Anti-Diabetic and Antioxidant Activities of Red Wine Concentrate Enriched with Polyphenol Compounds under Experimental Diabetes in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Wine Material Used in Research
2.3. Preparation of Concentrate of Red Wine Enriched with Natural Polyphenolic Compounds
2.4. Identification and Quantification of Compounds of PC Concentrate by the UPLC-qTOF-MS/MS and HPLC-PDA Methods
2.5. DPPH Radical Scavenging Assay
2.6. Liposome Oxidation Assay
2.7. Animals
2.8. Induction of Diabetes Mellitus Type 1
2.9. Determination of Glucose Concentration
2.10. The Glucose Tolerance Test
2.11. Blood Collection
2.12. Determination of the Number of Erythrocytes
2.13. Determination of Total Hemoglobin Concentration
2.14. Isolation of Erythrocytes
2.15. Obtaining of Erythrocytes Lysate
2.16. Determination of Glycated Hemoglobin Content
2.17. Determination of Protein Concentration by Lowry Method
2.18. Methods of Studyingthe Activity of Antioxidant Enzymes
2.18.1. Determination of Superoxide Dismutase Activity
2.18.2. Determination of Catalase Activity
2.18.3. Determination of Glutathione Peroxidase Activity
2.19. Determination of the Content of the Products of Oxidative Modification of Proteins
2.20. Determination of the Content of Lipids Peroxidation Products
2.21. Statistical Analysis of Results
3. Results
3.1. Qualitative and Quantitative Analysis of Red Wine Polyphenol Complex Concentrate
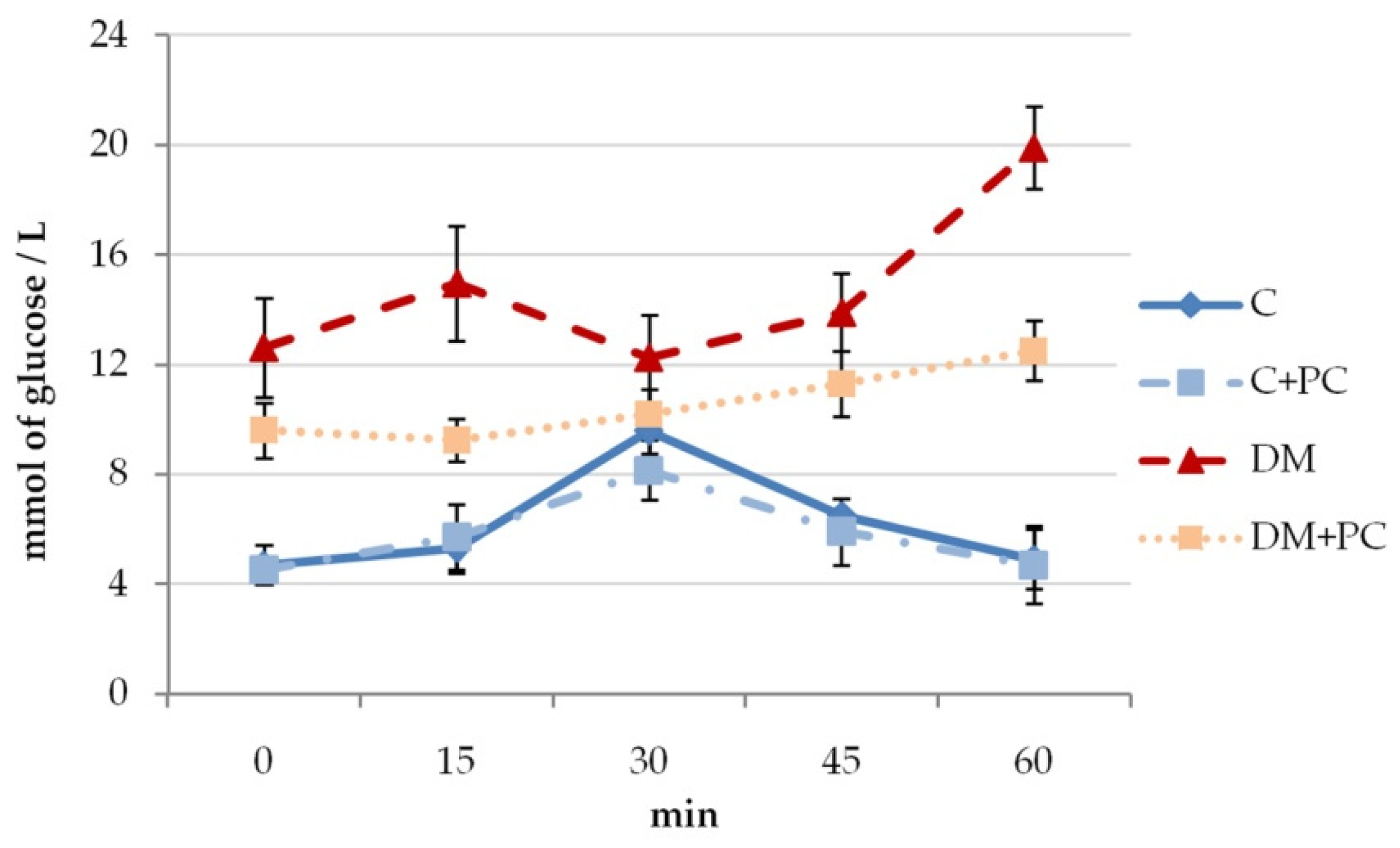
3.2. The Effect of Red Wine Polyphenol Complex Concentrate on Glycemia under Diabetes Mellitus
3.3. Influence of Red Wine Polyphenol Complex Concentrate on Hematological Indices of Rats with Diabetes Mellitus
3.4. Antioxidant Effect of Red Wine Polyphenol Complex Concentrate In Vitro and In Vivo
3.4.1. In Vitro Studies on the Radical Scavenging Activity of PC Concentrate
3.4.2. Liposome Oxidation Assay
3.4.3. In Vivo Studies of PC Concentrate Antioxidant Effect
3.4.4. The Activity of Antioxidant Enzymes of Plasma of Rats with Diabetes Mellitus under Red Wine Polyphenol Complex Concentrate Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramalakshmi, K.; Kubra, I.R.; Rao, L.J.M. Antioxidant potential of low-grade coffee beans. Int. Food Res. J. 2008, 41, 96–103. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Giovinazzo, G.; Carluccio, M.A.; Grieco, F. Wine Polyphenols and Health. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 1135–1155. [Google Scholar]
