Identification and Antioxidant Abilities of Enzymatic-Transesterification (−)-Epigallocatechin-3-O-gallate Stearyl Derivatives in Non-Aqueous Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EGCG Derivatives via Different Reaction Parameters
2.3. Determination of Conversion of EGCG Derivatives
2.4. Purification and Identification of EGCG Stearyl Derivatives
2.5. Determination of 1-Octanol/Water Partition Coefficient
2.6. Determination of Radical Scavenging Ability of 2,2-Diphenylpicrylhydrazyl (DPPH)
2.7. Determination of Radical Scavenging Ability of 2,2-Azino-bis (3-Ethylbenzothiazoline-6-sulfonate) (ABTS)
2.8. Reducing Power Assay
2.9. Determination of Hydroxyl Antioxidant Ability
2.10. Determination of Oxygen Radical Absorbance Capacity (ORAC)
2.11. Determination of Lipid Oxidation at Storage
2.12. Statistical Analysis
3. Results and Discussion
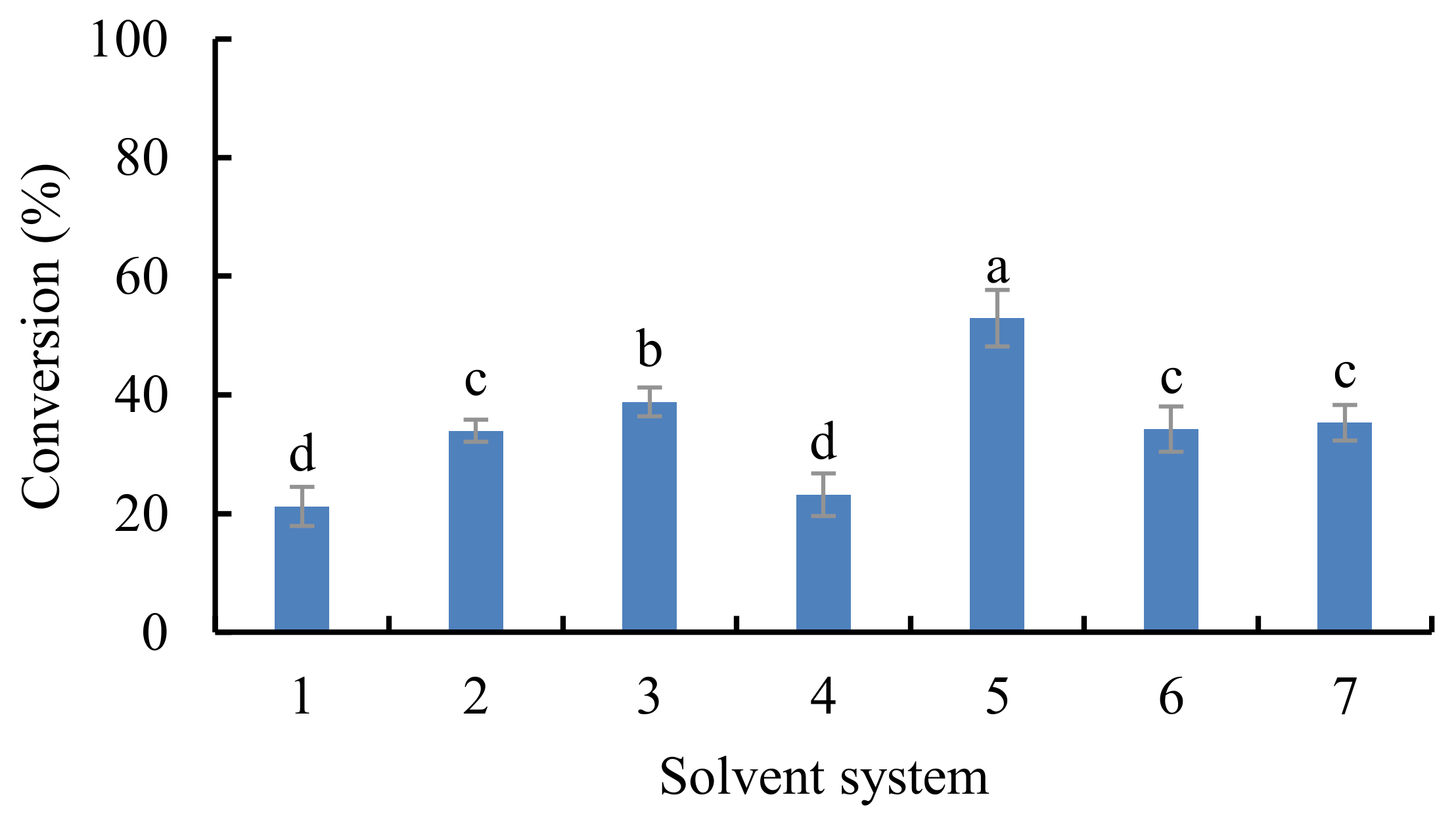
3.1. Effect of Various Extraction Factors on the Synthesis of EGCG Derivatives
3.2. Identification and Structure Analysis of EGCG Stearyl Derivatives
3.3. Lipophilicity of EGCG Stearyl Derivatives
3.4. Antioxidative Activities of EGCG Stearyl Derivatives and Synthetic Antioxidants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mozuraityte, R.; Kristinova, V.; Rustad, T. Oxidation of Food Components. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 186–190. [Google Scholar]
- Eskandani, M.; Hamishehkar, H.; Dolatabadi, J.E.N. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014, 153, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Echevarria, B.N.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-Tert-Butyl-Hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. Food Saf. 2014, 14, 67–80. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Forouharmehr, A.; Eskandani, M.; Barzegari, A.; Kafil, V.; Kashanian, S.; Dolatabadi, J.E.N. Cytotoxicity and DNA Fragmentation Properties of Butylated Hydroxyanisole. DNA Cell Biol. 2013, 32, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lue, B.-M.; Nielsen, N.S.; Jacobsen, C.; Hellgren, L.; Guo, Z.; Xu, X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010, 123, 221–230. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Danihelova, M.; Viskupicova, J.; Šturdík, E. Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chim. Slovaca 2012, 5, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Espinoza, M.-C.; Villeneuve, P. Phenolic Acids Enzymatic Lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Tobiason, F.L. MNDO and AM1 Molecular Orbital and Molecular Mechanics Analyses of (+)-Catechin, (-)-Epicatechin, and their 3-O-Acetyl Derivatives. In Plant Polyphenols; Springer: Boston, MA, USA, 1992; pp. 459–478. [Google Scholar]
- Ardhaoui, M.; Falcimaigne, A.; Ognier, S.; Engasser, J.; Moussou, P.; Pauly, G.; Ghoul, M. Effect of acyl donor chain length and substitutions pattern on the enzymatic acylation of flavonoids. J. Biotechnol. 2004, 110, 265–272. [Google Scholar] [CrossRef]
