Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer
Abstract
:1. Introduction
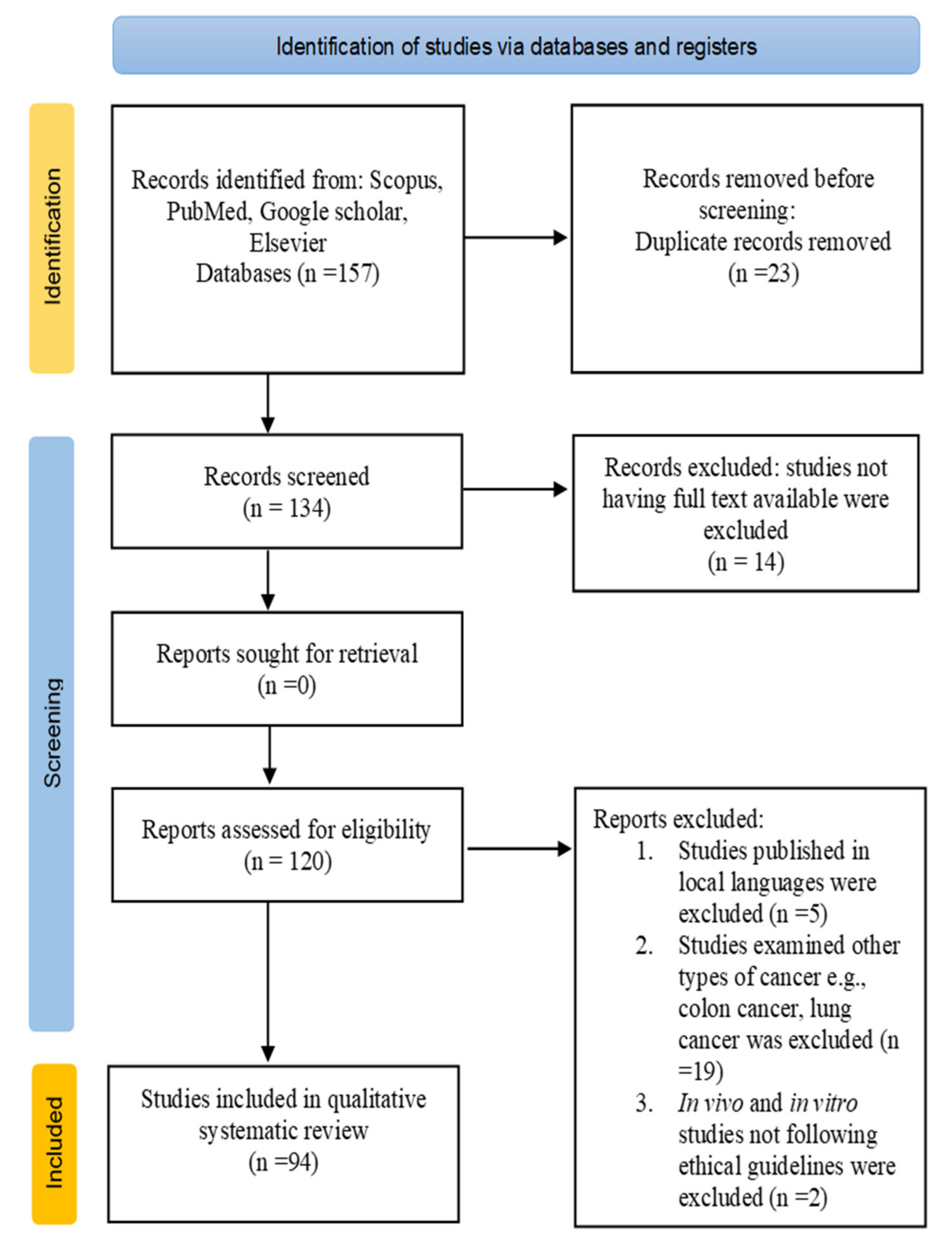
2. Methodology
3. Types of Oral Cancer
3.1. Oral Squamous Cell Carcinoma (OSCC)
3.2. Oral Verrucous Carcinoma (VC)
3.3. Oral Melanoma
3.4. Lymphoma
4. Plants with Beneficial Effects against Oral Cancer
4.1. Ocimum sanctum L.
4.2. Curcuma longa L.
4.3. Vaccinium corymbosum L.
4.4. Vaccinium macrocarpon Aiton
4.5. Momordica charantia L.
4.6. Azadirachta indica A. Juss
4.7. Senegalia Catechu (L. f.) P.J.H. Hurter & Mabb.
4.8. Dracaena cinnabari Balf.f.
4.9. Piper nigrum L.
4.10. Zingiber Officinale Roscoe
5. Phytoextracts/Phytoconstituents for the Treatment of Oral Cancer
5.1. Curcumin
5.2. Nimbolide
5.3. Resveratrol
5.4. Anthocyanin
5.5. Piperine
5.6. Eugenol
6. Safety of Phytoextracts in Oral Cancer Treatment
7. Antioxidants and Anticancer Activity Relationship
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hema, K.N.; Smitha, T.; Sheethal, H.S.; Mirnalini, S.A. Epigenetics in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Yasmine, G.; Elnaaj, A. Global incidence and risk factors of oral cancer. Harefuah 2017, 156, 645–649. [Google Scholar]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef] [Green Version]
- Irani, S. New insights into oral cancer—Risk factors and prevention: A review of literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Guan, X.; Wu, H.; Sun, Z.; Zeng, H. Immunosuppression induced by chronic inflammation and the progression to oral squamous cell carcinoma. Mediat. Inflamm. 2016, 2016, 5715719. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Prakash, S.; Bhatia, R.; Negi, M.; Singh, J.; Bishnoi, M.; Kondepudi, K.K. Generation of structurally diverse pectin oligosaccharides having prebiotic attributes. Food Hydrocoll. 2020, 108, 10. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Saurabh, V.; Sasi, M.; Punia, S.; Potkule, J.; Maheshwari, C.; Changan, S.; Radha, B.B.; Singh, S.; et al. Delineating the inherent functional descriptors and biofunctionalities of pectic polysaccharides. Carbohydr. Polym. 2021, 269, 5988. [Google Scholar] [CrossRef]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition 8319, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S.; et al. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Patil, S.; Mageshwaran, V.; Radha, S.V.; Berwal, M.K.; Mahapatra, A.; Saxena, S.; Ashtaputre, N.; Souza, C.D. Evaluation of detoxified cottonseed protein isolate for application as food supplement. Toxin Rev. 2021. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Dwivedi, J.; Jain, P.K.; Satpathy, S.; Patra, A. Medicinal plants for treatment of cancer: A brief review. Pharmacogn. J. 2016, 8, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.K.; Waske, S. Study of Medicinal Plants used by Local Herbal Healers in South Block of Seoni District (M.P.). Int. J. Theor. Appl. Sci. 2018, 10, 95–99. [Google Scholar]
- Radha, P.S.; Chandel, K.; Pundir, A.; Thakur, M.S.; Chauhan, B.; Simer, K.; Dhiman, N.; Shivani Thakur, Y.S.; Kumar, S. Diversity of ethnomedicinal plants in Churdhar Wildlife Sanctuary of district Sirmour of Himachal Pradesh, India. J. Appl. Pharm. Sci. 2019, 9, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Radha, P.S.; Kumar, V. Phytochemical screening of medicinal plants used by tribal migratory shepherds in Western Himalaya. Ann. Biol. 2019, 35, 11–14. [Google Scholar]
