Association of Serum Bilirubin with the Severity and Outcomes of Intracerebral Hemorrhages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Subject Selection Criteria
2.2. Data Extraction
2.3. Statistical Analysis
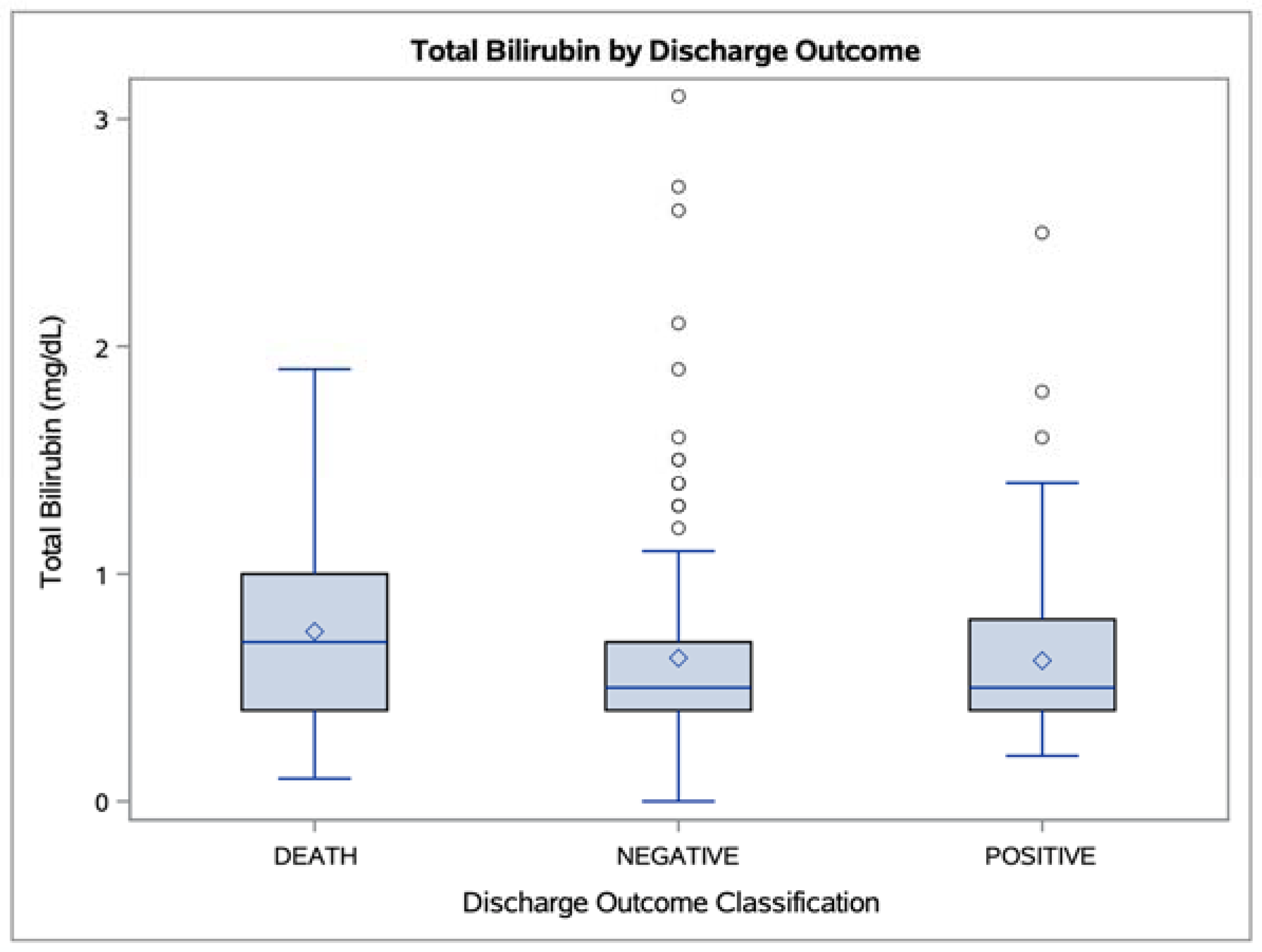
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, 139–596. [Google Scholar] [CrossRef]
- Marrugat, J.; Arboix, A.; García-Eroles, L.; Salas, T.; Vila, J.; Castell, C.; Tresserras, R.; Elosua, R. The Estimated Incidence and Case Fatality Rate of Ischemic and Hemorrhagic Cerebrovascular Disease in 2002 in Catalonia. Rev. Esp. Cardiol. 2007, 60, 573–580. [Google Scholar] [CrossRef]
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, Case Fatality, and Functional Outcome of Intracerebral Haemorrhage over Time, According to Age, Sex, and Ethnic Origin: A Systematic Review and Meta-Analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Pandey, A.S.; Thompson, B.G.; Keep, R.F.; Hua, Y.; Xi, G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 2018, 134, 240–248. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C.; Zhang, J.; Hu, Z. Mechanism and Therapy of Brain Edema after Intracerebral Hemorrhage. Cerebrovasc. Dis. 2016, 42, 155–169. [Google Scholar] [CrossRef]
- Right, C.; Bozza, M.T.; Oliveira, M.F.; Bozza, F.A. Molecular, cellular and clinical aspects of intracerebral hemorrhage: Are the enemies within? Curr. Neuropharmacol. 2016, 14, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemphill, J.C.; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guide-line for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [Green Version]
- Amin, S.B. Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin. Perinatol. 2004, 28, 340–347. [Google Scholar] [CrossRef]
- Ziberna, L.; Martelanc, M.; Franko, M.; Passamonti, S. Bilirubin is an endogenous antioxidant in human vascular endothelial cells. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dore, S.; Takahashi, M.; Ferris, C.D.; Zakhary, R.; Hester, L.D.; Guastella, D.; Snyder, S.H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA 1999, 96, 2445–2450. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-W.; Carey, D.; Wu, J.; Sugiyama, H. The cytoprotective effects of bilirubin and biliverdin on rat hepatocytes and human erythrocytes and the impact of albumin. Biochem. Cell Biol. 1991, 69, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Kapitulnik, J. Bilirubin: An endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol. Pharmacol. 2004, 66, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, T.W.; Snyder, S.H. Bilirubin benefits: Cellular protection by a biliverdin reductase antioxidant Cycle. Pediatrics 2004, 113, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Daiber, A. Direct Antioxidant Properties of Bilirubin and Biliverdin. Is there a Role for Biliverdin Reductase? Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Jansen, T.; Hortmann, M.; Oelze, M.; Opitz, B.; Steven, S.; Schell, R.; Knorr, M.; Karbach, S.; Schuhmacher, S.; Wenzel, P.; et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1—evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010, 49, 186–195. [Google Scholar] [CrossRef]
