A Blood Biomarker for Duchenne Muscular Dystrophy Shows That Oxidation State of Albumin Correlates with Protein Oxidation and Damage in Mdx Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Procedures
2.2. Taurine Treatment
2.3. Blood and Tissue Collection for Oxidative Stress Analyses
2.4. Protein Thiol Oxidation in Muscle
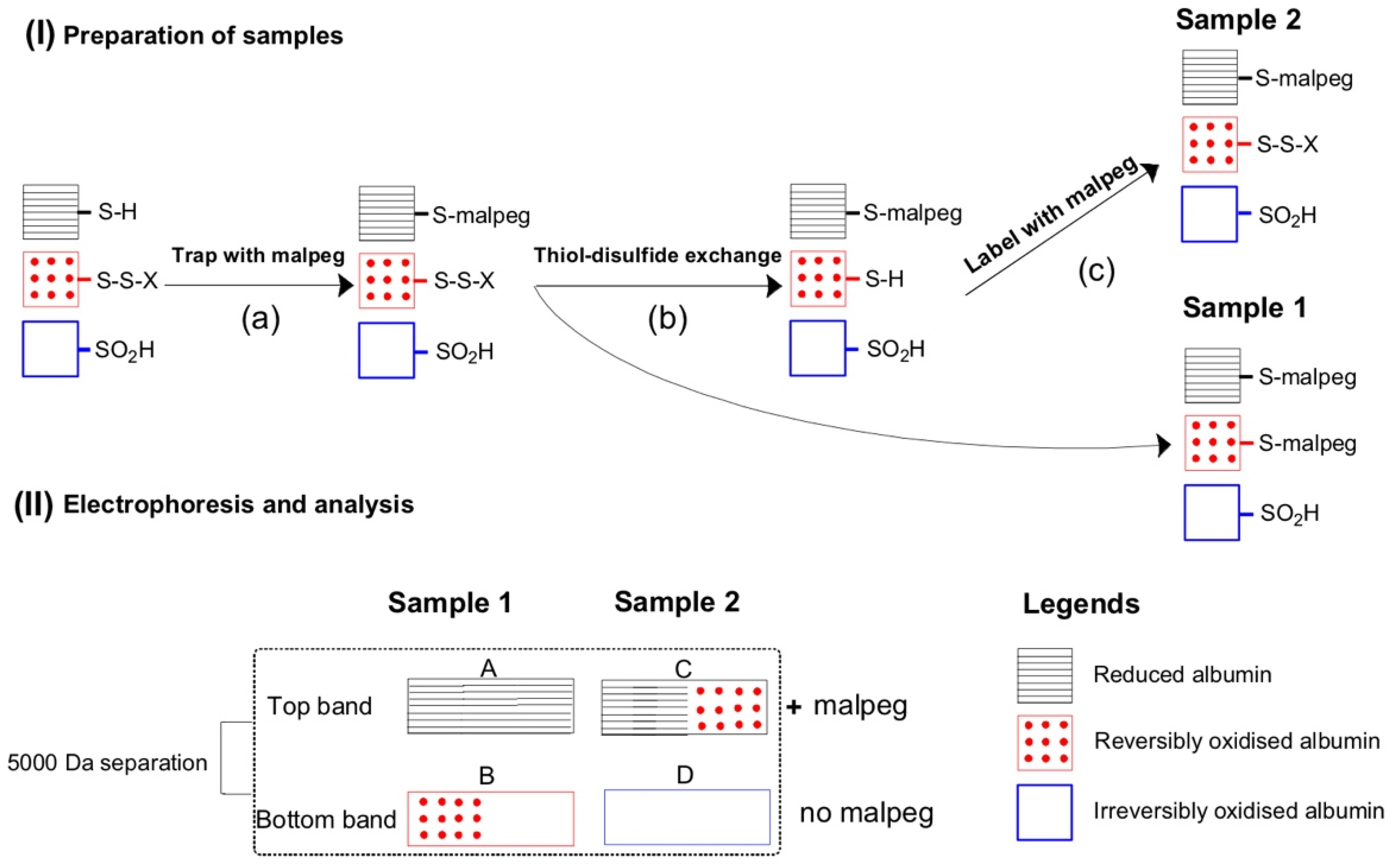
2.5. Plasma Albumin Thiol Oxidation
2.6. Protein Carbonylation in Muscle and Plasma
2.7. Creatine Kinase (CK) Assay in Plasma
2.8. Statistics
3. Results
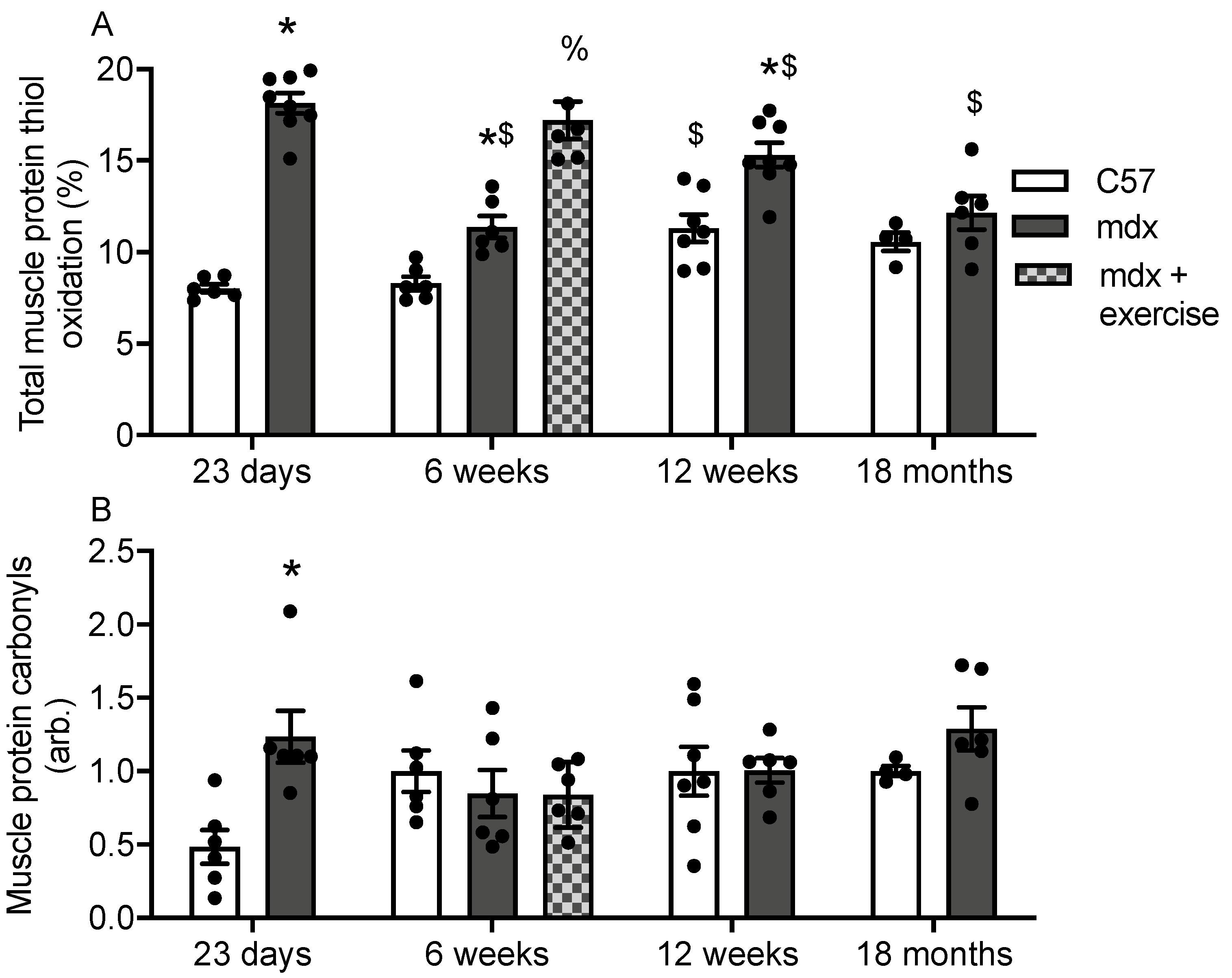
3.1. Protein Thiol Oxidation and Carbonylation in Muscles of C57 and Mdx Mice
3.2. Plasma Albumin Thiol Oxidation and Carbonylation in C57 and Mdx Mice
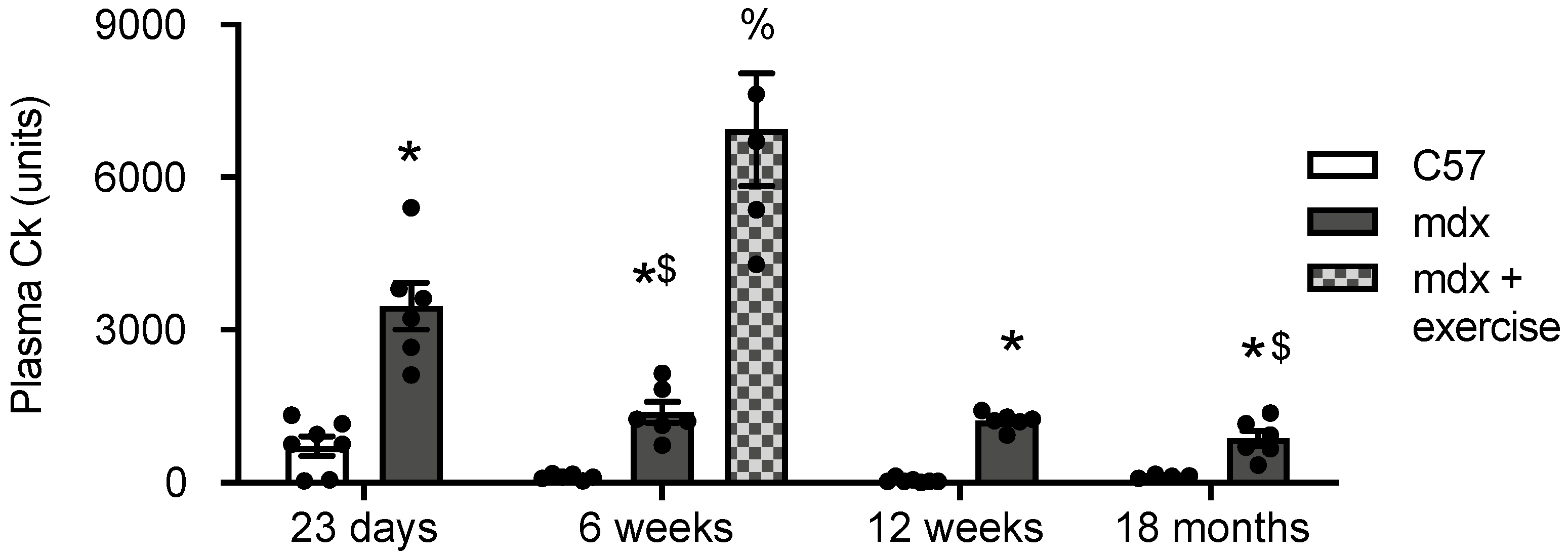
3.3. Creatine Kinase (CK) in Plasma of C57 and Mdx Mice
3.4. Correlation Analysis
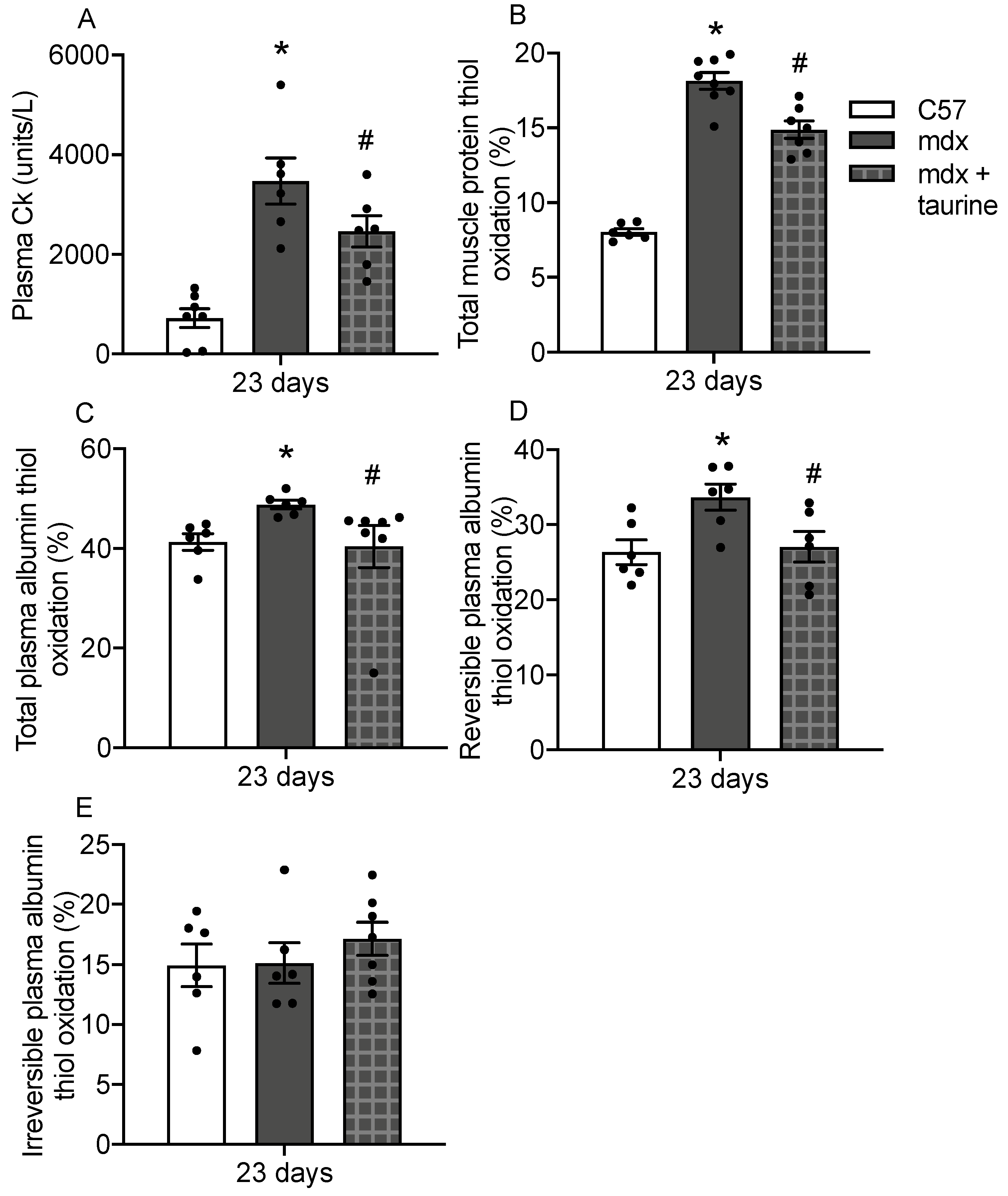
3.5. Albumin Thiol Oxidation in Plasma of Taurine Treated Mdx Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. Two-tiered hypotheses for Duchenne muscular dystrophy. Cell. Mol. Life Sci. 2008, 65, 1621–1625. [Google Scholar] [CrossRef]
- Kharraz, Y.; Guerra, J.; Pessina, P.; Serrano, A.L.; Munoz-Canoves, P. Understanding the process of fibrosis in Duchenne muscular dystrophy. Biomed. Res. Int. 2014, 2014, 965631. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Scotton, C.; Passarelli, C.; Ferlini, A. Duchenne Muscular Dystrophy: From Diagnosis to Therapy. Molecules 2015, 20, 18168–18184. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef] [Green Version]
- Arthur, P.G.; Grounds, M.D.; Shavlakadze, T. Oxidative stress as a therapeutic target during muscle wasting: Considering the complex interactions. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 408–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrill, J.R.; Radley-Crabb, H.G.; Iwasaki, T.; Lemckert, F.A.; Arthur, P.G.; Grounds, M.D. Oxidative stress and pathology in muscular dystrophies: Focus on protein thiol oxidation and dysferlinopathies. FEBS J. 2013, 280, 4149–4164. [Google Scholar] [CrossRef]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Targeting Nrf2 for the treatment of Duchenne Muscular Dystrophy. Redox Biol. 2021, 38, 101803. [Google Scholar] [CrossRef]
