Dry-Cured Ham-Derived Peptide (Asp–Leu–Glu–Glu) Exerts Cytoprotective Capacity in Human Intestinal Epithelial Caco-2 Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cellular Antioxidant Activity
2.4. ROS in Caco-2 Cells
2.5. Oxidative Stress Treatment
2.5.1. Antioxidant Enzyme Activity
2.5.2. RNA Isolation and PCR Array Analysis
2.5.3. Western Blotting
2.6. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
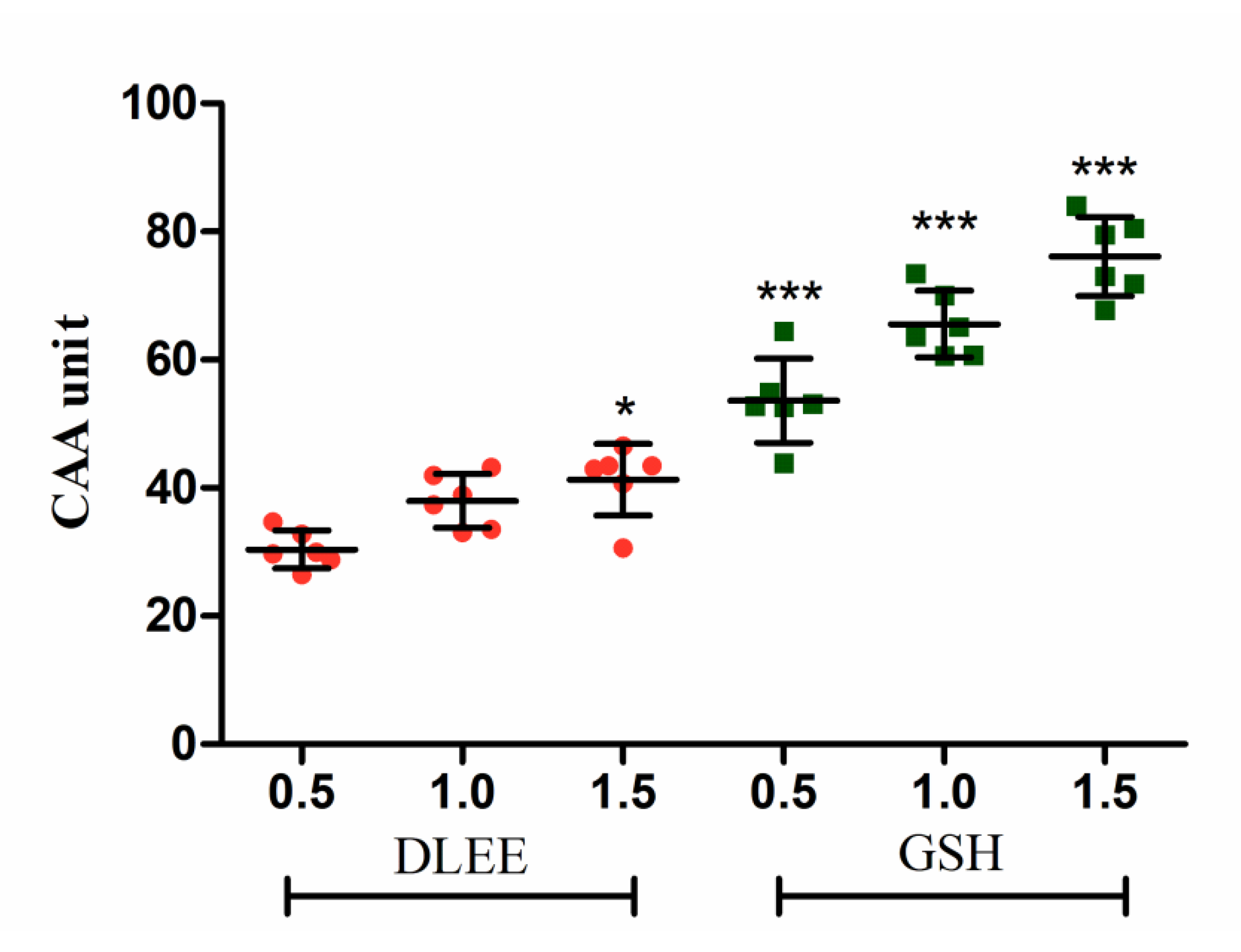
3.1. Cellular Antioxidant Activity of DLEE in Caco-2 Cells
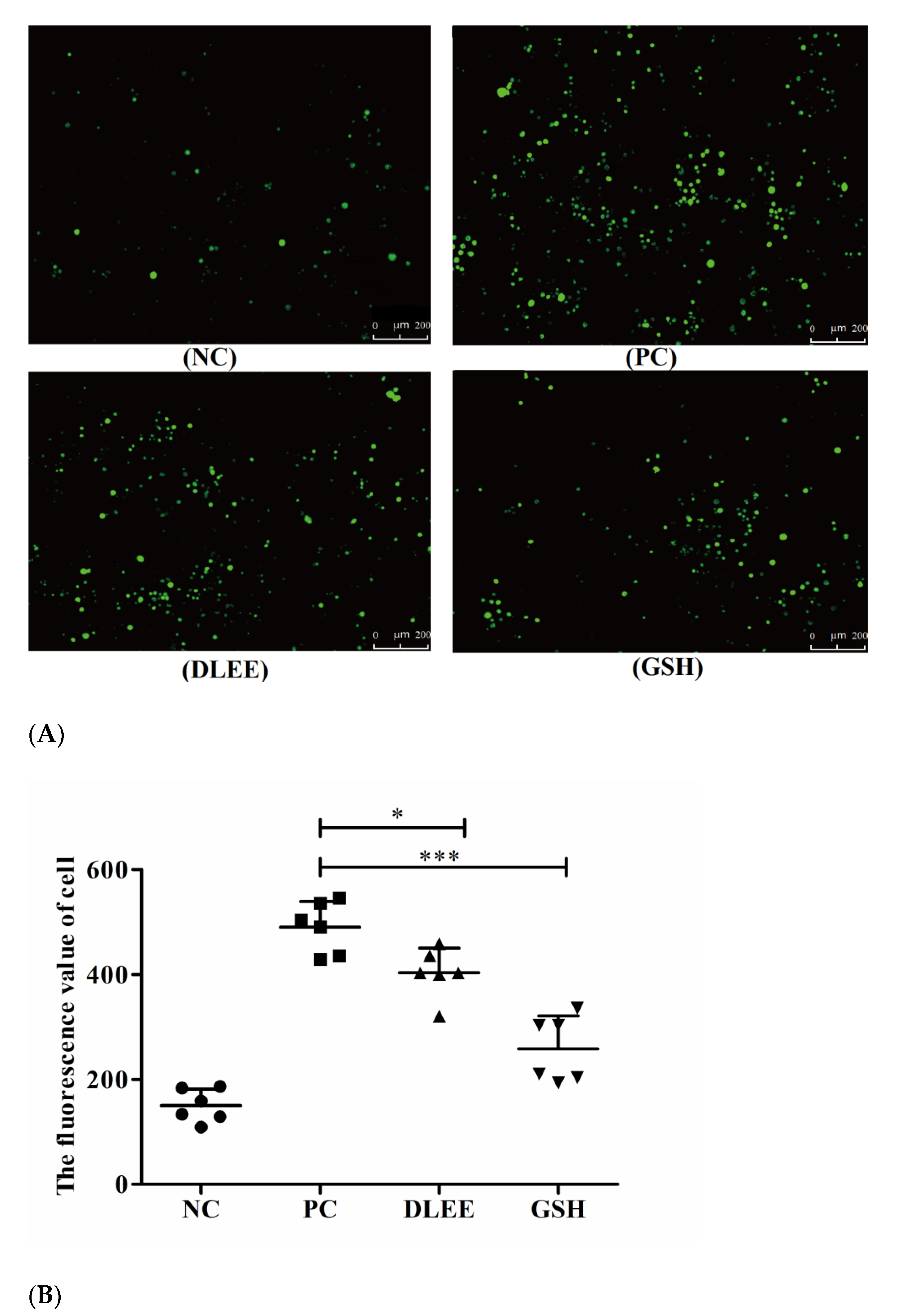
3.2. Cellular ROS Detection in Caco-2 Cells
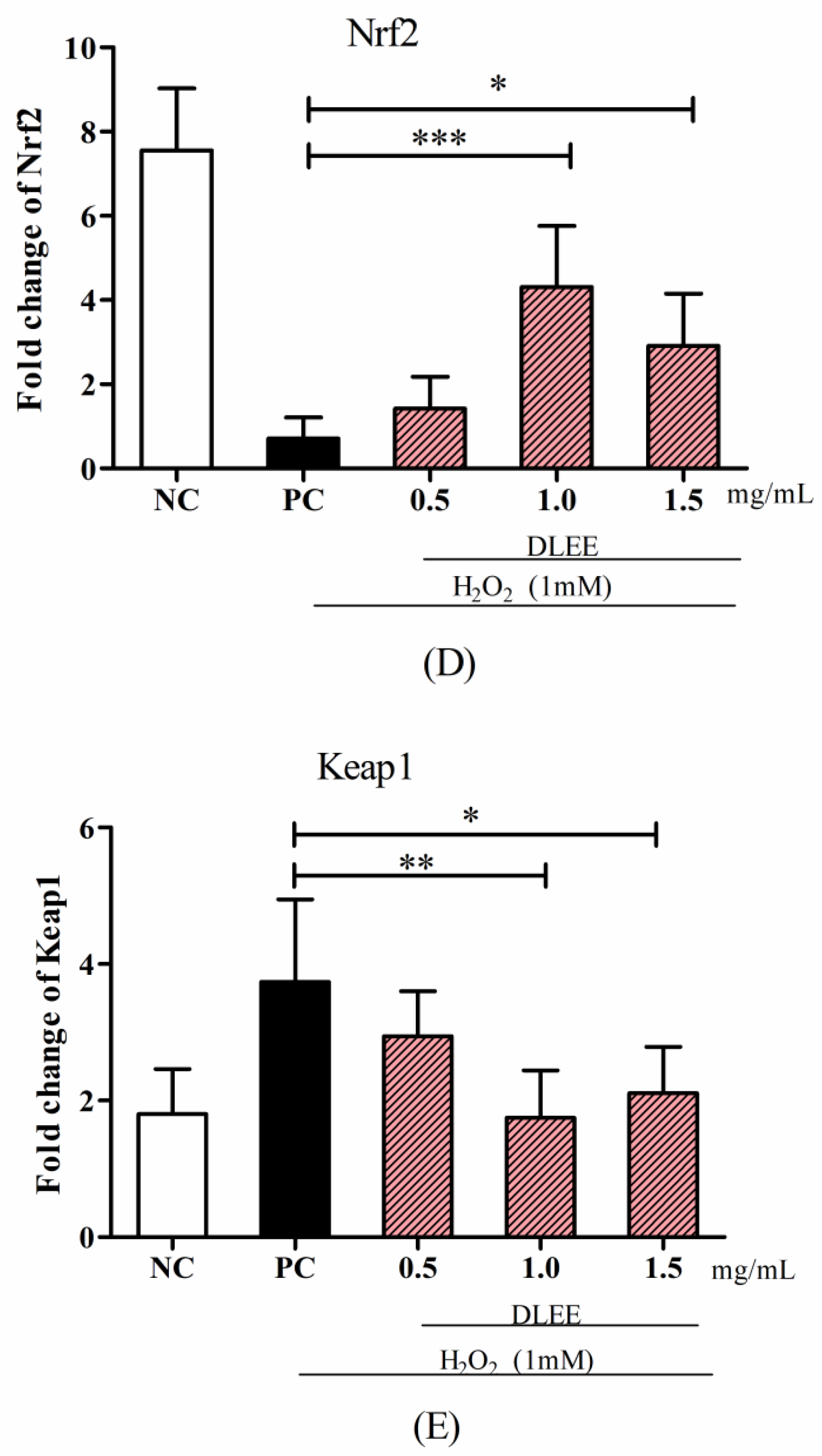
3.3. Gene Expression of Antioxidant Enzymes, Nrf2, and Keap1
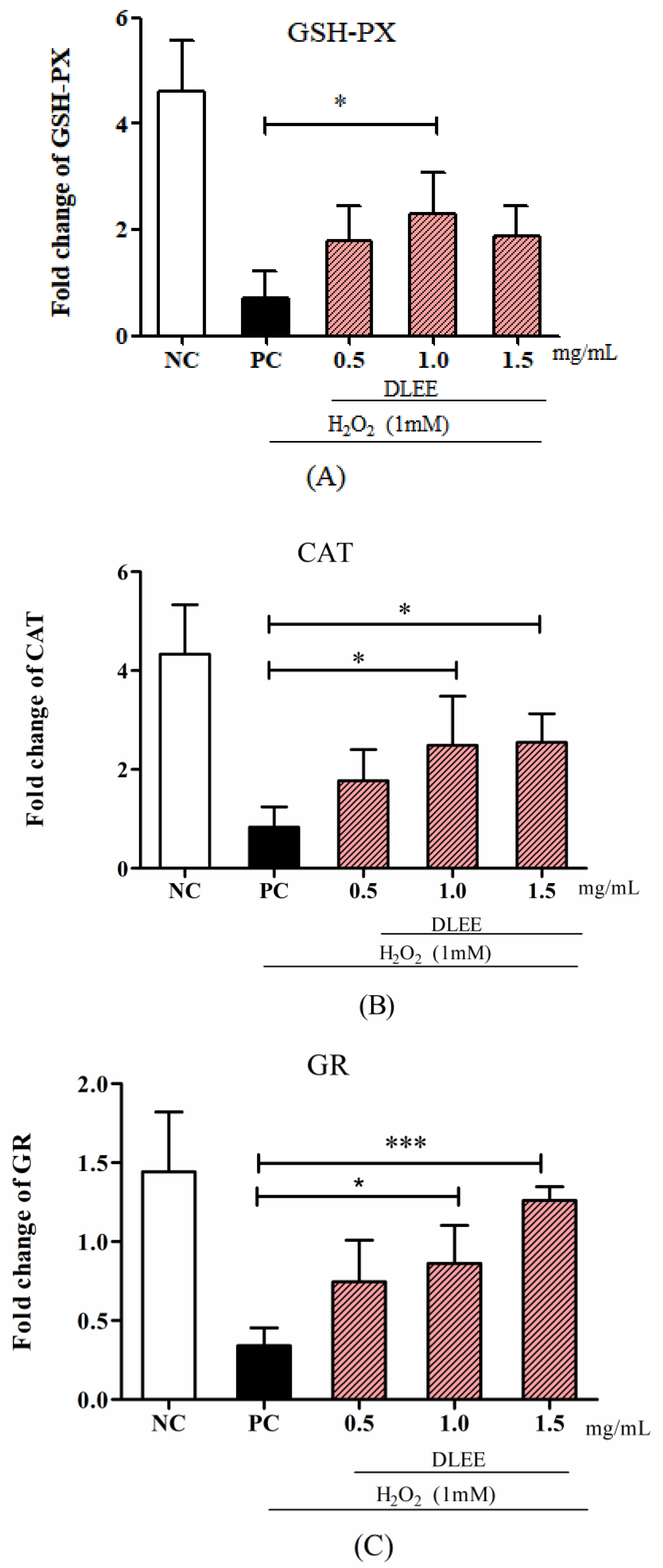
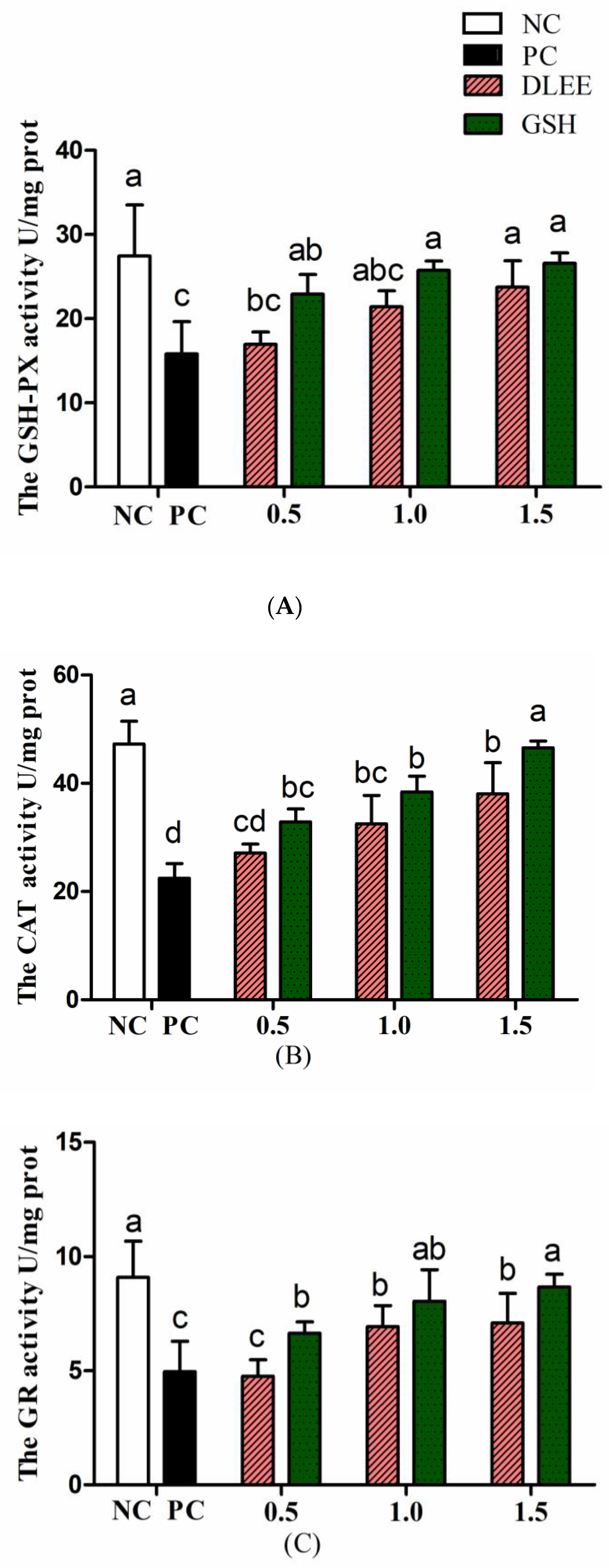
3.4. Activities of Antioxidant Enzymes
3.5. Expression of Antioxidant Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starkov, A.A. The Role of Mitochondria in Reactive Oxygen Species Metabolism and Signaling. Ann. N. Y. Acad. Sci. 2010, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Dong, Y.; Hou, W.; Yu, Z.; Weiner, C. The inflammatory profile and ROS levels in fetal brain differ by the cause of fetal inflammatory response syndrome (FIRS): Lipopolysaccharide (LPS) vs. chronic hypoxia (HPX). Am. J. Obstet. Gynecol. 2009, 2011, S157–S158. [Google Scholar] [CrossRef]