- Brouillard, R.; George, F.; Fougerousse, A. Polyphenols produced during red wine ageing. Biofactors 1997, 6, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Mateus, N.; Proença, S.; Ribeiro, P.; Machado, J.M.; Freitas, V.D. Grape and wine polyphenolic composition of red Vitis vinifera varieties concerning vineyard altitude. Cienc. Y Tecnol. Aliment. 2001, 3, 102–110. [Google Scholar] [CrossRef]
- Mazza, G.J. Anthocyanins and heart health. Ann. Ist. Super. Sanita 2007, 43, 369–374. [Google Scholar]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Moradi, S.; Jafarian Kerman, S.R.; Rohani, F.; Salari, F. Association between diabetes complications and leukocyte counts in Iranian patients. J. Inflamm. Res. 2012, 5, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Chang, S.-C.; Yang, W.-C.V. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit. Rev. Oncol. Hematol. 2016, 108, 146–153. [Google Scholar] [CrossRef]
- Navarro, J.; Mora, C. Role of inflammation in diabetic complications. Nephrol. Dial. Transplant. 2006, 20, 2601–2604. [Google Scholar] [CrossRef] [Green Version]
- Bento, C.F.; Pereira, P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia 2011, 54, 1946–1956. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Delmastro, M.M.; Piganelli, J.D. Oxidative stress and redox modulation potential in type 1 diabetes. Clin. Dev. Immunol. 2011, 2011, 593863. [Google Scholar] [CrossRef]
- Yan, L. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Anim. Models Exp. Med. 2018, 1, 7–13. [Google Scholar] [CrossRef]
- Al-Awwadi, N.; Azay, J.; Poucheret, P.; Cassanas, G.; Krosniak, M.; Auger, C.; Gasc, F.; Rouanet, J.M.; Cros, G.; Teissèdre, P.L. Antidiabetic activity of red wine polyphenolic extract, ethanol, or both in streptozotocin-treated rats. J. Agric. Food Chem. 2004, 52, 1008–1016. [Google Scholar] [CrossRef]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Role of red grape polyphenols as antidiabetic agents. Integr. Med. Res. 2014, 3, 119–125. [Google Scholar] [CrossRef] [Green Version]
- SCSU 4806:2007 Wines. Available online: http://www.sovietwine.com/history/gost/dstu_4806-2007.html (accessed on 1 March 2021).