- El-Shafey, A.F.; Armstrong, A.E.; Terrill, J.R.; Grounds, M.D.; Arthur, P.G. Screening for increased protein thiol oxidation in oxidatively stressed muscle tissue. Free Radic. Res. 2011, 45, 991–999. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, P.L.; Shavlakadze, T.; Grounds, M.D.; Arthur, P.G. Differential thiol oxidation of the signaling proteins Akt, PTEN or PP2A determines whether Akt phosphorylation is enhanced or inhibited by oxidative stress in C2C12 myotubes derived from skeletal muscle. Int. J. Biochem. Cell Biol. 2015, 62, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.E.; Zerbes, R.; Fournier, P.A.; Arthur, P.G. A fluorescent dual labeling technique for the quantitative measurement of reduced and oxidized protein thiols in tissue samples. Free Radic. Biol. Med. 2010, 50, 510–517. [Google Scholar] [CrossRef]
- Iwasaki, T.; Terrill, J.; Shavlakadze, T.; Grounds, M.D.; Arthur, P.G. Visualizing and quantifying oxidized protein thiols in tissue sections: A comparison of dystrophic mdx and normal skeletal mouse muscles. Free Radic. Biol. Med. 2013, 65, 1408–1416. [Google Scholar] [CrossRef]
- Maciazek-Jurczyk, M.; Sulkowska, A. Spectroscopic analysis of the impact of oxidative stress on the structure of human serum albumin (HSA) in terms of its binding properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136 Pt B, 265–282. [Google Scholar] [CrossRef]
- Terrill, J.R.; Radley-Crabb, H.G.; Grounds, M.D.; Arthur, P.G. N-Acetylcysteine treatment of dystrophic mdx mice results in protein thiol modifications and inhibition of exercise induced myofibre necrosis. Neuromuscul. Disord. 2012, 22, 427–434. [Google Scholar] [CrossRef]
- Radley-Crabb, H.G.; Terrill, J.R.; Shavlakadze, T.; Tonkin, J.; Arthur, P.G.; Grounds, M.D. A single 30 min treadmill exercise session is suitable for ‘proof-of concept studies’ in adult mdx mice: A comparison of the early consequences of two different treadmill protocols. Neuromuscul. Disord. 2012, 22, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Duong, M.N.; Turner, R.; Le Guiner, C.; Boyatzis, A.; Kettle, A.J.; Grounds, M.D.; Arthur, P.G. Levels of inflammation and oxidative stress, and a role for taurine in dystropathology of the Golden Retriever Muscular Dystrophy dog model for Duchenne Muscular Dystrophy. Redox Biol. 2016, 9, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Terrill, J.R.; Boyatzis, A.; Grounds, M.D.; Arthur, P.G. Treatment with the cysteine precursor l-2-oxothiazolidine-4-carboxylate (OTC) implicates taurine deficiency in severity of dystropathology in mdx mice. Int. J. Biochem. Cell Biol. 2013, 45, 2097–2108. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox. Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hondur, G.; Göktas, E.; Yang, X.; Al-Aswad, L.; Auran, J.D.