- Radha, S.P.; Pundir, A. Review on Ethnomedicinal Plant: Trillium govanianum Wall. Ex D. Don. Int. J. Theor. Appl. Sci. 2019, 11, 4–9. [Google Scholar]
- Radha, J.S.; Srivastava, S.; Negi, V. Ethnobotanical study of medicinal plants used in shikari devi wildlife sanctuary of Himachal Pradesh, India. Med. Plants 2020, 12, 666–673. [Google Scholar] [CrossRef]
- Radha, R.; Chauhan, P.; Puri, S.; Thakur, M.; Rathour, S.; Sharma, A.K.; Pundir, A. A study of wild medicinal plants used in Nargu Wildlife Sanctuary of district Mandi in Himachal Pradesh, India. J. Appl. Pharm. Sci. 2021, 11, 135–144. [Google Scholar] [CrossRef]
- Radha, K.M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S.; Choudhary, P.; Parameswari, E.; Alhariri, A.; Samota, M.K.; Damale, R.D.; et al. Evaluation of Nutritional, Phytochemical, and Mineral Composition of Selected Medicinal Plants for Therapeutic Uses from Cold Desert of Western Himalaya. Plants 2021, 10, 1429. [Google Scholar] [CrossRef]
- Radha, I.; Janjua, S.; Ali, M.; Thakur, M.; Jamwal, R.; Rathour, S.; Kumar, P.A.; Kumari, N.; Puri, S.; Pundir, A.; et al. Documenting Traditional Knowledge before they are Forgotten: A Study on the Ethnomedicinal uses of Wild Plants by Rural People of Jubbarhatti in District Shimla. Int. J. Theor. Appl. Sci. 2021, 13, 37–51. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Home–The Plant List. 2013. Available online: http://www.theplantlist.org/ (accessed on 22 May 2021).
- Markopoulos, A.K. Current Aspects on Oral Squamous Cell Carcinoma. Open Dent. J. 2012, 6, 126. [Google Scholar] [CrossRef]
- Yakop, F.; Abd Ghafar, S.A.; Yong, Y.K.; Saiful Yazan, L.; Mohamad, H.R.; Lim, V.; Eshak, Z. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borse, V.; Konwar, A.N.; Buragohain, P. Oral cancer diagnosis and perspectives in India. Sens. Int. 2020, 1, 100046. [Google Scholar] [CrossRef]
- Franklyn, J.; Janakiraman, R.; Tirkey, A.; Thankachan, C.; Muthusami, J. Oral verrucous carcinoma: Ten-year experience from a Tertiary Care Hospital in India. Indian J. Med Paediatr. Oncol. 2017, 38, 452. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.D.; Liu, O.S.; Tang, Z.G. Oral verrucous carcinoma: A retrospective clinical study of 29 Chinese patients. Int. J. Clin. Exp. Med. 2017, 10, 5228–5232. [Google Scholar]
- Shergill, A.K.; Solomon, M.C.; Carnelio, S.; Kamath, A.T.; Aramanadka, C.; Shergill, G.S. Verrucous carcinoma of the oral cavity: Current concepts. Int. J. Sci. Study 2015, 3, 114–118. [Google Scholar]
- Padhye, A.; D’Souza, J. Oral malignant melanoma: A silent killer. J. Indian Soc. Periodontol. 2011, 15, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.H.; Li, W.Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of melanoma. Exon Publ. 2017, 3–22. [Google Scholar] [CrossRef]
- Zito, P.M.; Mazzoni, T. Cancer, Melanoma, Oral. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30020648 (accessed on 22 May 2021).
- Guevara-Canales, J.O.; Morales-Vadillo, R.; de Faria, P.E.; Sacsaquispe-Contreras, S.J.; Leite, F.P.; Chaves, M.G. Systematic review of lymphoma in oral cavity and maxillofacial region. Acta Odontol. Latinoam. AOL 2011, 24, 245–250. Available online: https://pubmed.ncbi.nlm.nih.gov/22550817/ (accessed on 22 May 2021).