- Jacobsen, J.; Wennberg, R.P. Determination of unbound bilirubin in the serum of newborns. Clin. Chem. 1974, 20, 783–789. [Google Scholar] [CrossRef]
- Shimabuku, R.; Nakamura, H. Total and unbound bilirubin determination using an automated peroxidase micromethod. Kobe J. Med. Sci. 1982, 28, 91–104. [Google Scholar]
- Ahlfors, C.E.; Marshall, G.D.; Wolcott, D.K.; Olson, D.C.; Van Overmeire, B. Measurement of unbound bilirubin by the peroxidase test using Zone Fluidics. Clin. Chim. Acta 2006, 365, 78–85. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Vreman, H.J.; Wong, R.J.; Stevenson, D.K. Photoisomers: Obfuscating Factors in Clinical Peroxidase Measurements of Unbound Bilirubin? Pediatrics 2009, 123, 67–76. [Google Scholar] [CrossRef]
- Iskander, I.; Gamaleldin, R.; El Houchi, S.; El Shenawy, A.; Seoud, I.; El Gharbawi, N.; Abou-Youssef, H.; Aravkin, A.; Wennberg, R.P. Serum Bilirubin and Bilirubin/Albumin Ratio as Predictors of Bilirubin Encephalopathy. Pediatrics 2014, 134, 1330–1339. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Morioka, I.; Miwa, A.; Yokota, T.; Matsuo, K.; Koda, T.; Fujioka, K.; Morikawa, S.; Shibata, A.; Yokoyama, N.; et al. Is Bilirubin/Albumin Ratio Correlated with Unbound Bilirubin Concentration? Pediatr. Int. 2012, 54, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, S.B.; Dana, V.G.; Ziaee, V.; Ashtiani, M.-T.H.; Djavid, G.E.; Alijani, M. Bilirubin/Albumin Ratio for Predicting Acute Bilirubin-induced Neurologic Dysfunction. Iran. J. Pediatr. 2011, 21, 28–32. [Google Scholar]
- Thakkar, M.; Edelenbos, J.; Doré, S. Bilirubin and Ischemic Stroke: Rendering the Current Paradigm to Better Understand the Protective Effects of Bilirubin. Mol. Neurobiol. 2019, 56, 5483–5496. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Wu, D.; Ye, X.; Wang, X.; Zhou, Y.; Zhu, X.; Liu, X. Association of Circulating Total Bilirubin Level with Ischemic Stroke: A Systemic Review and Meta-Analysis of Observational Evidence. Ann. Transl. Med. 2019, 7. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Pande, R.L.; Creager, M.A.; Weuve, J.; Beckman, J.A. Serum Total Bilirubin Level, Prevalent Stroke, and Stroke Outcomes: NHANES 1999–2004. Am. J. Med. 2008, 121, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Asl, E.S.; Taheraghdam, A.; Rahmani, F.; Javadrashid, R.; Golzari, S.E.J.; Ghaemian, N.; Sadeghpour, Y.; Esfanjani, R.M.; Soleimanpour, H. Determination of the Predictive Value of Serum Bilirubin in Patients with Ischemic Stroke: A Prospective Descriptive Analytical Study. Adv. Pharm. Bull. 2018, 8, 715–719. [Google Scholar] [CrossRef]
- Arsalan; Ismail, M.; Khattak, M.B.; Khan, F.; Anwar, M.J.; Murtaza, Z.; Khan, A. Prognostic Significance of Serum Bilirubin in Stroke. J. Ayub Med. Coll. Abbottabad 2011, 23, 104–107. [Google Scholar]