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M.; Suh, L.H.; Trief, D.; et al. Oxidative Stress-Related Molecular Biomarker Candidates for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4078–4088. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.L.; Yin, H.; Hardy, K.D.; Davies, S.S.; Roberts, L.J., 2nd. Isoprostane generation and function. Chem. Rev. 2011, 111, 5973–5996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanidis, Y.; Priftis, A.; Stagos, D.; Stravodimos, G.A.; Leonidas, D.D.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Oxidation of human serum albumin exhibits inter-individual variability after an ultra-marathon mountain race. Exp. Ther. Med. 2017, 13, 2382–2390. [Google Scholar] [CrossRef] [Green Version]
- Argüelles, S.; García, S.; Maldonado, M.; Machado, A.; Ayala, A. Do the serum oxidative stress biomarkers provide a reasonable index of the general oxidative stress status? Biochim. Biophys. Acta 2004, 1674, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dumlu, E.G.; Tokaç, M.; Bozkurt, B.; Yildirim, M.B.; Ergin, M.; Yalçin, A.; Kiliç, M. Correlation between the serum and tissue levels of oxidative stress markers and the extent of inflammation in acute appendicitis. Clinics 2014, 69, 677–682. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Nikolaidis, M.G.; Kyparos, A.; Kouretas, D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic. Biol. Med. 2009, 47, 1371–1374. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Going retro: Oxidative stress biomarkers in modern redox biology. Free Radic. Biol. Med. 2016, 98, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood reflects tissue oxidative stress: A systematic review. Biomarkers 2015, 20, 97–108. [Google Scholar] [CrossRef]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Birner-Gruenberger, R.; Spindelboeck, W.; Stueger, H.P.; Dorn, L.; Stadlbauer, V.; Putz-Bankuti, C.; Krisper, P.; Graziadei, I.; Vogel, W.; et al. Oxidative albumin damage in chronic liver failure: Relation to albumin binding capacity, liver dysfunction and survival. J. Hepatol. 2013, 59, 978–983. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Ulivelli, M.; Bartalini, S.; Battistini, S.; Cerase, A.; Passero, S.; Summa, D.; Frosali, S.; Priora, R.; Margaritis, A.; et al. Regulation of redox forms of plasma thiols by albumin in multiple sclerosis after fasting and methionine loading test. Amino Acids 2010, 38, 1461–1471. [Google Scholar] [CrossRef]