- Domingues, T.; Silva, B.; Belo, C.; Ferreira, T.; Leite, G.B.; De Menezes Pontes, J.R.; Antunes, H.S. Oral manifestations of lymphoma: A systematic review. Ecancermedicalscience 2016. [Google Scholar] [CrossRef] [Green Version]
- Luke, A.M.; Patnaik, R.; Kuriadom, S.T.; Jaber, M.; Mathew, S. An in vitro study of Ocimum sanctum as a chemotherapeutic agent on oral cancer cell-line. Saudi J. Biol. Sci. 2021, 28, 887–890. [Google Scholar] [CrossRef]
- Shivpuje, P.; Ammanangi, R.; Bhat, K.; Katti, S. Effect of Ocimum sanctum on Oral Cancer Cell Line: An in vitro Study. J. Contemp. Dent. Pract. 2015, 16, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Niyomtham, N.; Yingyongnarongkul, B.E.; Koontongkaew, S. Ethanolic Extract of Ocimum sanctum Leaves Reduced Invasion and Matrix Metalloproteinase Activity of Head and Neck Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2020, 21, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Wang, H. Anticancer activity of Vicenin-2 against 7,12 dimethylbenz[a]anthracene-induced buccal pouch carcinoma in hamsters. J. Biochem. Mol. Toxicol. 2021, 35, e22673. [Google Scholar] [CrossRef]
- Sood, S.; Nagpal, M. Role of curcumin in systemic and oral health: An overview. J. Nat. Sci. Biol. Med. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.M.; Patel, V.; Shyur, L.F.; Lee, W.L. Copper supplementation amplifies the anti-tumor effect of curcumin in oral cancer cells. Phytomedicine 2016, 23, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Sakamoto, T.; Zhengguang, L.; Yasui, H.; Hamada, H.; Kubo, H.; Nakajima, M. Curcumin inhibits epithelial-mesenchymal transition in oral cancer cells via c-Met blockade. Oncol. Lett. 2020, 19, 4177–4182. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Arjmandi, B. Evidence for Anti-Cancer Properties of Blueberries: A Mini-Review. Anti Cancer Agents Med. Chem. 2013, 13, 1142–1148. [Google Scholar] [CrossRef]
- Baba, A.B.; Kowshik, J.; Krishnaraj, J.; Sophia, J.; Dixit, M.; Nagini, S. Blueberry inhibits invasion and angiogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters via suppression of TGF-β and NF-κB signaling pathways. J. Nutr. Biochem. 2016, 35, 37–47. [Google Scholar] [CrossRef]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef]
- Chang, H.P.; Lu, C.C.; Chiang, J.H.; Tsai, F.J.; Juan, Y.N.; Tsao, J.W.; Chiu, H.Y.; Yang, J.S. Pterostilbene modulates the suppression of multidrug resistance protein 1 and triggers autophagic and apoptotic mechanisms in cisplatin-resistant human oral cancer CAR cells via AKT signaling. Int. J. Oncol. 2018, 52, 1504–1514. [Google Scholar] [CrossRef]
- Ankola, A.; Kumar, V.; Thakur, S.; Singhal, R.; Smitha, T.; Sankeshwari, R. Anticancer and antiproliferative efficacy of a standardized extract of Vaccinium macrocarpon on the highly differentiating oral cancer KB cell line athwart the cytotoxicity evaluation of the same on the normal fibroblast L929 cell line. J. Oral Maxillofac. Pathol. 2020, 24, 258. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, K.; Chatelain, K.; Phippen, S.; McCabe, J.; Teeters, C.A.; O’Malley, S. Cranberry and grape seed extracts inhibit the proliferative phenotype of oral squamous cell carcinomas. Evid. Based Complementary Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Sur, S.; Ray, R.B. Bitter Melon (Momordica Charantia), a Nutraceutical Approach for Cancer Prevention and Therapy. Cancers 2020, 12, 2064. [Google Scholar] [CrossRef]
- Sur, S.; Steele, R.; Aurora, R.; Varvares, M.; Schwetye, K.E.; Ray, R.B. Bitter Melon Prevents the Development of 4-NQO–Induced Oral Squamous Cell Carcinoma in an Immunocompetent Mouse Model by Modulating Immune Signaling. Cancer Prev. Res. 2018, 11, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sur, S.; Nakanishi, H.; Flaveny, C.; Ippolito, J.E.; McHowat, J.; Ford, D.A.; Ray, R.B. Inhibition of the key metabolic pathways, glycolysis and lipogenesis, of oral cancer by bitter melon extract. Cell Commun. Signal. 2019, 17, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Prakash, S.; Radha, K.N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; Pradhan, P.C.; et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Tanagala, K.K.K.; Baba, A.B.; Kowshik, J.; Reddy, G.B.; Nagini, S. Gedunin, A Neem Limonoid in Combination with Epalrestat Inhibits Cancer Hallmarks by Attenuating Aldose Reductase-Driven Oncogenic Signaling in SCC131 Oral Cancer Cells. Anti Cancer Agents Med. Chem. 2018, 18, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Popli, D.B.; Sircar, K.; Chowdhry, A. A review of the anticancer activity of Azadirachta indica (Neem) in oral cancer. J. Oral Biol. Craniofac. Res. 2020, 10, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Antioxidant, Anti-inflammatory, and Chemoprotective Properties of Acacia catechu Heartwood Extracts. Phytother. Res. 2015, 29, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, T.; Ezhilarasan, D.; Vijayaragavan, R.; Bhullar, S.K.; Rajendran, R. Acacia catechu ethanolic bark extract induces apoptosis in human oral squamous carcinoma cells. J. Adv. Pharm. Technol. Res. 2017, 8, 143–149. [Google Scholar] [CrossRef]
- Lakshmi, T.; Ezhilarasan, D.; Nagaich, U.; Vijayaragavan, R. Acacia catechu ethanolic seed extract triggers apoptosis of SCC-25 cells. Pharmacogn. Mag. 2017, 13, 405–411. [Google Scholar] [CrossRef]
- Al-Fatimi, M. Ethnobotanical survey of Dracaena cinnabari and investigation of the pharmacognostical properties, antifungal and antioxidant activity of its resin. Plants 2018, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Alabsi, A.M.; Lim, K.L.; Paterson, I.C.; Ali-Saeed, R.; Muharram, B.A. Cell Cycle Arrest and Apoptosis Induction via Modulation of Mitochondrial Integrity by Bcl-2 Family Members and Caspase Dependence in Dracaena cinnabari—Treated H400 Human Oral Squamous Cell Carcinoma. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Al-Afifi, N.; Alabsi, A.; Kaid, F.; Bakri, M.; Ramanathan, A. Prevention of oral carcinogenesis in rats by Dracaena cinnabari resin extracts. Clin. Oral Investig. 2018, 23, 2287–2301. [Google Scholar] [CrossRef]
