeast. Bioresour. Technol. 2013, 136, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zheng, Y.-L.; Yang, S.-K.; Zhang, J.-R.; Cheng, X.-Y.; Ghiladi, R.; Ma, Z.; Wang, J.; Deng, W.-W. One-Pot Method Based on Deep Eutectic Solvent for Extraction and Conversion of Polydatin to Resveratrol from Polygonum Cuspidatum. Food Chem. 2021, 343, 128498. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Wang, X.; Wang, X.; Thomsen, B.; Kadarmideen, H.N.; Lan, X.; Huang, Y.; Chen, H. Transcriptomic Changes in Bovine Skeletal Muscle Cells after Resveratrol Treatment. Gene 2020, 754, 144849. [Google Scholar] [CrossRef]
- Mendes, K.L.; Lelis, D.d.F.; Santos, S.H.S. Nuclear Sirtuins and Inflammatory Signaling Pathways. Cytokine Growth Factor Rev. 2017, 38, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Mohar, D.S. The Sirtuin System: The Holy Grail of Resveratrol? J. Clin. Exp. Cardiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-Y.; Zhao, Y.-H.; Hao, H.-S.; Wang, H.-Y.; Huang, J.-M.; Yan, C.-L.; Du, W.-H.; Pang, Y.-W.; Zhang, P.-P.; Liu, Y.; et al. Resveratrol Significantly Improves the Fertilisation Capacity of Bovine Sex-Sorted Semen by Inhibiting Apoptosis and Lipid Peroxidation. Sci. Rep. 2018, 8, 7603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Wang, K.; Liu, H.; Hu, F.; Zhao, F.; Liu, J. Protection of Bovine Mammary Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Cell Damage by Resveratrol. Oxid. Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, W.; Hu, G.; Qiu, L.; Chen, J. Transcriptome Analysis of Juvenile Tilapia (Oreochromis Niloticus) Blood, Fed With Different Concentrations of Resveratrol. Front. Physiol. 2020, 11, 600730. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, S.; Luo, Z.; Shi, B.; Shan, A.; Cheng, B. Maternal Dietary Resveratrol Alleviates Weaning-Associated Diarrhea and Intestinal Inflammation in Pig Offspring by Changing Intestinal Gene Expression and Microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Zhao, X.H.; Chen, X.Y.; Yang, L.; Geng, Z.Y. Dietary Resveratrol Supplementation Prevents Transport-Stress-Impaired Meat Quality of Broilers through Maintaining Muscle Energy Metabolism and Antioxidant Status. Poult. Sci. 2017, 96, 2219–2225. [Google Scholar] [CrossRef]
- Rawat, D.; Shrivastava, S.; Naik, R.A.; Chhonker, S.K.; Koiri, R.K. SIRT1-mediated Amelioration of Oxidative Stress in Kidney of Alcohol-aflatoxin-B1-induced Hepatocellular Carcinoma by Resveratrol Is Catalase Dependent and GPx Independent. J. Biochem. Mol. Toxicol. 2020, 34. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Z.; Zhuo, R.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol Attenuates Aflatoxin B1-Induced ROS Formation and Increase of M6A RNA Methylation. Animals 2020, 10, 677. [Google Scholar] [CrossRef]
- Omur, A.; Yildirim, B.; Saglam, Y.; Comakli, S.; Ozkaraca, M. Activity of Resveratrol on the Influence of Aflatoxin B1 on the Testes of Sprague Dawley Rats. Pol. J. Vet. Sci. 2019, 22, 313–320. [Google Scholar] [CrossRef]