- Róka, R.; Gecse, K.; Wittmann, T. Recent observations related to the pathogenesis of irritable bowel syndrome. Eur. Gastroenterol. Hepatol. Rev. 2011, 7, 26–30. [Google Scholar]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal. Res. 2010, 36, 1–9. [Google Scholar] [CrossRef]
- Datta, G.; Basu, S.; Sen, A.; Nath, P. Role of α -tocopherol, ß -carotene and ascorbic acid against alcohol induced hepatotoxicity: A comparative analysis. J. Pharm. Res. 2012, 20125, 2485–2488. [Google Scholar]
- Xu, Y.W.; Cheng, W.; Gong, P.S.; Yan, G.L.; Xu, J.J. Preparation and antioxidant characters of selenium-containing soybean peptide with glutathione peroxidase activity. In Proceedings of the International Symposium on Information Technology in Medicine & Education, Hokkaido, Japan, 3–5 August 2012. [Google Scholar]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected Lactic Acid Bacteria Synthesize Antioxidant Peptides during Sourdough Fermentation of Cereal Flours. Appl. Environ. Microb. 2012, 78, 1087. [Google Scholar] [CrossRef] [Green Version]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Xing, L.; Hu, Y.; Hu, H.; Ge, Q.; Zhou, G.; Zhang, W. Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Makkar, S.K.; Liyanage, R.; Kannan, L.; Packialakshmi, B.; Rath, N.C. Chicken egg shell membrane associated proteins and peptides. J. Agric. Food Chem. 2015, 63, 9888–9898. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef]
- Toldra, F.; Gallego, M.; Reig, M.; Aristoy, M.; Mora, L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.C.; Toldra, F. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef]
- Xing, L.; Rui, L.; Gao, X.; Zheng, J.; Chong, W.; Zhou, G.; Zhang, W. The proteomics homology of antioxidant peptides extracted from dry-cured Xuanwei and Jinhua ham. Food Chem. 2018, 266, 420–426. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Del Mar Contreras, M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhakar, S.; Nazeer, R.A. Structural characterization of an Indian squid antioxidant peptide and its protective effect against cellular reactive oxygen species. J. Funct. Foods 2015, 14, 502–512. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Peptides derived from eggshell membrane improve antioxidant enzyme activity and glutathione synthesis against oxidative damage in Caco-2 cells. J. Funct. Foods 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Wang, M.; Zhou, G.; Zhang, W. Effect of protein S-nitrosylation on autolysis and catalytic ability of mu-calpain. Food Chem. 2016, 213, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintás, G.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant activity of enzymatic hydrolysates from eggshell membrane proteins and its protective capacity in human intestinal epithelial Caco-2 cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Bakken, H.E. Preventive effect of Nile tilapia hydrolysate against oxidative damage of HepG2 cells and DNA mediated by H2O2 and AAPH. J. Food Sci. Tech. Mys. 2015, 52, 6194–6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Phenolic compounds protect HepG2 cells from oxidative damage: Relevance of glutathione levels. Life Sci. 2006, 79, 2056–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiriyaphan, C.; Chitsomboon, B.; Yongsawadigul, J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012, 132, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Liu, R.; Tang, C.; Pereira, J.; Zhou, G.; Zhang, W. The antioxidant activity and transcellular pathway of Asp-Leu-Glu-Glu in a Caco-2 cell monolayer. Int. J. Food Sci. Tech. 2018, 53, 2405–2414. [Google Scholar] [CrossRef]