- Patent for a Utility Model No 90347. Available online: https://iprop-ua.com/inv/3hm6i6j7/ (accessed on 2 March 2021).
- Kucharska, A.; Sokół-Łętowska, A.; Oszmiańsk, I.J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [Green Version]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with Vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Calderón, F.; González, M.C.; Gómez-Cordovés, M.C.; Suárez, J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Radler, F. Yeast. Metabolism of organic acids. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Camberwell, Australia, 1992; pp. 165–182. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity in vitro of quercetin glycoside obtained in Beauveria bassiana culture and its interaction with liposome membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [Green Version]
- Yeh, S.-T. Using Trapezoidal Rule for the Area Under a Curve Calculation. In Proceedings of the NESUG 2002, SAS Conference, Buffalo, NY, USA, 29 September–2 October 2002. [Google Scholar]
- Belosludtsev, K.N.; Trudovishnikov, A.S.; Belosludtseva, N.V.; Agafonov, A.V.; Mironova, G.D. Palmitic Acid Induces the Opening of a Ca2+-Dependent Pore in the Plasma Membrane of Red Blood Cells: The Possible Role of the Pore in Erythrocyte Lysis. J. Membr. Biol. 2010, 237, 13–19. [Google Scholar] [CrossRef]
- Sybirna, N.O.; Burda, V.A.; Chaika, Y.P. Methods of Blood System Research: Textbook [In Ukrainian]; Publishing Center of Ivan Franko LNU: Lviv, Ukraine, 2005. [Google Scholar]
- Vitak, T.Y.; Wasser, S.P.; Nevo, E.; Sybirna, N.O. The Effect of the Medicinal Mushrooms Agaricusbrasiliensis and Ganoderma lucidum (Higher Basidiomycetes) on the Erythron System in Normal and Streptozotocin-Induced Diabetic Rats. Int. J. Med. Mushrooms 2015, 17, 277–286. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Csóvári, S.; Andyal, T.; Strenger, J. Determination of the antioxidant properties of the blood and their diagnostic significance in the elderly [Article in Russian]. Lab. Delo 1991, 10, 9–13. [Google Scholar]
- Koroliuk, M.A.; Ivanova, L.I.; Maĭorova, I.G.; Tokarev, V.E. A method of determining catalase activity [Article in Russian]. Lab. Delo 1988, 1, 16–19. [Google Scholar]
- Moin, V.M. A simple and specific method for determining glutathione peroxidase activity in erythrocytes [Article in Russian]. Lab. Delo 1986, 12, 724–727. [Google Scholar]
- Dubinina, E.E.; Burmistrov, S.O.; Khodov, D.A.; Porotov, I.G. Oxidative modification of human serum proteins. A method of determining it [Article in Russian]. Vopr. Med. Khim. 1995, 41, 24–26. [Google Scholar]
- Timirbulatov, R.A.; Seleznev, E.I. Method for increasing the intensity of free radical oxidation of lipid-containing components of the blood and its diagnostic significance [Article in Russian]. Lab. Delo 1981, 4, 209–211. [Google Scholar]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. ISRN Spectrosc. 2013, 2013, e813563. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Pati, S.; Crupi, P.; Benucci, I.; Antonacci, D.; Di Luccia, A.; Esti, M. HPLC-DAD–MS/MS characterization of phenolic compounds in white wine stored without added sulfite. Int. Food Res. J. 2014, 66, 207–215. [Google Scholar] [CrossRef]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological activity of Japanese quince extract and its Interactions with lipids, erythrocyte membrane, and human albumin. J. Membr. Biol. 2016, 249, 393–410. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative Stress as a Major Culprit in Kidney Disease in Diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.-Y. The importance and strategy of diabetes prevention. Chronic. Dis. Transl. Med. 2016, 2, 204–207. [Google Scholar] [CrossRef]