- Oettl, K.; Marsche, G. Redox state of human serum albumin in terms of cysteine-34 in health and disease. Methods Enzymol. 2010, 474, 181–195. [Google Scholar]
- Belinskaia, D.A.; Voronina, P.A.; Batalova, A.A.; Goncharov, N.V. Serum Albumin. Encyclopedia 2021, 1, 9. [Google Scholar] [CrossRef]
- Medina-Navarro, R.; Corona-Candelas, I.; Barajas-González, S.; Díaz-Flores, M.; Durán-Reyes, G. Albumin antioxidant response to stress in diabetic nephropathy progression. PLoS ONE 2014, 9, e106490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candiano, G.; Petretto, A.; Bruschi, M.; Santucci, L.; Dimuccio, V.; Prunotto, M.; Gusmano, R.; Urbani, A.; Ghiggeri, G.M. The oxido-redox potential of albumin methodological approach and relevance to human diseases. J. Proteom. 2009, 73, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Imafuku, T.; Otagiri, M.; Maruyama, T. Clinical Implications Associated With the Posttranslational Modification-Induced Functional Impairment of Albumin in Oxidative Stress-Related Diseases. J. Pharm. Sci. 2017, 106, 2195–2203. [Google Scholar] [CrossRef] [Green Version]
- Bocedi, A.; Cattani, G.; Stella, L.; Massoud, R.; Ricci, G. Thiol disulfide exchange reactions in human serum albumin: The apparent paradox of the redox transitions of Cys34. FEBS J. 2018, 285, 3225–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonanata, J.; Turell, L.; Antmann, L.; Ferrer-Sueta, G.; Botasini, S.; Méndez, E.; Alvarez, B.; Coitiño, E.L. The thiol of human serum albumin: Acidity, microenvironment and mechanistic insights on its oxidation to sulfenic acid. Free Radic. Biol. Med. 2017, 108, 952–962. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox. Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Ali, R. Reactive oxygen species damaged human serum albumin in patients with type 1 diabetes mellitus: Biochemical and immunological studies. Life Sci. 2006, 79, 2320–2328. [Google Scholar] [CrossRef]
- Terawaki, H.; Yoshimura, K.; Hasegawa, T.; Matsuyama, Y.; Negawa, T.; Yamada, K.; Matsushima, M.; Nakayama, M.; Hosoya, T.; Era, S. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004, 66, 1988–1993. [Google Scholar] [CrossRef] [Green Version]
- Oettl, K.; Reibnegger, G.; Schmut, O. The redox state of human serum albumin in eye diseases with and without complications. Acta Ophthalmol. 2011, 89, e174–e179. [Google Scholar] [CrossRef] [PubMed]