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef] [Green Version]
- Tkachev, V.O.; Menshchikova, E.B.; Zenkov, N.K. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochem. Mosc. 2011, 76, 407–422. [Google Scholar] [CrossRef]
- Chen, X.L.; Kunsch, C. Induction of Cytoprotective Genes Through Nrf2/Antioxidant Response Element Pathway: A New Therapeutic Approach for the Treatment of Inflammatory Diseases. Curr. Pharm. Des. 2004, 10, 879–891. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keapl-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Nie, S.; Ding, L.; Wang, L.; Liu, J.; Liu, W.; Zhang, T. Direct inhibition of Keap1–Nrf2 interaction by egg-derived peptides DKK and DDW revealed by molecular docking and fluorescence polarization. RSC Adv. 2017, 7, 34963–34971. [Google Scholar] [CrossRef] [Green Version]
- Majumder, K.; Fukuda, T.; Zhang, H.; Sakurai, T.; Taniguchi, Y.; Watanabe, H.; Mitsuzumi, H.; Matsui, T.; Mine, Y. Intervention of Isomaltodextrin Mitigates Intestinal Inflammation in a Dextran Sodium Sulfate-Induced Mouse Model of Colitis via Inhibition of Toll-like Receptor-4. J. Agr. Food Chem. 2017, 65, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]



| DLEE | GSH | |