- El-Agamy, D.S. Comparative Effects of Curcumin and Resveratrol on Aflatoxin B1-Induced Liver Injury in Rats. Arch. Toxicol. 2010, 84, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Gleich, A.; Kaiser, B.; Schumann, J.; Fuhrmann, H. Establishment and Characterisation of a Novel Bovine SV40 Large T-Antigen-Transduced Foetal Hepatocyte-Derived Cell Line. In Vitro Cell. Dev. Biol. 2016, 52, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, M.; Tolosi, R.; Giantin, M.; Guerra, G.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Insights into Aflatoxin B1 Toxicity in Cattle: An In Vitro Whole-Transcriptomic Approach. Toxins 2020, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R Package for Comparing Biological Themes among Gene Clusters. OMICS J. Integrat. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and Its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Zancanella, V.; Giantin, M.; Dacasto, M. Absolute Quantification and Modulation of Cytochrome P450 3A Isoforms in Cattle Liver. Vet. J. 2014. [Google Scholar] [CrossRef]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M.E.; Xiao, D.; Weinstein, I.B. Resveratrol Induces Growth Inhibition, S-Phase Arrest, Apoptosis, and Changes in Biomarker Expression in Several Human Cancer Cell Lines. Clin. Cancer Res. 2002, 8, 893–903. [Google Scholar] [PubMed]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [Green Version]
- El Khoury, A.; Atoui, A.; Yaghi, J. Analysis of Aflatoxin M1 in Milk and Yogurt and AFM1 Reduction by Lactic Acid Bacteria Used in Lebanese Industry. Food Control 2011, 22, 1695–1699. [Google Scholar] [CrossRef]
- Kuilman, M.E.M.; Maas, R.F.M.; Fink-Gremmels, J. Cytochrome P450-Mediated Metabolism and Cytotoxicity of Aflatoxin B1 in Bovine Hepatocytes. Toxicology Vitr. 2000, 14, 321–327. [Google Scholar] [CrossRef]
- Min, L.; Fink-Gremmels, J.; Li, D.; Tong, X.; Tang, J.; Nan, X.; Yu, Z.; Chen, W.; Wang, G. An Overview of Aflatoxin B1 Biotransformation and Aflatoxin M1 Secretion in Lactating Dairy Cows. Anim. Nutr. 2021, 7, 42–48. [Google Scholar] [CrossRef]
- Nakazato, M.; Morozumi, S.; Saito, K.; Fujinuma, K.; Nishima, T.; Kasai, N. Interconversion of Aflatoxin B1 and Aflatoxicol by Several Fungi. Appl. Environ. Microbiol. 1990, 56, 1465–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murcia, H.W.; Diaz, G.J. In Vitro Hepatic Aflatoxicol Production Is Related to a Higher Resistance to Aflatoxin B1 in Poultry. Sci. Rep. 2020, 10, 5508. [Google Scholar] [CrossRef] [Green Version]
- Kühn, G.; Pallauf, K.; Schulz, C.; Birringer, M.; Diaz-Rica, B.; de Pascual-Teresa, S.; Rimbach, G. Resveratrol Modulates Desaturase Expression and Fatty Acid Composition of Cultured Hepatocytes. Front. Nutr. 2018, 5, 106. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Fu, W.; Wu, W.; Xu, J. Suppression of FADS1 Induces ROS Generation, Cell Cycle Arrest, and Apoptosis in Melanocytes: Implications for Vitiligo. Aging 2019, 11, 11829–11843. [Google Scholar] [CrossRef]
- Inoue, H.; Jiang, X.-F.; Katayama, T.; Osada, S.; Umesono, K.; Namura, S. Brain Protection by Resveratrol and Fenofibrate against Stroke Requires Peroxisome Proliferator-Activated Receptor α in Mice. Neurosci. Lett. 2003, 352, 203–206. [Google Scholar] [CrossRef]