- De Araújo, M.E.M.B.; Franco, Y.E.M.; Messias, M.C.F.; Longato, G.; Pamphile, J.A.; de Carvalho, P. Biocatalytic Synthesis of Flavonoid Esters by Lipases and Their Biological Benefits. Planta Medica 2016, 83, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Li, Y.; Li, Z.; Ma, C.; Lou, Z.; Yokoyama, W.; Wang, H. Lipase-catalyzed synthesis of acetylated EGCG and antioxidant properties of the acetylated derivatives. Food Res. Int. 2014, 56, 279–286. [Google Scholar] [CrossRef]
- Viskupicova, J.; Danihelova, M.; Ondrejovic, M.; Liptaj, T.; Sturdik, E. Lipophilic rutin derivatives for antioxidant protection of oil-based foods. Food Chem. 2010, 123, 45–50. [Google Scholar] [CrossRef]
- Matsumura, K.; Kaihatsu, K.; Mori, S.; Cho, H.H.; Kato, N.; Hyon, S.H. Enhanced antitumor activities of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives in vitro and in vivo. Biochem. Biophys. Res. Commun. 2008, 377, 1118–1122. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Kontogianni, A.; Skouridou, V.; Sereti, V.; Stamatis, H.; Kolisis, F.N. Regioselective acylation of flavonoids catalyzed by lipase in low toxicity media. Eur. J. Lipid Sci. Technol. 2011, 103, 655–660. [Google Scholar] [CrossRef]
- Chen, M.; Yu, S. Lipophilized Grape Seed Proanthocyanidin Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2017, 65, 1598–1605. [Google Scholar] [CrossRef]
- Gordon, M.H.; Paiva-Martins, F.; Almeida, M. Antioxidant Activity of Hydroxytyrosol Acetate Compared with That of Other Olive Oil Polyphenols. J. Agric. Food Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Taira, I.; Inafuku, S.; Sumi, H.; Sawamura, M.; Takara, K.; Wada, K. Volatile Aroma Components and Antioxidant Activities of the Flavedo Peel Extract of Unripe Shiikuwasha (Citrus depressa Hayata). J. Food Sci. 2012, 77, C469–C475. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Screening of antiradical and antioxidant activity of monodesmosides and crude extract from Leontice smirnowii tuber. Phytomedicine 2006, 13, 343–351. [Google Scholar] [CrossRef]
- Kong, S.; Cao, P.; Guo, J.; Su, Z. Antioxidant ofsmallmolecular weightchitosan oligosaccharidein vitro. BIO Web Conf. 2017, 8, 1028. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Du, L.-H.; Luo, X.-P. Lipase-catalyzed regioselective acylation of sugar in microreactors. RSC Adv. 2012, 2, 2663–2665. [Google Scholar] [CrossRef]
- Yadav, G.D.; Devendran, S. Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process. Biochem. 2012, 47, 496–502. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ahmad, S.; Ariff, A.B. Comparative study of stirred and fluidized tank reactor for hydroxyl-kojic acid derivatives synthesis and their biological activities. Turk. J. Biochem. 2017, 43, 205–219. [Google Scholar] [CrossRef]