- Nasif, W.A.; Mukhtar, M.H.; El-Emshaty, H.M.; Alwazna, A.H. Redox State of Human Serum Albumin and Inflammatory Biomarkers in Hemodialysis Patients with Secondary Hyperparathyroidism During Oral Calcitriol Supplementation for Vitamin D. Open J. Med. Chem. 2018, 12, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.; Duong, M.N.; Boyatzis, A.E.; Golden, E.; Vrielink, A.; Fournier, P.A.; Arthur, P.G. Oxidation of cysteine 34 of plasma albumin as a biomarker of oxidative stress. Free Radic. Res. 2020, 54, 91–103. [Google Scholar] [CrossRef]
- McGeachie, J.K.; Grounds, M.D.; Partridge, T.A.; Morgan, J.E. Age-related changes in replication of myogenic cells in mdx mice: Quantitative autoradiographic studies. J. Neurol. Sci. 1993, 119, 169–179. [Google Scholar] [CrossRef]
- Grounds, M.D.; Torrisi, J. Anti-TNF alpha (Remicade (R)) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004, 18, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radley, H.G.; Grounds, M.D. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol. Dis. 2006, 23, 387–397. [Google Scholar] [CrossRef]
- Radley, H.G.; Davies, M.J.; Grounds, M.D. Reduced muscle necrosis and long-term benefits in dystrophic mdx mice after cV1q (blockade of TNF) treatment. Neuromuscul. Disord. 2008, 18, 227–238. [Google Scholar] [CrossRef]
- Brussee, V.; Tardif, F.; Tremblay, J.P. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul. Disord. 1997, 7, 487–492. [Google Scholar] [CrossRef]
- Vilquin, J.T.; Brussee, V.; Asselin, I.; Kinoshita, I.; Gingras, M.; Tremblay, J.P. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve 1998, 21, 567–576. [Google Scholar] [CrossRef]
- De Luca, A.; Pierno, S.; Liantonio, A.; Cetrone, M.; Camerino, C.; Fraysse, B.; Mirabella, M.; Servidei, S.; Ruegg, U.T.; Conte Camerino, D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003, 304, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Yoshida, K.; Nakamura, A.; Sasazawa, F.; Oide, T.; Takeda, S.; Ikeda, S. Chronic exercise accelerates the degeneration-regeneration cycle and downregulates insulin-like growth factor-1 in muscle of mdx mice. Muscle Nerve 2005, 32, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.D.; Vargas, C.C.; Anderson, J.E. Persistent and improved functional gain in mdx dystrophic mice after treatment with L-arginine and deflazacort. FASEB J. 2006, 20, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Payne, E.T.; Yasuda, N.; Bourgeois, J.M.; Devries, M.C.; Rodriguez, M.C.; Yousuf, J.; Tarnopolsky, M.A. Nutritional therapy improves function and complements corticosteroid intervention in mdx mice. Muscle Nerve 2006, 33, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Burdi, R.; Rolland, J.F.; Fraysse, B.; Litvinova, K.; Cozzoli, A.; Giannuzzi, V.; Liantonio, A.; Camerino, G.M.; Sblendorio, V.; Capogrosso, R.F. Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: Outcome of a large array of in vivo and ex vivo tests. J. Appl. Physiol. 2009, 106, 1311–1324. [Google Scholar] [CrossRef] [Green Version]