- Resar, L.M.S. The High Mobility Group A1 Gene: Transforming Inflammatory Signals into Cancer? Cancer Res. 2010, 70, 436–439. [Google Scholar] [CrossRef] [Green Version]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The Role of Osteopontin in Inflammatory Processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in Immune Regulation and Stress Responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, A.; Fedele, M. Roles of HMGA Proteins in Cancer. Nat. Rev. Cancer 2007, 7, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Peng, F. Association of SIRT1 and HMGA1 Expression in Non-Small Cell Lung Cancer. Oncol. Lett. 2016, 11, 782–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; Lian, N.; Zhang, F.; Chen, L.; Chen, Q.; Lu, C.; Bian, M.; Shao, J.; Wu, L.; Zheng, S. Activation of PPARγ/P53 Signaling Is Required for Curcumin to Induce Hepatic Stellate Cell Senescence. Cell Death Dis. 2016, 7, e2189. [Google Scholar] [CrossRef] [PubMed]
- Casarin, R.C.; Casati, M.Z.; Pimentel, S.P.; Cirano, F.R.; Algayer, M.; Pires, P.R.; Ghiraldini, B.; Duarte, P.M.; Ribeiro, F.V. Resveratrol Improves Bone Repair by Modulation of Bone Morphogenetic Proteins and Osteopontin Gene Expression in Rats. Int. J. Oral Maxillofac. Surg. 2014, 43, 900–906. [Google Scholar] [CrossRef]
- Ozcan-Kucuk, A.; Alan, H.; Gul, M.; Yolcu, U. Evaluating the Effect of Resveratrol on the Healing of Extraction Sockets in Cyclosporine A–Treated Rats. J. Oral Maxillofac. Surg. 2018, 76, 1404–1413. [Google Scholar] [CrossRef]
- Tu, C.; Su, Y.; Huang, Y.; Tsai, M.; Li, L.; Chen, Y.; Cheng, C.; Dai, D.; Yang, R. Localization and Characterization of a Novel Secreted Protein SCUBE1 in Human Platelets. Cardiovasc. Res. 2006, 71, 486–495. [Google Scholar] [CrossRef] [Green Version]
- Erdoğan, M.; Findikli, H.A.; Okuducu Teran, İ. A Novel Biomarker for Predicting Sepsis Mortality: SCUBE-1. Medicine 2021, 100, e24671. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A Severity of Disease Classification System. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Thajeb, P.; Tu, C.-F.; Chiang, F.-T.; Chen, C.-H.; Yang, R.-B.; Chen, J.-J. Plasma Concentration of SCUBE1, a Novel Platelet Protein, Is Elevated in Patients With Acute Coronary Syndrome and Ischemic Stroke. J. Am. Coll. Cardiol. 2008, 51, 2173–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erol, O.; Ellidağ, H.Y.; Özel, M.K.; Uysal Derbent, A.; Eren, E.; Yılmaz, N. Circulating SCUBE1 Levels in Women with Polycystic Ovary Syndrome. J. Turk. Soc. Obstet. Gynecol. 2018, 15, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Uyanikoglu, H.; Hilali, N.G.; Yardimciel, M.; Koyuncu, I. A New Biomarker for the Early Diagnosis of Ovarian Torsion: SCUBE-1. Clin. Exp. Reprod. Med. 2018, 45, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.M.; Hofmann, S.; Askew, D.S.; Davidson, W.S. High Density Lipoprotein: It’s Not Just about Lipid Transport Anymore. Trends Endocrinol. Metab. 2011, 22, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keith, R.L.; Miller, Y.E.; Hudish, T.M.; Girod, C.E.; Sotto-Santiago, S.; Franklin, W.A.; Nemenoff, R.A.; March, T.H.; Nana-Sinkam, S.P.; Geraci, M.W. Pulmonary Prostacyclin Synthase Overexpression Chemoprevents Tobacco Smoke Lung Carcinogenesis in Mice. Cancer Res. 2004, 64, 5897–5904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Yang, Y.; Meng, H.; Li, H.; Chen, X.; Huang, H.; Bu, F.; Yu, H.; Wang, Q.; Huang, C.; et al. DNA Methylation of PTGIS Enhances Hepatic Stellate Cells Activation and Liver Fibrogenesis. Front. Pharmacol. 2018, 9, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Ho, N.; Santos, C.; Dubois, P.; Mamo, J.; Croft, K.; Allister, E. Red Wine Polyphenolics Increase LDL Receptor Expression and Activity and Suppress the Secretion of ApoB100 from Human HepG2 Cells. J. Nutr. 2003, 133, 700–706. [Google Scholar] [CrossRef]