- Pineda, S.; Bang, O.Y.; Saver, J.L.; Starkman, S.; Yun, S.W.; Liebeskind, D.S.; Kim, D.; Ali, L.K.; Shah, S.H.; Ovbiagele, B. Association of Serum Bilirubin with Ischemic Stroke Outcomes. J. Stroke Cerebrovasc. Dis. 2008, 17, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohi, K.; Mochizuki, Y.; Satoh, K.; Jimbo, H.; Hayashi, M.; Toyoda, I.; Ikeda, Y.; Abe, T.; Aruga, T. Transient Elevation of Serum Bilirubin (a Heme Oxygenase-1 Metabolite) Level in Hemorrhagic Stroke: Bilirubin Is a Marker of Oxidant Stress. In Brain Edema XII. Acta Neurochirurgica Supplements; Kuroiwa, T., Ed.; Springer: Vienna, Austria, 2003; Volume 86, pp. 247–249. [Google Scholar] [CrossRef]
- Li, H.; Dai, B.; Shen, G.; Liu, W.; Fu, R.; He, M. Original Article Serum Bilirubin Levels in Acute Stroke in Chinese Population: A Meta-Analysis. Int. J. Clin. Exp. Med. 2017, 10, 905–912. [Google Scholar]
- Wang, R.; He, M.; Xu, J. Serum Bilirubin Level Correlates with Mortality in Patients with Traumatic Brain Injury. Medicine 2020, 99, e21020. [Google Scholar] [CrossRef]
- Morotti, A.; Marini, S.; Lena, U.K.; Crawford, K.; Schwab, K.; Kourkoulis, C.; Ayres, A.M.; Edip Gurol, M.; Viswanathan, A.; Greenberg, S.M.; et al. Significance of Admission Hypoalbuminemia in Acute Intracerebral Hemorrhage. J. Neurol. 2017, 264, 905–911. [Google Scholar] [CrossRef]
- Dziedzic, T.; Slowik, A.; Szczudlik, A. Serum Albumin Level as a Predictor of Ischemic Stroke Outcome. Stroke 2004, 35, e156–e158. [Google Scholar] [CrossRef] [Green Version]
- Seet, R.C.S.; Lim, E.C.H.; Chan, B.P.L.; Ong, B.K.C. Serum Albumin Level as a Predictor of Ischemic Stroke Outcome [6] (multiple letters). Stroke 2004, 35, 2435–2436. [Google Scholar] [CrossRef]
- Babu, M.S.; Kaul, S.; Dadheech, S.; Rajeshwar, K.; Jyothy, A.; Munshi, A. Serum Albumin Levels in Ischemic Stroke and Its Subtypes: Correlation with Clinical Outcome. Nutrition 2013, 29, 872–875. [Google Scholar] [CrossRef]
- Idicula, T.T.; Waje-Andreassen, U.; Brøgger, J.C.; Naess, H.; Thomassen, L. Serum Albumin in Ischemic Stroke Patients: The Higher the Better. The Bergen Stroke Study. Cerebrovasc. Dis. 2009, 28, 13–17. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.-L.; Mira, J.-P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensiv. Care 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Belayev, L.; Liu, Y.; Zhao, W.; Busto, R.; Ginsberg, M.D. Human Albumin Therapy of Acute Ischemic Stroke: Marked Neuroprotective Efficacy at Moderate Doses and with a Broad Therapeutic Window. Stroke 2001, 32, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Belayev, L.; Busto, R.; Zhao, W.; Clemens, J.A.; Ginsberg, M.D. Effect of Delayed Albumin Hemodilution on Infarction Volume and Brain Edema after Transient Middle Cerebral Artery Occlusion in Rats. J. Neurosurg. 