- Stern, M.P.; Williams, K.; Haffner, S.M. Identification of persons at high risk for type 2 diabetes mellitus: Do we need the oral glucose tolerance test? Ann. Intern. Med. 2002, 136, 575–581. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.; Clifton, P. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, D.P.; Doble, M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 2009, 16, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jimenez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Abe, H.; Katada, K.; Orita, M.; Nishikibe, M. Effects of calcium antagonists on the erythrocyte membrane. J. Pharm. Pharmacol. 1991, 43, 22–26. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Bors, M.; Podsędek, A.; Koter-Michalak, M.; Broncel, M. Effect of polyphenols extracts from Brassica vegetables on erythrocyte membranes (in vitro study). Environ. Toxicol. Pharmacol. 2012, 34, 783–790. [Google Scholar] [CrossRef] [Green Version]
- García-Becerra., L.; Mitjans, M.; Rivas-Morales, C.; Verde-Star, J.; Oranday-Cárdenas, A.; Vinardell, M.P. Antioxidant comparative effects of two grape pomace Mexican extracts from vineyards on erythrocytes. Food Chem. 2016, 194, 1081–1088. [Google Scholar] [CrossRef]
- Liao, K.; Yin, M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: Importance of the partition coefficient. J. Agric. Food Chem. 2000, 48, 2266–2270. [Google Scholar] [CrossRef]
- Pazzini, C.E.F.; Colpo, A.C.; Poetini, M.R.; Pires, C.F.; de Camargo, V.B.; Mendez, A.S.; Azevedo, M.L.; Soares, J.C.; Folmer, V. Effects of Red Wine Tannat on Oxidative Stress Induced by Glucose and Fructose in Erythrocytes in Vitro. Int. J. Med. Sci. 2015, 12, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Vera, J.C.; Reyes, A.M.; Cárcamo, J.G.; Velásquez, F.V.; Rivas, C.I.; Zhang, R.H.; Strobel, P.; Iribarren, R.; Scher, H.I.; Slebe, J.C.; et al. Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J. Biol. Chem. 1996, 271, 8719–8724. [Google Scholar] [CrossRef] [Green Version]
- Obi, B.C.; Okoye, T.C.; Okpashi, V.E.; Igwe, C.N.; Alumanah, E.O. Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxaninduced diabetic rats. J. Diabetes Res. 2016, 2016, 1635361. [Google Scholar]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. Antiaging effect of metformin on brain in naturally aged and accelerated senescence model of rat. Rejuvenation Res. 2017, 20, 173–182. [Google Scholar] [CrossRef]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. Metformin alleviates altered erythrocyte redox status during aging in rats. Rejuvenation Res. 2017, 20, 15–24. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Suhaimi, M.; Wahab, A.; Salam, S.K.N.; Salleh, M.S.M.; Gurtu, S. Comparison of Antioxidant Effects of Honey, Glibenclamide, Metformin, and Their Combinations in the Kidneys of Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2011, 12, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Sangi, S.M.A.; Jalaud, N.A.A. Prevention and treatment of brain damage in streptozotocin induced diabetic rats with Metformin, Nigella sativa, Zingiber officinale, and Punica granatum. Biomed. Res. Ther. 2019, 7, 3274–3285. [Google Scholar] [CrossRef] [Green Version]
- Yogalakshmi, B.; Bhuvaneswari, S.; Sreeja, S.; Anuradha, C.V. Grape seed proanthocyanidins and metformin act by different mechanisms to promote insulin signaling in rats fed high calorie diet. J. Cell Commun. Signal. 2014, 8, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Oboh, G.; Ogunbadejo, M.D.; Ogunsuyi, O.B.; Oyeleye, S.I. Can gallic acid potentiate the antihyperglycemic effect of acarboseand metformin? Evidence from streptozotocin-induced diabetic rat model. Arch. Physiol. Biochem. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hacioglu, C.; Kar, F.; Kara, Y.; Yucel, E.; Donmez, D.B.; Sentürk, H.; Kanbak, G. Comparative effects of metformin and Cistus laurifolius L. extractin streptozotocin-induced diabetic rat model: Oxidative, inflammatory, apoptotic, and histopathological analyzes. Environ. Sci. Pollut. Res. 2021, 2021, 1–14. [Google Scholar]
- Hebert, S.L.; Nair, K.S. Protein and energy metabolism in type 1 diabetes. Clin. Nutr. 2010, 29, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, O.; Ramírez, E.; Picatoste, B.; Egido, J.; Tuñón, J. Alteration of Energy Substrates and ROS Production in Diabetic Cardiomyopathy. Mediat. Inflamm. 2013, 2013, 11. [Google Scholar] [CrossRef]