- Yashiro, T.; Nanmoku, M.; Shimizu, M.; Inoue, J.; Sato, R. Resveratrol Increases the Expression and Activity of the Low Density Lipoprotein Receptor in Hepatocytes by the Proteolytic Activation of the Sterol Regulatory Element-Binding Proteins. Atherosclerosis 2012, 220, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-T.; Lin, C.-F.; Huang, Y.-L.; Chong, K.-Y.; Hsieh, M.-F.; Huang, T.-H.; Cheng, C.-Y. Protective Mechanisms of Resveratrol Derivatives against TNF-α-Induced Inflammatory Responses in Rat Mesangial Cells. Cytokine 2019, 113, 380–392. [Google Scholar] [CrossRef]
- Bellezza, I.; Roberti, R.; Gatticchi, L.; Del Sordo, R.; Rambotti, M.G.; Marchetti, M.C.; Sidoni, A.; Minelli, A. A Novel Role for Tm7sf2 Gene in Regulating TNFα Expression. PLoS ONE 2013, 8, e68017. [Google Scholar] [CrossRef] [Green Version]
- Gatticchi, L.; Bellezza, I.; Del Sordo, R.; Peirce, M.J.; Sidoni, A.; Roberti, R.; Minelli, A. The Tm7sf2 Gene Deficiency Protects Mice against Endotoxin-Induced Acute Kidney Injury. PLoS ONE 2015, 10, e0141885. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Liu, C.; Tang, K.; Dong, X.; Xue, L.; Su, G.; Zhang, W.; Jin, Y. Aquaporin-1 Attenuates Macrophage-Mediated Inflammatory Responses by Inhibiting P38 Mitogen-Activated Protein Kinase Activation in Lipopolysaccharide-Induced Acute Kidney Injury. Inflamm. Res. 2019, 68, 1035–1047. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, W.; Yu, G.; Liu, Q.; Jin, Y. Cytoprotective Effect of Aquaporin 1 against Lipopolysaccharide-induced Apoptosis and Inflammation of Renal Epithelial HK-2 Cells. Exp. Ther. Med. 2018. [Google Scholar] [CrossRef]
- Ray, N.; Kuwahara, M.; Takada, Y.; Maruyama, K.; Kawaguchi, T.; Tsubone, H.; Ishikawa, H.; Matsuo, K. C-Fos Suppresses Systemic Inflammatory Response to Endotoxin. Int. Immunol. 2006, 18, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Thiel, G.; Rössler, O.G. Resveratrol Stimulates C-Fos Gene Transcription via Activation of ERK1/2 Involving Multiple Genetic Elements. Gene 2018, 658, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Gadaleta, R.M.; Moschetta, A. Deciphering the Nuclear Bile Acid Receptor FXR Paradigm. Nucl. Recept. Signal. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschini, L.; Gobbo, G.; Gatti, S.; Caccamo, L.; Prato, P.; Maggioni, M.; Braidotti, P.; Di Stefano, R.; Fassati, L.R. Endothelial Targeting with C1-Inhibitor Reduces Complement Activation in vitro and during ex vivo Reperfusion of Pig Liver: Reduction of Ischaemia-Reperfusion Injury by Cell-Bound C1-INH. Clin. Exp. Immunol. 2001, 126, 412–420. [Google Scholar] [CrossRef]
- Bosma, M.; Gerling, M.; Pasto, J.; Georgiadi, A.; Graham, E.; Shilkova, O.; Iwata, Y.; Almer, S.; Söderman, J.; Toftgård, R.; et al. FNDC4 Acts as an Anti-Inflammatory Factor on Macrophages and Improves Colitis in Mice. Nat. Commun. 2016, 7, 11314. [Google Scholar] [CrossRef]