1997, 87, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.H.; Yeatts, S.D.; Hill, M.D.; Moy, C.S.; Ginsberg, M.D.; Palesch, Y.Y. ALIAS Parts 1 and 2 and NETT Investigators ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data from Parts 1 and 2. Stroke 2016, 47, 2355–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.S.; Heeley, E.; Huang, Y.; Wang, J.; Stapf, C.; Delcourt, C.; Lindley, R.; Robinson, T.; Lavados, P.; Neal, B.; et al. Rapid Blood-Pressure Lowering in Patients with Acute Intracerebral Hemorrhage. N. Engl. J. Med. 2013, 368, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Wasay, M.; Khealani, B.A.; Shafqat, S.; Kamal, A.; Syed, N.A. Hypotension at Presentation Is an Indicator of Poor Prognosis in Acute Intracerebral Haemorrhage. J. Pak. Med. Assoc. 2008, 58, 359–361. [Google Scholar] [PubMed]
- Besmertis, L.; Bonovich, D.C.; HemphillIII, J.C. The Role of Hypotension in Secondary Brain Injury after Intracerebral Hemorrhage. Stroke 2000, 32. [Google Scholar] [CrossRef]
- Kim, H.C.; Kang, D.R.; Nam, C.M.; Hur, N.W.; Shim, J.S.; Jee, S.H.; Suh, I. Elevated Serum Aminotransferase Level as a Predictor of Intracerebral Hemorrhage: Korea Medical Insurance Corporation Study. Stroke 2005, 36, 1642–1647. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Takeuchi, S.; Tanaka, R.; Koike, T.; Sasaki, O.; Minakawa, T. Liver Dysfunction in Spontaneous Intracerebral Hemorrhage. Neurosurgery 1994, 35, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Hazlewood, J.; DeVivo, M.J.; Rosner, M. Elevated Liver Enzymes after Nontraumatic Intracranial Hemorrhages. Arch. Phys. Med. Rehabil. 1998, 79, 766–771. [Google Scholar] [CrossRef]
- Arboix, A.; García-Eroles, L.; Massons, J.; Oliveres, M.; Targa, C. Hemorrhagic Lacunar Stroke. Cerebrovasc. Dis. 2000, 10, 229–234. [Google Scholar] [CrossRef]
- Daood, M.J.; McDonagh, A.F.; Watchko, J.F. Calculated Free Bilirubin Levels and Neurotoxicity. J. Perinatol. 2009, 29 (Suppl. 1), 14–19. [Google Scholar] [CrossRef]
- Ahlfors, C.E. The Bilirubin Binding Panel: A Henderson-Hasselbalch Approach to Neonatal Hyperbilirubinemia. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Mean (SD) or n (%) |
|---|---|
| Age (years) | 65.8 (16.2) |
| Sex | |
| Male | 148 (54.6%) |
| Female | 128 (46.4%) |
| Medical history | |
| Hypertension | 184 (70.2%) |
| Diabetes | 56 (21.0%) |
| Hyperlipidemia | 49 (18.7%) |
| History of stroke | 43 (21.6%) |
| Atrial fibrillation | 42 (16.0%) |
| Coronary artery disease | 31 (11.8%) |
| Thyroid disease | 21 (8.0%) |
| Chronic obstructive pulmonary disease | 21 (8.0%) |
| Congestive heart failure | 20 (7.6%) |
| Chronic kidney disease | 18 (6.9%) |
| Asthma | 16 (6.1%) |
| Myocardial infarction | 12 (4.6%) |
| Liver disease | 8 (3.0%) |
| Deep vein thrombosis | 8 (3.0%) |
| Obstructive sleep apnea | 7 (2.7%) |
| Valve disease or surgery | 7 (2.7%) |
| Social history | |
| Tobacco use | |
| Smoker | 87 (52.1%) |
| Nonsmoker | 80 (47.9%) |
| Current alcohol use | 68 (39.5%) |
| Current marijuana use | 10 (5.9%) |
| Variable | Mean (SD) or n (%) |
|---|---|
| Vital signs | |
| Heart rate (beats/minute) | 84.4 (18.9) |
| Systolic pressure (mmHg) | 155.0 (35.2) |