- Al-Afifi, N.A.; Alabsi, A.M.; Shaghayegh, G.; Ramanathan, A.; Ali, R.; Alkoshab, M.; Bakri, M.M. The in vitro and in vivo antitumor effects of Dracaena cinnabari resin extract on oral cancer. Arch. Oral Biol. 2019, 104, 77–89. [Google Scholar] [CrossRef]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahamad, M.S.; Jafri, A.; Afzal, M.; Arshad, M. Piperine Triggers Apoptosis of Human Oral Squamous Carcinoma Through Cell Cycle Arrest and Mitochondrial Oxidative Stress. Nutr. Cancer 2017, 69, 791–799. [Google Scholar] [CrossRef]
- Macedo, A.L.; da Silva, D.P.D.; Moreira, D.L.; de Queiroz, L.N.; Vasconcelos, T.R.A.; Araujo, G.F.; Kaplan, M.A.C.; Pereira, S.S.C.; de Almeida, E.C.P.; Valverde, A.L.; et al. Cytotoxicity and selectiveness of Brazilian Piper species towards oral carcinoma cells. Biomed. Pharmacother. 2019, 110, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainal, N.S.; Gan, C.P.; Lau, B.F.; Yee, P.S.; Tiong, K.H.; Abdul Rahman, Z.A.; Patel, V.; Cheong, S.C. Zerumbone targets the CXCR4-RhoA and PI3K-mTOR signaling axis to reduce motility and proliferation of oral cancer cells. Phytomedicine 2018, 39, 33–41. [Google Scholar] [CrossRef]
- Annamalai, G.; Suresh, K. [6]-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis. Biomed. Pharmacother. 2018, 98, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, J.; Wang, F. [6]-Gingerol impedes 7,12-dimethylbenz(a)anthracene-induced inflammation and cell proliferation-associated hamster buccal pouch carcinogenesis through modulating Nrf2 signaling events. J. Biochem. Mol. Toxicol. 2021, 35, e22689. [Google Scholar] [CrossRef]
- Alok, A.; Singh, I.; Singh, S.; Jha, A. Curcumin: Pharmacological actions and its role in head and neck squamous cell carcinoma—A review. J. Indian Acad. Oral Med. Radiol. 2017, 29, 115. [Google Scholar] [CrossRef]
- Ardito, F.; Perrone, D.; Giuliani, M.; Testa, N.F.; Muzio, L.L. Effects of Curcumin on Squamous Cell Carcinoma of Tongue: An In Vitro Study. Curr. Top. Med. Chem. 2018, 18, 233–243. [Google Scholar] [CrossRef]
- Maulina, T.; Hadikrishna, I.; Hardianto, A.; Sjamsudin, E.; Pontjo, B.; Yusuf, H.Y. The therapeutic activity of curcumin through its anti-cancer potential on oral squamous cell carcinoma: A study on Sprague Dawley rat. SAGE Open Med. 2019, 7, 205031211987598. [Google Scholar] [CrossRef]
- Kowshik, J.; Mishra, R.; Sophia, J.; Rautray, S.; Anbarasu, K.; Reddy, G.D.; Dixit, M.; Mahalingam, S.; Nagini, S. Nimbolide upregulates RECK by targeting miR-21 and HIF-1α in cell lines and in a hamster oral carcinogenesis model. Sci. Rep. 2017, 7, 2045. [Google Scholar] [CrossRef] [Green Version]
- Sophia, J.; Kowshik, J.; Dwivedi, A.; Bhutia, S.K.; Manavathi, B.; Mishra, R.; Nagini, S. Nimbolide, a neem limonoid inhibits cytoprotective autophagy to activate apoptosis via modulation of the PI3K/Akt/ GSK-3β signalling pathway in oral cancer. Cell Death Dis. 2018, 9, 1087. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.; Gonzales, C.B.; De La Chapa, J.J.; Cabang, A.B.; Fountzilas, C.; Patel, M.; Orozco, S.; Wargovich, M.J. The Highly Pure Neem Leaf Extract, SCNE, Inhibits Tumorigenesis in Oral Squamous Cell Carcinoma via Disruption of Pro-tumor Inflammatory Cytokines and Cell Signaling. Front. Oncol. 2019, 9, 890. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.; Yang, G.; Xiang, W.; Pei-Jun, W.; Bin, Z. Effects of resveratrol on oral squamous cell carcinoma (OSCC) cells in vitro. J. Cancer Res. Clin. Oncol. 2014, 140, 371–374. [Google Scholar] [CrossRef]
- Yu, X.D.; Yang, J.L.; Zhang, W.L.; Liu, D.X. Resveratrol inhibits oral squamous cell carcinoma through induction of apoptosis and G2/M phase cell cycle arrest. Tumor Biol. 2016, 37, 2871–2877. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, K.H.; Lee, H.Y. Effect of Resveratrol on Oral Cancer Cell Invasion Induced by Lysophosphatidic Acid. J. Dent. Hyg. Sci. 2018, 18, 188–193. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, K.H.; Jeong, B.Y.; Park, C.G.; Lee, H.Y. Experimental & Molecular Medicine Zeb1 for RCP-induced oral cancer cell invasion and its suppression by resveratrol. Exp. Mol. Med. 2020, 52, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.M.; Frond, A.D.; Ştirbu, I.; Rugina, D.; Socaciu, C. Anthocyanins-Smart Molecules for Cancer Prevention. In Phytochemicals—Source of Antioxidants and Role in Disease Prevention; InTech: London, UK, 2018. [Google Scholar]
- Madanakumar, A.J.; Lawarence, B.; Manoj, G.S.; Kumaraswamy, M. Purified Anthocyanin from in vitro Culture of Bridelia retusa (L.) Spreng. Capable of Inhibiting the Growth of Human Oral Squamous Cell Carcinoma Cells. Pharmacogn. J. 2018, 10, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Yue, E.; Tuguzbaeva, G.; Chen, X.; Qin, Y.; Li, A.; Sun, X.; Dong, C.; Liu, Y.; Yu, Y.; Zahra, S.M.; et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Setzer, W.N.; Jafari, S. Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies. Curr. Med. Chem. 2017, 25, 4918–4928. [Google Scholar] [CrossRef]
- Manoharan, S.; Balakrishnan, S.; Menon, V.P.; Alias, L.M.; Reena, A.R. Chemopreventive & efficacy of curcumin and piperine during 7,12-dimethylbenz [a]anthracene-induced hamster buccal pouch carcinogenesis. Singap. Med J. 2009, 50, 139–146. Available online: https://europepmc.org/article/med/19296028 (accessed on 22 May 2021).