- Koniaris, L.G.; Zimmers-Koniaris, T.; Hsiao, E.C.; Chavin, K.; Sitzmann, J.V.; Farber, J.M. Cytokine-Responsive Gene-2/IFN-Inducible Protein-10 Expression in Multiple Models of Liver and Bile Duct Injury Suggests a Role in Tissue Regeneration. J. Immunol. 2001, 167, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Bone-Larson, C.L.; Hogaboam, C.M.; Evanhoff, H.; Strieter, R.M.; Kunkel, S.L. IFN-γ-Inducible Protein-10 (CXCL10) Is Hepatoprotective During Acute Liver Injury Through the Induction of CXCR2 on Hepatocytes. J. Immunol. 2001, 167, 7077–7083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.; Han, B.; Liu, H.; Zhang, S. Curcumin Protects Mice against Concanavalin A-Induced Hepatitis by Inhibiting Intrahepatic Intercellular Adhesion Molecule-1 (ICAM-1) and CXCL10 Expression. Mol. Cell. Biochem. 2011, 358, 53–60. [Google Scholar] [CrossRef]
- Kutty, R.K.; Samuel, W.; Abay, R.; Cherukuri, A.; Nagineni, C.N.; Duncan, T.; Jaworski, C.; Vijayasarathy, C.; Redmond, T.M. Resveratrol Attenuates CXCL11 Expression Induced by Proinflammatory Cytokines in Retinal Pigment Epithelial Cells. Cytokine 2015, 74, 335–338. [Google Scholar] [CrossRef] [Green Version]
- Zeremski, M.; Petrovic, L.M.; Chiriboga, L.; Brown, Q.B.; Yee, H.T.; Kinkhabwala, M.; Jacobson, I.M.; Dimova, R.; Markatou, M.; Talal, A.H. Intrahepatic Levels of CXCR3-Associated Chemokines Correlate with Liver Inflammation and Fibrosis in Chronic Hepatitis C. Hepatology 2008, 48, 1440–1450. [Google Scholar] [CrossRef] [Green Version]
- Simpson, K.J.; Henderson, N.C.; Bone-Larson, C.L.; Lukacs, N.W.; Hogaboam, C.M.; Kunkel, S.L. Chemokines in the Pathogenesis of Liver Disease: So Many Players with Poorly Defined Roles. Clin. Sci. 2003, 104, 47–63. [Google Scholar] [CrossRef]
- Azhar, N.; Ziraldo, C.; Barclay, D.; Rudnick, D.A.; Squires, R.H.; Vodovotz, Y. Analysis of Serum Inflammatory Mediators Identifies Unique Dynamic Networks Associated with Death and Spontaneous Survival in Pediatric Acute Liver Failure. PLoS ONE 2013, 8, e78202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Wang, J.; Ma, L.; Tang, X.; Qiao, Y.; Pan, Q.; Yu, Y.; Sun, F. CD166 Plays a Pro-Carcinogenic Role in Liver Cancer Cells via Inhibition of FOXO Proteins through AKT. Oncol. Rep. 2014, 32, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Zendehdel, E.; Abdollahi, E.; Momtazi-Borojeni, A.A.; Korani, M.; Alavizadeh, S.H.; Sahebkar, A. The Molecular Mechanisms of Curcumin’s Inhibitory Effects on Cancer Stem Cells. J. Cell. Biochem. 2019, 120, 4739–4747. [Google Scholar] [CrossRef]
- Li, M.; Xu, D.; Xia, X.; Ni, B.; Zhu, C.; Zhao, G.; Cao, H. Sema3C Promotes Hepatic Metastasis and Predicts Poor Prognosis in Gastric Adenocarcinoma. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef]
- Papic, N.; Zidovec Lepej, S.; Gorenec, L.; Grgic, I.; Gasparov, S.; Filipec Kanizaj, T.; Vince, A. The Association of Semaphorins 3C, 5A and 6D with Liver Fibrosis Stage in Chronic Hepatitis C. PLoS ONE 2018, 13, e0209481. [Google Scholar] [CrossRef] [Green Version]