- Frinchi, M.; Morici, G.; Mudó, G.; Bonsignore, M.R.; Di Liberto, V. Beneficial Role of Exercise in the Modulation of mdx Muscle Plastic Remodeling and Oxidative Stress. Antioxidants 2021, 10, 558. [Google Scholar] [CrossRef]
- Pastoret, C.; Sebille, A. Mdx mice show progressive weakness and muscle deterioration with age. J. Neurol. Sci. 1995, 129, 97–105. [Google Scholar] [CrossRef]
- Chamberlain, J.S.; Metzger, J.; Reyes, M.; Townsend, D.; Faulkner, J.A. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007, 21, 2195–2204. [Google Scholar] [CrossRef] [Green Version]
- Lefaucheur, J.P.; Pastoret, C.; Sebille, A. Phenotype of dystrophinopathy in old mdx mice. Anat. Rec. 1995, 242, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, M.; Barkhouse, M.; Uaesoontrachoon, K.; Giri, M.; Hathout, Y.; Dang, U.J.; Gordish-Dressman, H.; Nagaraju, K.; Hoffman, E.P. Biomarker-focused multi-drug combination therapy and repurposing trial in mdx mice. PLoS ONE 2021, 16, e0246507. [Google Scholar] [CrossRef]
- Dang, U.J.; Ziemba, M.; Clemens, P.R.; Hathout, Y.; Conklin, L.S.; Hoffman, E.P. Serum biomarkers associated with baseline clinical severity in young steroid-naïve Duchenne muscular dystrophy boys. Hum. Mol. Genet. 2020, 29, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D.; Terrill, J.R.; Al-Mshhdani, B.A.; Duong, M.N.; Radley-Crabb, H.G.; Arthur, P.G. Biomarkers for Duchenne muscular dystrophy: Myonecrosis, inflammation and oxidative stress. Dis. Model. Mech. 2020, 13, dmm043638. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Terrill, J.R.; Pinniger, G.J.; Graves, J.A.; Grounds, M.D.; Arthur, P.G. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J. Physiol. 2016, 594, 3095–3110. [Google Scholar] [CrossRef] [Green Version]
- Terrill, J.R.; Pinniger, G.J.; Nair, K.V.; Grounds, M.D.; Arthur, P.G. Beneficial effects of high dose taurine treatment in juvenile dystrophic mdx mice are offset by growth restriction. PLoS ONE 2017, 12, e0187317. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Webb, S.M.; Arthur, P.G.; Hackett, M.J. Investigation of the effect of taurine supplementation on muscle taurine content in the mdx mouse model of Duchenne muscular dystrophy using chemically specific synchrotron imaging. Analyst 2020, 145, 7242–7251. [Google Scholar] [CrossRef]