- Grinevicius, V.M.; Andrade, K.S.; Mota, N.S.; Bretanha, L.C.; Felipe, K.B.; Ferreira, S.R.; Pedrosa, R.C. CDK2 and Bcl-xL inhibitory mechanisms by docking simulations and anti-tumor activity from piperine enriched supercritical extract. Food Chem. Toxicol. 2019, 132, 110644. [Google Scholar] [CrossRef]
- Rekha, U.V.; Anita, M.; Jayamathi, G.; Sadhana, K.; Deepa, S.; Hussain, S.; Bhuvaneswari, J.; Ramya, V.; Selvaraj, J.; Naveenraj, N.S. Molecular docking analysis of piperine with CDK2, CDK4, Cyclin D and Cyclin T proteins. Bioinformation 2020, 16, 359–362. [Google Scholar] [CrossRef]
- Koh, T.; Murakami, Y.; Tanaka, S.; Machino, M.; Onuma, H.; Kaneko, M.; Sugimoto, M.; Soga, T.; Tomita, M.; Sakagami, H. Changes of Metabolic Profiles in an Oral Squamous Cell Carcinoma Cell Line Induced by Eugenol. In Vivo 2013, 27, 233–243. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23422484 (accessed on 22 May 2021). [PubMed]
- Dhawan, N.; Kumar, K.; Kalia, A.N.; Arora, S. N-Succinyl Chitosan as Buccal Penetration Enhancer for Delivery of Herbal Agents in Treatment of Oral Mucositis. Curr. Drug Deliv. 2014, 11, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.; Narasimhan, M.; Balaji, T.M.; Chamundeeswari, D.P.; Sakthisekaran, D. In Vitro Anticancer Effects of Cinnamomum verum J. Presl, Cinnamaldehyde, 4 Hydroxycinnamic Acid and Eugenol on an Oral Squamous Cell Carcinoma Cell Line. J. Contemp. Dent. Pract. 2020, 21, 1027–1033. [Google Scholar] [CrossRef]
- Dos Santos Filho, E.X.; Arantes, D.A.C.; Oton Leite, A.F.; Batista, A.C.; Mendonça, E.F.; de Marreto, R.N.; Naves, L.N.; Lima, E.M.; Valadares, M.C. Randomized clinical trial of a mucoadhesive formulation containing curcuminoids (Zingiberaceae) and Bidens pilosa Linn (Asteraceae) extract (FITOPROT) for prevention and treatment of oral mucositis—Phase I study. Chem. Biol. Interact. 2018, 291, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Bera, A.; Yoon, A.J.; Morselli, M.; Jeong, C.; Tosevska, A.; Dong, T.S.; Eklund, M.; Russ, E.; Nasser, H. A randomized, phase 1, placebo-controlled trial of APG-157 in oral cancer demonstrates systemic absorption and an inhibitory effect on cytokines and tumor-associated microbes. Cancer 2020, 126, 1668–1682. [Google Scholar] [CrossRef]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary phytochemicals with antioxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects—Involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]



| Botanical Name | Study Type (in vitro/in vivo/ Clinical Trial) and Extract | Objective | Observation | Reference | |
|---|---|---|---|---|---|
| Ocimum sanctum L. (Holy Basil) | Study—In vitro on KB mouth cell line Extract—Leaves aqueous extract | To analyse dose dependent cytotoxic activity of O. sanctum aqueous extract of leaves on oral cancer cell line | O. sanctum treatment show cytotoxic effect against oral cancer KB mouth cell with IC50 value of 10 µg/mL (aqueous extract of light leaves) and 20 µg/mL (aqueous extract of dark leaves) in 48 h MTT assay. | [36] | |
| Study—In vitro on HNSCC cell lines, HN4, HN30, HN12, HN31 Extract—Leaf ethanolic extract | To study the cytotoxic and anti-invasive effect of O. sanctum leaf ethanolic extract on HNSCC cell lines. | O. sanctum ethanolic extract (0.8 mg/mL) treatment shows decrease in cell viability of HNSCC cell lines HN30 (40%), HN31 (53%), HN4 (52%), and HN12 (40%). | [37] | ||
| O. sanctum ethanolic extract with 0.4 mg/mL conc. inhibited MMP-2 activity in HN12, HN4 cells by 71% and 65% and MMP-9 activity in HN12, HN4 cells by 85% and 44%. | |||||
| The invasion activity of HN12 and HN4 cells is inhibited by 30%. | |||||
| Study—In vivo on DMBA induced OSCC hamsters Extract—Vicenin | To study anticancer effect of Vicenin-2 on DMBA-induced oral carcinogenesis in hamsters. | Vicenin-2 treatment (30 mg/kg) with DMBA-induced OSCC hamster improved antioxidant level, inhibited lipid peroxidation, and stopped tumor incidence. | [38] | ||
| DMBA-induced hamsters with treatment of vicenin-2, halts the production of proinflammatory cytokines (TNF-α, IL-1β, IL-6) and inhibited immunohistochemical expression of cyclin-D1, Bcl-2, PCNA | |||||
| Study—In vitro on OSCC cell line Ca9-22 Extract—Leaves (dry and aqueous) | To study the effect of dry leaves and aqueous extract of O. sanctum on OSCC cell line. | In 24 h MTT assay O. sanctum aqueous extract treatment, result shows HPC value 30 mg/L and MIC value of 5 mg/L and for dry extract HPC value is 35 mg/L and MIC value is 5mg/L. | [35] | ||
| Curcuma longa L. (Turmeric) | Study—In vitro on OSCC cell lines, H314, ORL-115. Extract—Curcumin | To access the correlation between intracellular copper levels and response to curcumin treatment in OSCC cell lines obtained from oral cancer patients. | Copper (250 µM) supplementation shows decrease in curcumin concentrations 25 μM to 5.3 μM at 48 h and 50 μM to 40.3 μM at 24 h, which inhibited 50% OSCC cell viability (IC50) 24 h MTT assay. | [41] | |
| Increase of copper level in OSCC cells treated with curcumin shows increase in Nrf2 level and significant induction of intracellular ROS. Combined treatment of curcumin with copper early apoptosis is observed. | |||||
| Study—In vitro on OSCC cell lines, Ca9-22 and HSC-4. Extract—Curcumin | To study the effects of curcumin on HGF-induced EMT in OSCC cell lines. | Curcumin treatment in OSCC cells inhibited cell motility, HGF-induced EMT via c-Met blockade and reduced the expression of phosphorylated c-Met/ERK pathway which inhibit HGF-induced increase in vimentin level. | [42] | ||