- Gilkerson, R.; De La Torre, P.; Vallier, S.S. Mitochondrial OMA1 and OPA1 as Gatekeepers of Organellar Structure/Function and Cellular Stress Response. Front. Cell Dev. Biol. 2021, 9, 626117. [Google Scholar] [CrossRef]
- Garcia, I.; Innis-Whitehouse, W.; Lopez, A.; Keniry, M.; Gilkerson, R. Oxidative Insults Disrupt OPA1-Mediated Mitochondrial Dynamics in Cultured Mammalian Cells. Redox Rep. 2018, 23, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Qin, M.; Hu, P.; Ji, K.; Xiao, R.; Sun, N.; Pan, X.; Zhang, X. Resveratrol Protects Retinal Ganglion Cells against Ischemia Induced Damage by Increasing Opa1 Expression. Int. J. Mol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Delucchi, A.B. Resveratrol, a Red Wine Constituent, Is a Mechanism-Based Inactivator of Cytochrome P450 3A4. Life Sci. 2000, 67, 3103–3112. [Google Scholar] [CrossRef]
- Boshuizen, M.; van der Ploeg, K.; von Bonsdorff, L.; Biemond, B.J.; Zeerleder, S.S.; van Bruggen, R.; Juffermans, N.P. Therapeutic Use of Transferrin to Modulate Anemia and Conditions of Iron Toxicity. Blood Rev. 2017, 31, 400–405. [Google Scholar] [CrossRef]
- Chen, Y.-I.; Wei, P.-C.; Hsu, J.-L.; Su, F.-Y.; Lee, W.-H. NPGPx (GPx7): A Novel Oxidative Stress Sensor/Transmitter with Multiple Roles in Redox Homeostasis. Am. J. Transl. Res. 2016, 8, 1626–1640. [Google Scholar]
- Hanmer, K.L.; Mavri-Damelin, D. Peroxidasin Is a Novel Target of the Redox-Sensitive Transcription Factor Nrf2. Gene 2018, 674, 104–114. [Google Scholar] [CrossRef]
- Dougan, J.; Hawsawi, O.; Burton, L.J.; Edwards, G.; Jones, K.; Zou, J.; Nagappan, P.; Wang, G.; Zhang, Q.; Danaher, A.; et al. Proteomics-Metabolomics Combined Approach Identifies Peroxidasin as a Protector against Metabolic and Oxidative Stress in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, L.; Lopes Costa, A.; Le Bachelier, C.; Slama, A.; Lebre, A.-S.; Taylor, R.W.; Bastin, J.; Djouadi, F. Resveratrol Attenuates Oxidative Stress in Mitochondrial Complex I Deficiency: Involvement of SIRT3. Free Radic. Biol. Med. 2016, 96, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol Rescues Cadmium-Induced Mitochondrial Injury by Enhancing Transcriptional Regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a Pathway in TCMK-1 Cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, M.; Zeng, X.; Yang, J.; Deng, H.; Yi, L.; Mi, M. Resveratrol Regulates Mitochondrial Reactive Oxygen Species Homeostasis through Sirt3 Signaling Pathway in Human Vascular Endothelial Cells. Cell Death Dis. 2014, 5, e1576. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, R.; Zhang, J.; Johansson, K. Microsomal Glutathione Transferase 1: Mechanism and Functional Roles. Drug Metabol. Rev. 2011, 43, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary Resveratrol Improves Antioxidant Status of Sows and Piglets and Regulates Antioxidant Gene Expression in Placenta by Keap1-Nrf2 Pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Chanas, S.A.; Jiang, Q.; McMahon, M.; McWalter, G.K.; McLellan, L.I.; Elcombe, C.R.; Henderson, C.J.; Wolf, C.R.; Moffat, G.J.; Itoh, K.; et al. Loss of the Nrf2 Transcription Factor Causes a Marked Reduction in Constitutive and Inducible Expression of the Glutathione S-Transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 Genes in the Livers of Male and Female Mice. Biochem. J. 2002, 365, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Ma, J.Z.; Zeng, Q.; Cechova, S.; Gantz, A.; Nievergelt, C.; O’Connor, D.; Lipkowitz, M.; Le, T.H. Loss of GSTM1, a NRF2 Target, Is Associated with Accelerated Progression of Hypertensive Kidney Disease in the African American Study of Kidney Disease (AASK). Am. J. Physiol. Renal Physiol. 