|---|---|---|
| ORAC a | 1031.54 ± 53.87 * | 1588.84 ± 141.89 |
| ABTS+ a | 148.34 ± 7.90 | 157.98 ± 8.24 |
| Superoxide radical b | 65.05 ± 3.94 * | 56.05 ± 3.79 |
| DPPH radical b | 70.53 ± 3.91 * | 83.25 ± 2.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, L.; Fu, L.; Hao, Y.; Zhang, W. Dry-Cured Ham-Derived Peptide (Asp–Leu–Glu–Glu) Exerts Cytoprotective Capacity in Human Intestinal Epithelial Caco-2 Cells. Antioxidants 2021, 10, 1354. https://doi.org/10.3390/antiox10091354
Xing L, Fu L, Hao Y, Zhang W. Dry-Cured Ham-Derived Peptide (Asp–Leu–Glu–Glu) Exerts Cytoprotective Capacity in Human Intestinal Epithelial Caco-2 Cells. Antioxidants. 2021; 10(9):1354. https://doi.org/10.3390/antiox10091354
Chicago/Turabian StyleXing, Lujuan, Lijuan Fu, Yuejing Hao, and Wangang Zhang. 2021. "Dry-Cured Ham-Derived Peptide (Asp–Leu–Glu–Glu) Exerts Cytoprotective Capacity in Human Intestinal Epithelial Caco-2 Cells" Antioxidants 10, no. 9: 1354. https://doi.org/10.3390/antiox10091354
APA StyleXing, L., Fu, L., Hao, Y., & Zhang, W. (2021). Dry-Cured Ham-Derived Peptide (Asp–Leu–Glu–Glu) Exerts Cytoprotective Capacity in Human Intestinal Epithelial Caco-2 Cells. Antioxidants, 10(9), 1354. https://doi.org/10.3390/antiox10091354