- Arcos, J.A.; Hill, C.G., Jr.; Otero, C. Kinetics of the lipase-catalyzed synthesis of glucose esters in acetone. Biotechnol. Bioeng. 2001, 73, 104–110. [Google Scholar] [CrossRef]
- Hazarika, S.; Goswami, P.; Dutta, N.N. Lipase catalysed transesterification of 2-o-benzylglycerol with vinyl acetate: Solvent effect. Chem. Eng. J. 2003, 94, 1–10. [Google Scholar] [CrossRef]
- Yang, T.; Rebsdorf, M.; Engelrud, U.; Xu, X. Enzymatic Production of Monoacylglycerols Containing Polyunsaturated Fatty Acids through an Efficient Glycerolysis System. J. Agric. Food Chem. 2005, 53, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Huang, Y.; Zhang, H.; Hou, L. Lipase-Catalyzed Transesterification Synthesis of Geranyl Acetate in Organic Solvents and Its Kinetics. Food Sci. Technol. Res. 2014, 20, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Jakovetić, S.M.; Jugovic, B.; Gvozdenović, M.M.; Bezbradica, D.I.; Antov, M.G.; Mijin, D..; Knežević-Jugović, Z.D. Synthesis of Aliphatic Esters of Cinnamic Acid as Potential Lipophilic Antioxidants Catalyzed by Lipase B from Candida antarctica. Appl. Biochem. Biotechnol. 2013, 170, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yang, F.; Xie, J.; Zhang, M.; Liu, W.; Fu, L. A Rapid and Practical Catalytic Esterification for the Preparation of Caffeic Acid Esters. J. Chem. Res. 2014, 38, 695–700. [Google Scholar] [CrossRef]
- Zengin, G. Synthesis, antimicrobial activity, and structure–activity relationships of eugenol, menthol, and genistein esters. Chem. Nat. Compd. 2011, 47, 550–555. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Zhong, Y.J.; Perera, N.; Shahidi, F. Antiglycation activity of lipophilized epigallocatechin gallate (EGCG) derivatives. Food Chem. 2016, 190, 1022–1026. [Google Scholar] [CrossRef]
- Huang, D.; Ou, A.B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Rezende, M.C.; Arenas, A. How meaningful is the assessment of antioxidant activities in microheterogeneous media? Food Chem. 2009, 113, 1083–1087. [Google Scholar] [CrossRef]
- Iglesias, J.; Pazos, M.; Lois, S.; Medina, I. Contribution of Galloylation and Polymerization to the Antioxidant Activity of Polyphenols in Fish Lipid Systems. J. Agric. Food Chem. 2010, 58, 7423–7431. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Pan, Y.L.; Qin, A.X.; Kang, X.H.; Li, X.Y.; Jia, Y.N.; Chen, C.; Cui, T. Synthesis of Oil-Soluble Aliphatic Acylated Epicatechin and their Free Radical Scavenging Activity. Adv. Mater. Res. 2013, 781-784, 1424–1429. [Google Scholar] [CrossRef]
- Yuji, H.; Weiss, J.; Villeneuve, P.; Giraldo, L.J.L.; Figueroa-Espinoza, M.-C.; Decker, E.A. Ability of Surface-Active Antioxidants to Inhibit Lipid Oxidation in Oil-in-Water Emulsion. J. Agric. Food Chem. 2007, 55, 11052–11056. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Chen, B.; LeComte, J.; Villeneuve, P.; McClements, D.; Decker, E.A. Antioxidant Properties of Chlorogenic Acid and Its Alkyl Esters in Stripped Corn Oil in Combination with Phospholipids and/or Water. J. Agric. Food Chem. 2011, 59, 10361–10366. [Google Scholar] [CrossRef]