2013, 304, F348–F355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodson, M.; Anandhan, A.; Zhang, D.D. MGST1, a New Soldier of NRF2 in the Battle against Ferroptotic Death. Cell Chem. Biol. 2021, 28, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Hai, O.; Littlefield, M.J.; Carsons, S.; Reiss, A.B. Resveratrol Mediates Anti-Atherogenic Effects on Cholesterol Flux in Human Macrophages and Endothelium via PPARγ and Adenosine. Eur. J. Pharmacol. 2013, 698, 299–309. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Ni, Z.; Sun, J.; Gao, H.; Cheng, Z.; Xu, J.; Yin, P. Resveratrol Induces AMPK-Dependent MDR1 Inhibition in Colorectal Cancer HCT116/L-OHP Cells by Preventing Activation of NF-ΚB Signaling and Suppressing CAMP-Responsive Element Transcriptional Activity. Tumor Biol. 2015, 36, 9499–9510. [Google Scholar] [CrossRef] [PubMed]
- Lorico, A.; Nesland, J.; Emilsen, E.; Fodstad, O.; Rappa, G. Role of the Multidrug Resistance Protein 1 Gene in the Carcinogenicity of Aflatoxin B1: Investigations Using Mrp1-Null Mice. Toxicology 2002, 171, 201–205. [Google Scholar] [CrossRef]
- Van Herwaarden, A.E.; Wagenaar, E.; Karnekamp, B.; Merino, G.; Jonker, J.W.; Schinkel, A.H. Breast Cancer Resistance Protein (Bcrp1/Abcg2) Reduces Systemic Exposure of the Dietary Carcinogens Aflatoxin B1, IQ and Trp-P-1 but Also Mediates Their Secretion into Breast Milk. Carcinogenesis 2005, 27, 123–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Na, Z.; Chen, D.; Huang, G.; Cao, H. Investigation of the Transport of Aflatoxin M1 by the Transporter ABCG2 in Bovine Mammary Epithelial Cells. Med. Weter. 2020, 76, 646–651. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Discovering the Protective Effects of Resveratrol on Aflatoxin B1-Induced Toxicity: A Whole Transcriptomic Study in a Bovine Hepatocyte Cell Line. Antioxidants 2021, 10, 1225. https://doi.org/10.3390/antiox10081225
Pauletto M, Giantin M, Tolosi R, Bassan I, Barbarossa A, Zaghini A, Dacasto M. Discovering the Protective Effects of Resveratrol on Aflatoxin B1-Induced Toxicity: A Whole Transcriptomic Study in a Bovine Hepatocyte Cell Line. Antioxidants. 2021; 10(8):1225. https://doi.org/10.3390/antiox10081225
Chicago/Turabian StylePauletto, Marianna, Mery Giantin, Roberta Tolosi, Irene Bassan, Andrea Barbarossa, Anna Zaghini, and Mauro Dacasto. 2021. "Discovering the Protective Effects of Resveratrol on Aflatoxin B1-Induced Toxicity: A Whole Transcriptomic Study in a Bovine Hepatocyte Cell Line" Antioxidants 10, no. 8: 1225. https://doi.org/10.3390/antiox10081225
APA StylePauletto, M., Giantin, M., Tolosi, R., Bassan, I., Barbarossa, A., Zaghini, A., & Dacasto, M. (2021). Discovering the Protective Effects of Resveratrol on Aflatoxin B1-Induced Toxicity: A Whole Transcriptomic Study in a Bovine Hepatocyte Cell Line. Antioxidants, 10(8), 1225. https://doi.org/10.3390/antiox10081225