| Vaccinium corymbosum L. (Blueberry) | Study—In vitro and in vivo on OSCC cell line SCC131 and DMBA painted hamster Extract—Malvidin, Blueberry powder | To access the potential of malvidin and blueberry to target STAT-3 (oncogenic transcription factor) | Blueberry and malvidin suppress STAT-3 phosphorylation in SCC131 oral cancer cell line and induced mitochondrial-mediated apoptosis and G1/S phase cell cycle arrest. | [45] | |
| Blueberry treatment to DMBA painted hamster with 200 mg/kg concentration, increased tumor growth delay to 68.22%, reduced the multiplicity (0.87±0.22) | |||||
| Study—In vitro on CAR cell line CAL 27 Extract—Pterostilbene | To analyse anticancer effects of pterostilbene on cisplatin-resistant human oral cancer (CAR) cells. | Pterostilbene with 50, 75 µM concentration (24 h) treatment in CAR cells reduced the expression of MDR1, mRNA and phosphorylation of AKT on Ser473 site. | [46] | ||
| Vaccinium macrocarpon Aiton (Cranberry) | Study—In vitro on OSCC cell lines, SCC25, CAL27 Extract—Cranberry | To study anti-proliferative effect of cranberry against OSCC cell lines. | Cranberry extract treatment with CAL27 cells shows upregulation in key regulator of apoptosis, caspase-8 (+181%) and caspase-2 (+327%) and expression of c-myc (+29%), ODC (+371%), p53 (+44%). | [48] | |
| GImax value of cranberry extract 70 µg/mL reduced SCC25 proliferation by 36.3% in comparison to baseline treatment control. | |||||
| Study—In vitro on KB oral cancer cell line Extract—Fruit (hydroalcoholic) | To examine the anticancer effect of V. macrocarpon hydroalcoholic fruit extract against KB oral cancer cell line is examined. | V. macrocarpon extract kills 50% oral cancer cells with IC50 value of 3.564 µg/mL (24 h incubation, MTT cytotoxicity assay). | [47] | ||
| Momordica charantia L. (Bitter melon) | Study—In vivo on 4-NQO–induced cancer model Extract—Bitter melon extract | To access chemo preventive effect of bitter melon extract (BME) in HNSCC induced by 4-nitroquinoline 1-oxide (4-NQO) carcinogen | BME treatment (600 mg/mouse) suppress the expression of immune check point gene PDCD1/PD1, proinflammatory genes IL1b, IL23a, s100a9 and downregulate MMP9 pathway. | [50] | |
| Study—In vitro on human oral cancer cell line Cal27 and JHU022 Extract - Bitter melon extract | To analyse effect of Bitter melon extract on lipid metabolism and glycolysis pathways in human oral cancer cells. | BME treatment on oral cancer cell lines shows downregulation in protein and mRNA expression levels of PFKB, PKM, PDK3, SLC2A1/GLUT-1 and LDHA, induced mitochondrial ROS generation and CHOP expression associated with endoplasmic reticulum (ER)-stress, facilitated cell death via apoptosis in oral cancer. | [51] | ||
| Azadirachta indica A. Juss. (Neem tree) | Study—In vitro on oral cancer cell line SCC131 Extract—Gedunin | To study the potential of gedunin alone or with epalrestat to prevent hallmarks of cancer by inhibiting downstream PI3K/Akt/mTOR/ERK/NF-κB signalling axis and ARase in oral cancer cells. | Gedunin and epalrestat treatment shows, downregulation of proangiogenic and pro-invasive proteins in SCC131 cells and inhibited ROS generation, ARase expression. G1/S phase cell cycle arrest is associated with autophagy cell death following apoptosis | [53] | |
| Senegalia catechu (L.f.) P.J.H. Hurter & Mabb. (Black cutch) | Study—In vitro on HSCC cell line (SCC-25) Extract—Bark ethanolic extract | To analyse cytotoxic activity of ethanolic extract of S. catechu bark against HSCC cells. | Ethanolic extract of S. catechu bark shows cytotoxic activity in MTT assay (24 h) against HSCC cell line (SCC-25) with IC50 value of 52.09 µg/mL and 25 µg/mL concentration shows cell cycle arrest with 25% cell accumulation at S phase. | [56] | |
| Study—In vitro on HSCC cell line (SCC-25) Extract—Seed ethanolic extract | To analyse cytotoxic activity of ethanolic extract of S. catechu seed against HSCC cells. | Ethanolic seed extract treatment with SCC-25 cell line caused cytotoxicity (24 h, MTT assay) with IC50 value of 100 µg/mL and shows downregulation in Bcl-2 gene expression and upregulation in apoptotic gene marker expressions including cytochrome c, Bax, caspase 8 and 9. | [57] | ||
| Dracaena cinnabari Balf.f.(Dragon blood tree) | Study—In vitro on OSCC cell line -H400 Extract—Resin methanolic extract | To evaluate the apoptosis-inducing and cytotoxic effects of D. cinnabari on OSCC cells. | D. cinnabari treatment shows cytotoxic effect (MTT assay, 72 h) against OSCC (H400) cell line with IC50 value of 5.9 µg/mL, increase in caspase 8, caspase 9, caspase 3/7 activity, depolarization of mitochondrial membrane potential (MMP) and cell cycle arrest at S phase. | [59] | |
| Study—In vivo on 4-NQO–induced cancer model Extract—Resin methanolic extract | To study the chemo preventive efficacy of D. cinnabari on a 4NQO-induced oral cancer animal model. | D. cinnabari extract treatment with 1000 mg/kg inhibits expression of Ki-67, Bcl-2, p53 and cyclin D1 proteins and induced apoptosis by downregulation of Bcl-2, Cox-2, Tp53, upregulation of Casp3 and Bax genes. | [60] | ||
| Study—In vivo on 4NQO-induced tongue carcinogenesis and in vitro on tongue squamous cell carcinoma cell line (H103) Extract—Resin methanolic extract | To study the chemo preventive activity of D. cinnabari against 4NQO-induced tongue carcinogenesis in rat and apoptosis induction of D. cinnabari on tongue squamous cell carcinoma cell line. | D. cinnabari methanolic extract treated rats shows incidence of SCC 100 mg/kg (57.1%), 500 mg/kg (28.6%) and 1000 mg/kg (14.3%) as compared to 4NQO induced cancer rats 85.7%. | [61] | ||