- Budilarto, E.S.; Kamal-Eldin, A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1095–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai-Li, J.; Chang-Hai, W. Antioxidant properties of natural components from Salvia plebeia on oxidative stability of ascidian oil. Process. Biochem. 2006, 41, 1111–1116. [Google Scholar] [CrossRef]






| EGCG | Di-Stearyl EGCG | |||
|---|---|---|---|---|
| C/H Position | 1H | 13C | 1H | 13C |
| 2 | 4.99 | 77.22 | 4.99 | 76.88 |
| 3 | 5.55 | 68.54 | 5.57 | 68.38 |
| 4 | 3.00 | 25.43 | 3.01 | 25.36 |
| 2.86 | 2.88 | |||
| 5 | 155.83 | 155.55 | ||
| 6 | 5.98 | 94.49 | 5.98 | 94.30 |
| 7 | 156.47 | 156.42 | ||
| 8 | 5.98 | 95.15 | 5.98 | 95.01 |
| 9 | 156.47 | 156.42 | ||
| 10 | 98.04 | 97.98 | ||
| 1’ | 120.14 | 120.03 | ||
| 2’ | 6.52 | 105.50 | 6.51 | 105.51 |
| 3’ | 144.89 | 145.11 | ||
| 4’ | 132.40 | 132.41 | ||
| 5’ | 144.89 | 145.11 | ||
| 6’ | 6.52 | 105.50 | 6.51 | 105.51 |
| 1” | 129.42 | 129.28 | ||
| 2” | 6.97 | 108.88 | 6.86 | 108.89 |
| 3” | 145.29 | 149.75 | ||
| 4” | 138.39 | 136.74 | ||
| 5” | 145.29 | 149.75 | ||
| 6” | 6.97 | 108.88 | 6.86 | 108.89 |
| COO | 166.26 | 166.21 | ||
| 170.68 | ||||
| 172.44 | ||||
| Octanol/Water Partition Coefficient (logP) | |||
|---|---|---|---|
| EGCG Stearyl Derivatives | 3.49 | ± | 0.34 a |
| EGCG | 0.69 | ± | 0.08 b |
| DPPH Inhibition(%) | ABTS Inhibition (%) | Reducing Power (OD) | Hydroxyl Inhibition (%) | |
|---|---|---|---|---|
| EGCG | 90.89 ± 2.56 a | 90.36 ± 3.30 a | 2.845 ± 0.086 a | 30.71 ± 1.90 b |
| EGCG Stearyl Derivatives | 51.49 ± 1.80 c | 65.16 ± 1.28 b | 1.857 ± 0.044 c | 16.84 ± 1.18 c |
| BHT | 9.74 ± 0.65 d | 56.30 ± 2.30 c | 1.710 ± 0.033 c | 11.33 ± 0.80 d |
| BHA | 72.38 ± 2.31 b | 60.06 ± 2.88 bc | 2.260 ± 0.063 b | 7.18 ± 0.87 e |
| TBHQ | 92.99 ± 2.06 a | 63.01 ± 1.64 b | 2.689 ± 0.061 a | 73.86 ± 2.87 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Wang, L.; Huang, X.; Zhu, S.; Ma, C.; Wang, H. Identification and Antioxidant Abilities of Enzymatic-Transesterification (−)-Epigallocatechin-3-O-gallate Stearyl Derivatives in Non-Aqueous Systems. Antioxidants 2021, 10, 1282. https://doi.org/10.3390/antiox10081282
Jiang C, Wang L, Huang X, Zhu S, Ma C, Wang H. Identification and Antioxidant Abilities of Enzymatic-Transesterification (−)-Epigallocatechin-3-O-gallate Stearyl Derivatives in Non-Aqueous Systems. Antioxidants. 2021; 10(8):1282. https://doi.org/10.3390/antiox10081282
Chicago/Turabian StyleJiang, Chengyu, Li Wang, Xin Huang, Song Zhu, Chaoyang Ma, and Hongxin Wang. 2021. "Identification and Antioxidant Abilities of Enzymatic-Transesterification (−)-Epigallocatechin-3-O-gallate Stearyl Derivatives in Non-Aqueous Systems" Antioxidants 10, no. 8: 1282. https://doi.org/10.3390/antiox10081282