| Diastolic pressure (mmHg) | 82.7 (23.6) |
| Temperature (°C) | 37.7 (1.0) |
| SpO2 (%) | 97.1 (2.9) |
| Admission GCS | n = 275 |
| 8 or lower | 83 (30.2%) |
| 9 or higher | 192 (69.8%) |
| Admission ICH Score | n = 250 |
| Score 0 | 52 (20.8%) |
| Score 1 | 64 (25.6%) |
| Score 2 | 55 (22.0%) |
| Score 3 | 48 (19.2%) |
| Score 4 | 20 (8.0%) |
| Score 5 | 11 (4.4%) |
| Primary location of hemorrhage | n = 276 |
| Lobar | 111 (40.2%) |
| Deep | 84 (30.4%) |
| Brainstem and cerebellum | 30 (10.9%) |
| Uncertain | 51 (18.5%) |
| Surgical interventions | n = 276 |
| Yes | 68 (24.6%) |
| No | 208 (75.4%) |
| Variable | Mean (SD) or n (%) |
|---|---|
| Length of hospital stay (days) | 8.7 (8.5) |
| In-hospital deaths | 61 (22.1%) |
| Discharge GCS | n = 271 |
| 8 or lower | 80 (29.5%) |
| 9 or higher | 191 (70.5%) |
| Discharge mRS | n = 209 |
| Score 0 (no symptoms) | 12 (5.7%) |
| Score 1 (no significant disability despite symptoms) | 21 (10.1%) |
| Score 2 (slight disability) | 23 (11.0%) |
| Score 3 (moderate disability) | 18 (8.6%) |
| Score 4 (moderate severe disability) | 42 (20.1%) |
| Score 5 (severe disability; requiring constant care) | 32 (15.3%) |
| Score 6 (deceased) | 61 (29.2%) |
| Discharge destination outcome classification | n = 276 |
| Positive | 80 (29.0%) |
| Negative | 135 (48.9%) |
| Death | 61 (22.1%) |
| rs p-Value (n) 1 | Total Bilirubin | Direct Bilirubin | Indirect Bilirubin | Albumin |
|---|---|---|---|---|
| Admission GCS | −0.08 0.1978 (275) | −0.17 0.0114 * (225) | −0.03 0.6722 (225) | 0.13 0.0269 * (271) |
| Admission ICH Score | 0.07 0.2537 (250) | 0.19 0.0075 ** (204) | 0.03 0.6332 (204) | −0.06 0.3822 (247) |
| Discharge GCS | −0.07 0.2668 (271) | −0.12 0.0661 (222) | 0.01 0.8940 (222) | 0.15 0.0133 * (267) |
| Discharge mRS | 0.09 0.1991 (209) | 0.15 0.0454 * (171) | 0.06 0.4146 (171) | −0.16 0.0227 * (207) |
| Length of hospital stay | −0.09 0.1553 (276) | 0.01 0.8882 (226) | −0.07 0.3274 (226) | −0.03 0.5774 (272) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Garvan, C.S.; Heaton, S.C.; Nagaraja, N.; Doré, S. Association of Serum Bilirubin with the Severity and Outcomes of Intracerebral Hemorrhages. Antioxidants 2021, 10, 1346. https://doi.org/10.3390/antiox10091346
Fu K, Garvan CS, Heaton SC, Nagaraja N, Doré S. Association of Serum Bilirubin with the Severity and Outcomes of Intracerebral Hemorrhages. Antioxidants. 2021; 10(9):1346. https://doi.org/10.3390/antiox10091346
Chicago/Turabian StyleFu, Kai, Cynthia S. Garvan, Shelley C. Heaton, Nandakumar Nagaraja, and Sylvain Doré. 2021. "Association of Serum Bilirubin with the Severity and Outcomes of Intracerebral Hemorrhages" Antioxidants 10, no. 9: 1346. https://doi.org/10.3390/antiox10091346
APA StyleFu, K., Garvan, C. S., Heaton, S. C., Nagaraja, N., & Doré, S. (2021). Association of Serum Bilirubin with the Severity and Outcomes of Intracerebral Hemorrhages. Antioxidants, 10(9), 1346. https://doi.org/10.3390/antiox10091346