- Terrill, J.R.; Grounds, M.D.; Arthur, P.G. Increased taurine in pre-weaned juvenile mdx mice greatly reduces the acute onset of myofibre necrosis and dystropathology and prevents inflammation. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Ladner, C.L.; Yang, J.; Turner, R.J.; Edwards, R.A. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal. Biochem. 2004, 326, 13–20. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Shacter, E.; Williams, J.A.; Lim, M.; Levine, R.L. Differential susceptibility of plasma proteins to oxidative modification: Examination by western blot immunoassay. Free Radic. Biol. Med. 1994, 17, 429–437. [Google Scholar] [CrossRef]
- Burch, P.M.; Pogoryelova, O.; Goldstein, R.; Bennett, D.; Guglieri, M.; Straub, V.; Bushby, K.; Lochmüller, H.; Morris, C. Muscle-derived proteins as serum biomarkers for monitoring disease progression in three forms of muscular dystrophy. J. Neuromuscul. Dis. 2015, 2, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Mukherjee, K.; Chio, T.I.; Sackett, D.L.; Bane, S.L. Detection of oxidative stress-induced carbonylation in live mammalian cells. Free Radic. Biol. Med. 2015, 84, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipak Gasparovic, A.; Zarkovic, N.; Zarkovic, K.; Semen, K.; Kaminskyy, D.; Yelisyeyeva, O.; Bottari, S.P. Biomarkers of oxidative and nitro-oxidative stress: Conventional and novel approaches. Br. J. Pharmacol. 2017, 174, 1771–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Himmelfarb, J.; McMonagle, E. Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int. 2001, 60, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavone, B.; Sirolli, V.; Giardinelli, A.; Bucci, S.; Forli, F.; Di Cesare, M.; Sacchetta, P.; Di Pietro, N.; Pandolfi, A.; Urbani, A.; et al. Plasma protein carbonylation in chronic uremia. J. Nephrol. 2011, 24, 453–464. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Goldfarb, A.H.; Bloomer, R.J.; Nguyen, L.; Sha, X.; McKenzie, M.J. Oxidative stress response in normal and antioxidant supplemented rats to a downhill run: Changes in blood and skeletal muscles. Can. J. Appl. Physiol. 2005, 30, 677–689. [Google Scholar] [CrossRef]