| Cytotoxic effect (72 h, MTT assay) on H103 cells with IC50 value of 5.5 µg/mL in time and dose dependent manner, apoptosis induction is through intrinsic (mitochondrial) pathway and G2/M and S phase cell cycle arrest is observed. | |||||
| Piper nigrum L. (Black pepper) | Study—In vitro on OSCC cell line: SCC9, SCC25, SCC4 Extract- Leaves | To analyze cytotoxicity of four Piper species extract, Piper truncatum (PT), Piper arboretum (PA), Piper cernnum (PC), Piper mollicomum (PM). | In MTT assay, 48 h treatment cytotoxicity against OSCC cells with IC50 value of PC (-L-D) 47.2 µg/mL, PM (-L-D 94.2 µg/mL, PCa (-L-D) 47.5 µg/mL is observed. | [64] | |
| Zingiber officinale Roscoe (Ginger) | Study—In vitro on OSCC cell line ORL-48 and ORL-115 Extract—Zerumbone | To examine OSCC cells were sensitive to zerumbone treatment and find the molecular pathways involved in the mechanism of action. | Zerumbone treatment on normal keratinocyte cells and OSCC cells shows cytotoxicity (MTT assay, 72 h) with IC50 value of 25 µM and 5 µM, inhibit activation of PI3K-mTOR and CXCR4-RhoA signalling pathway and induced apoptosis in OSCC cells, and shows cell cycle arrest at G2/M phase. | [66] | |
| Study—In vivo on DMBA induced hamster buccal pouch carcinogenesis Extract - Shogaol | To study the effect of (6)-Shogaol on cell proliferation and inflammation by inhibiting the translocation of AP-1 and NF-κB in DMBA induced HBP carcinogenesis | (6)-Shogaol treatment in DMBA induced hamsters shows degradation in IκB-α, aberrant activation of AP-1, IKKβ, c-jun, c-fos and NF-κB and upregulation of cell proliferative markers (PCNA, Ki-67 and Cyclin-D1), inflammatory markers (interleukin-1 and -6, COX-2, TNF-α, iNOS. | [67] | ||
| Study—In vivo on DMBA induced hamster buccal pouch carcinogenesis Extract—Gingerol | To examine the chemo preventive effect of (6)-gingerol on DMBA induced hamster buccal pouch carcinogenesis models. | Oral supplementation of (6)-gingerol 20 mg/kg shows reduction in tumor incidence, tumor volume and tumor burden as compared to DMBA treatment tumor burden (1346.84 ± 81.19), tumor volume (429.19 ± 28.29). | [68] | ||
| (6)-Gingerol treatment prevent HBP carcinogenesis, by enhanced nuclear factor erythroid-2- related factor-2 (Nrf2) expression as DMBA induced hamster shows depletion of Nrf2 signalling. | |||||
| Phyto-Extract | Source | Study Type (in vitro/in vivo/ Clinical Trial) | Objective | Observation | Reference |
|---|---|---|---|---|---|
| Curcumin | Curcuma longa L. | Study—In vitro on HNSCC cells | To study therapeutic activity of curcumin against HNSCC cells. | Curcumin shows stimulatory effect on extrinsic apoptotic pathway activated by binding of Fas ligand and TNF-α to their conforming cell surface receptors. It induces apoptosis in cancer cells at the G2 cell cycle phase by upregulation of p53 gene expression. | [69] |
| Study—In vitro study on TSCC cell line | To study anticancer potential of curcumin against TSCC cells. | Curcumin with IC50 value of 10 µM (MTT assay, 24 h) reduces the progression and migration of TSCC cells, inhibits tumorigenesis, and promotes apoptosis. | [70] | ||
| Study—In vivo on DMBA-induced OSCC in Sprague Dawley rats | To evaluate the anti-cancer potential of curcumin on OSCC based on the expression of cyclooxygenase 2 and nuclear factor kappa B during epithelial dysplasia stage. | Curcumin treatment found to be effective in suppressing cyclooxygenase 2 (p = 0.03) and nuclear factor kappa B (NFKB) (p < 0.01). | [71] | ||
| Nimbolide | Azadirachta indica | Study—In vitro study on OSCC cell line SCC4 and in vivo on DMBA-induced HBP carcinogenesis. | To study the chemotherapeutic effect of nimbolide based on modulation of the expression of key molecules involved in angiogenesis, invasion, and the upregulation of RECK. | Nimbolide treatment upregulates—RECK by reducing miR-21 and HIF-1α expression leads to downregulation of MMP activity, blockade of notch signalling and VEGF. Nimbolide significantly inhibits DMBA-induced HBP carcinomas. | [72] |
| Study—In vitro study on OSCC cell lines SCC131 and SCC4. | To analyze the effect of nimbolide on autophagy and the time point at which the phosphorylation status of PI3K, GSK-3β determine the choice between apoptosis and autophagy in oral cancer cells. | Nimbolide treatment activates apoptosis by inhibiting shielding effects of cytoprotective autophagy through modulation in the phosphorylation status of GSK-3β and Akt in oral cancer cells SCC131 and SCC4, as well as in miR-126, ncRNAs, and HOTAIR. | [73] | ||
| Super critical CO2 Neem leaf extract (SCNE) | Study—In vitro study on OSCC cell line SCC4, Cal27, and HSC3. | To study anticancer effects of SCNE and nimbolide against OSCC cell lines while inflammation, migration and proliferation were analyzed over time. | Nimbolide treatment caused disruption of cell migration, cell signaling and efficiently reduced pro-cancer inflammatory cytokines. Use of nimbolide and SCNE decreased NFkBp65, COX2 expression, and downregulated pAKT, pERK1/2, and pSTAT3 in SCC4 cells. | [74] | |
| Resveratrol | Vaccinium corymbosum | Study—In vitro on OSCC cell lines, SCC-VII, SCC-25, and YD-38. | To examine the chemo preventive effect of resveratrol against oral squamous cancer cell lines, | Resveratrol shows an inhibitory effect on the growth of OSCC oral cancer cells by enhanced expression of cyclin A2, cyclin B1, and phosphor-cdc2 (Tyr 15) and the induction of G2/M phase cell cycle arrest and apoptosis. | [76] |
| IC50 value of resveratrol (48-h treatment, MTT assay) against SCC-25, YD-38 and SCC-VII cell lines were found to be 0.7, 1.0, and 0.5 μg/mL. | |||||
| Study—In vitro on OSCC cell line YD-10B. | To analyze the potential therapeutic efficiency of resveratrol in oral cancer patients. | Resveratrol treatment decrease metastasis and invasion of OSCC cells, as it proficiently inhibited LPA- induced oral cancer cell invasion and EMT by downregulating TWIST1 and SLUG expression. | [77] | ||
| Study—In vitro on OSCC cell line YD-9, YD-38 and YD-10B. | To study chemo preventive effect of resveratrol on RCP-induced OSCC. | Resveratrol treatment inhibited the RCP—induced OSCC invasion by Zeb1 and β1 integrin expression downregulation. | [78]] | ||
| Anthocyanin | Bridelia retusa | Study—In vitro on OSCC cell line SCC4, SCC9 and SCC25 | To study anti-metastatic potential of anthocyanin against oral squamous carcinoma cells. | Cell cycle arrest of SCC25 cells in S-G2/M and G0/G1 stages with up-regulation of the sub-G1 fraction is observed, indicate cell death by apoptosis. Anthocyanin treatment in SCC25 cells (MTS assay, 42 h), reported caspase-3 expression level between 1.5—3 folds (IC50—134%, IC80—267.5%) in comparison with control cells (89.6%). | [80] |
| Vaccinium corymbosum | Study—In vitro on OSCC cell lines SCC15, HaCaT and Tca8113 | To examine the potential inhibitory effects of anthocyanin on OSCC and find effective targets for therapy. | Anthocyanin shows, reduction in viability of OSCC cells with 250 µg/mL of concentration (CCK8 assay, 48 h). Anthocyanin treatment in OSCC cells shows a significant increase in protein expression of caspase-1, IL-1β, NLRP3. | [81] | |
| Piperine | Piper longum | Study- In vivo on DMBA induced HBP carcinogenesis | To study the chemo-preventive efficacy piperine against (DMBA)-induced hamster buccal pouch carcinogenesis. | Piperine treatment on DMBA induced HBP carcinoma, prevents OSCC formation in DMBA—painted hamsters with a dose of 50 mg/kg body weight, given alternate days to DMBA painting for 14 weeks completely. Piperine restored the status of detoxifying agents, antioxidants, and lipid peroxidation in DMBA- painted hamsters | [83] |
| Study—In vitro on OSCC KB cell line | To study the anti-cancer potential of piperine on human OSCC cells. | Piperine induce apoptosis via the reduction in MMP and ROS liberation following caspase-3 activation and cell cycle arrest. Cell cycle study showed that piperine reduced the DNA content and arrest cells in the G2/M phase. | [63] | ||
| Study- In silico on CDK-2 and Bcl-xL | To analyze the anti-tumor activity of piperine rich extract by SFE from black pepper. | In silico docking simulations reported, inside the ATP binding site in CDK2 there is hydrogen bonding between residue Ser5 and protein. | [84] | ||
| Eugenol | Study—In vitro on human OSCC cell line HSC-2 | To study the effect of eugenol treatment on the metabolic profiles of a human OSCC cell line. | Eugenol treatment in OSCC cells, induce oxidative stress by increase in oxidized form of glutathione (69%) and methionine sulfoxide/methione ratio (37%), increase in glycolytic metabolites and polyamines, decline of ATP utilization by 53% decrease in ADP/ATP ratio and 70% decrease in AMP/ATP ratio. | [86] | |
| Study—In vitro and Ex vivo on rat buccal mucosal tissue. | To study N-succinyl chitosan gel delivery system of micro emulsified honey, sodium hyaluronate and eugenol for synergistic effects on various pathological factors of oral mucositis. | N-succinyl chitosan orogel shows inflammation reduction in oral mucosa of animals (p < 0.05) compared to disease control. Eugenol release from N-succinyl chitosan were 87.45±0.14% in PBS buffer (pH-6.4) after 8 h. | [87] | ||
| Cinnamomum verum | Study—In vitro on OSCC cell line SCC25. | To study the anticancer effects of eugenol against OSCC cells. | Eugenol treatment on OSCC cells using MTT cytotoxic assay (72 h) shows IC50 value of 24.71 µM with concentration ranging between 1.9 µg/mL and 1000 µg/mL. In cell cycle analysis, 25 µM eugenol treatment with SSC 25 cell line shows subsequent increase in sub-G0 population and cell cycle arrest at S Phase. | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, S.; Radha; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. https://doi.org/10.3390/antiox10091358
Prakash S, Radha, Kumar M, Kumari N, Thakur M, Rathour S, Pundir A, Sharma AK, Bangar SP, Dhumal S, et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants. 2021; 10(9):1358. https://doi.org/10.3390/antiox10091358
Chicago/Turabian StylePrakash, Suraj, Radha, Manoj Kumar, Neeraj Kumari, Mamta Thakur, Sonia Rathour, Ashok Pundir, Abhishek Kumar Sharma, Sneh Punia Bangar, Sangram Dhumal, and et al. 2021. "Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer" Antioxidants 10, no. 9: 1358. https://doi.org/10.3390/antiox10091358
APA StylePrakash, S., Radha, Kumar, M., Kumari, N., Thakur, M., Rathour, S., Pundir, A., Sharma, A. K., Bangar, S. P., Dhumal, S., Singh, S., Thiyagarajan, A., Sharma, A., Sharma, M., Changan, S., Sasi, M., Senapathy, M., Pradhan, P. C., Garg, N. K., ... Mekhemar, M. (2021). Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants, 10(9), 1358. https://doi.org/10.3390/antiox10091358