- Liu, J.; Yeo, H.C.; Overvik-Douki, E.; Hagen, T.; Doniger, S.J.; Chyu, D.W.; Brooks, G.A.; Ames, B.N. Chronically and acutely exercised rats: Biomarkers of oxidative stress and endogenous antioxidants. J. Appl. Physiol. 2000, 89, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.G.; Wyckelsma, V.L.; Xu, H.; Murphy, R.M. Mitochondrial content is preserved throughout disease progression in the mdx mouse model of Duchenne muscular dystrophy, regardless of taurine supplementation. Am. J. Physiol. Cell Physiol. 2018, 314, C483–C491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.Y.; Lee, J.W.; Suh, M.R.; Choi, W.A.; Kang, S.W.; Oh, H.J. Correlation of Serum Creatine Kinase Level with Pulmonary Function in Duchenne Muscular Dystrophy. Ann. Phys. Rehabil. Med. 2017, 41, 306–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zellweger, H.; Antonik, A. Newborn screening for Duchenne muscular dystrophy. Pediatrics 1975, 55, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterbourn, C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002, 181–182, 223–227. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef] [Green Version]

| Indices 1 | Indices 1 | r | p |
|---|---|---|---|
| Muscle PTO | Plasma total PTO | 0.5 | <0.0001 * |
| Muscle PTO | Plasma revers. PTO | 0.15 | 0.3303 |
| Muscle PTO | Plasma irrevers. PTO | 0.5 | 0.0002 * |
| Muscle prot. carb. | Plasma prot. carb. | 0.05 | 0.7 |
| Plasma CK | Plasma total PTO | 0.4 | 0.007 * |
| Plasma CK | Plasma revers. PTO | 0.04 | 0.8 |
| Plasma CK | Plasma irrevers. PTO | 0.4 | 0.004 * |
| Plasma CK | Plasma prot. carb. | -0.1 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mshhdani, B.A.; Grounds, M.D.; Arthur, P.G.; Terrill, J.R. A Blood Biomarker for Duchenne Muscular Dystrophy Shows That Oxidation State of Albumin Correlates with Protein Oxidation and Damage in Mdx Muscle. Antioxidants 2021, 10, 1241. https://doi.org/10.3390/antiox10081241
Al-Mshhdani BA, Grounds MD, Arthur PG, Terrill JR. A Blood Biomarker for Duchenne Muscular Dystrophy Shows That Oxidation State of Albumin Correlates with Protein Oxidation and Damage in Mdx Muscle. Antioxidants. 2021; 10(8):1241. https://doi.org/10.3390/antiox10081241
Chicago/Turabian StyleAl-Mshhdani, Basma A., Miranda D. Grounds, Peter G. Arthur, and Jessica R. Terrill. 2021. "A Blood Biomarker for Duchenne Muscular Dystrophy Shows That Oxidation State of Albumin Correlates with Protein Oxidation and Damage in Mdx Muscle" Antioxidants 10, no. 8: 1241. https://doi.org/10.3390/antiox10081241
APA StyleAl-Mshhdani, B. A., Grounds, M. D., Arthur, P. G., & Terrill, J. R. (2021). A Blood Biomarker for Duchenne Muscular Dystrophy Shows That Oxidation State of Albumin Correlates with Protein Oxidation and Damage in Mdx Muscle. Antioxidants, 10(8), 1241. https://doi.org/10.3390/antiox10081241