- Strugała, P.; Urbaniak, A.; Kuryś, P.; Włoch, A.; Kral, T.; Ugorski, M.; Hof, M.; Gabrielska, J. Antitumor and antioxidant activities of purple potato ethanolic extract and its interaction with liposomes, albumin and plasmid DNA. Food Funct. 2021, 12, 1271–1290. [Google Scholar] [CrossRef]
- Strugała, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A Comprehensive study on the biological activity of elderberry extract and cyanidin 3-O-glucoside and their interactions with membranes and human serum albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef] [Green Version]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Filho, D.W.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Phenolic profile and effect of regular consumption of Brazilian red wines on in vivo antioxidant activity. J. Food Compos. Anal. 2013, 31, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Strugala, P.; Dudra, A.; Gabrielska, J. Activity of blackcurrant and chokeberry extracts and two major cyanidin glycosides against lipid membrane oxidation and their binding properties to albumin. Acta Pol. Pharm. 2017, 74, 679–687. [Google Scholar]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Samra, M.A.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 11, 45–49. [Google Scholar] [CrossRef]
- Alén-Ruiz, F.; García-Falcón, M.S.; Pérez-Lamela, M.C.; Martínez-Carballo, E.; Simal-Gándara, J. Influence of major polyphenols on antioxidant activity in Mencía and Brancellao red wines. Food Chem. 2009, 113, 53–60. [Google Scholar] [CrossRef]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Pedrosa, R.C.; Wilhelm-Filho, D.; Bordignon-Luiz, M.T. Stilbenes and tyrosol as target compounds in the assessment of antioxidant and hypolipidemic activity of Vitis vinifera red wines from Southern Brazil. J. Agric. Food Chem. 2011, 59, 7954–7961. [Google Scholar] [CrossRef]
- Macedo, L.F.; Rogero, M.M.; Guimarães, J.P.; Granato, D.; Lobato, L.P.; Castro, I.A. Effect of red wines with different in vitro antioxidant activity on oxidative stress of high-fat diet rats. Food Chem. 2013, 137, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2014, 18, 820–897. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.A.; Marra, G.; Giardina, B.; Cotroneo, P.; Mordente, A.; Martorana, G.E.; Manto, A.; Ghirlanda, G. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes 1997, 46, 1853–1858. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Mishra, N. Anti-Oxidant Effect of Quercetin on Type 2 Diabetic Erythrocytes. J. Food Biochem. 2009, 33, 404–415. [Google Scholar] [CrossRef]
- Pandey, K.B.; Mishra, N.; Rizvi, S.I. Myricetin may provide protection against oxidative stress in type 2 diabetic erythrocytes. Z. Naturforsch. C J. Biosci. 2009, 64, 626–630. [Google Scholar] [CrossRef]
- Saleh, D.O.; Bayoumi, A.R.; El-Eraky, W.I.; El-Khatib, A.S. Streptozotocin-induced vascular and biochemical changes in rats: Effects of rosiglitazone vs. metformin. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Berg, R.M.G.; Møller, K.; Bailey, D.M. Neuro-oxidative-nitrosative stress in sepsis. J. Cereb. Blood Flow. Metab. 2011, 31, 1532–1544. [Google Scholar] [CrossRef] [Green Version]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 42, w13659. [Google Scholar] [CrossRef]
- Huang, T.-T.; Zou, Y.; Corniola, R. Oxidative stress and adult neurogenesis—Effects of radiation and superoxide dismutase deficiency. Semin. Cell Dev. Biol. 2012, 23, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Siomek, A. NF-κB signaling pathway and free radical impact. Acta Biochim. Pol. 2012, 59, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotake, M.; Shinohara, R.; Kato, K.; Hayakawa, N.; Hayashi, R.; Uchimura, K.; Makino, M.; Nagata, M.; Kakizawa, H.; Nakagawa, H.; et al. Reduction of activity, but no decrease in concentration, of erythrocyte Cu,Zn-superoxide dismutase by hyperglycaemia in diabetic patients. Diabet. Med. 1998, 15, 668–671. [Google Scholar] [CrossRef]
- Pitocco, D.; Zaccardi, F.; Di Stasio, E.; Romitelli, F.; Santini, S.A.; Zuppi, C.C.; Ghirlanda, G. Oxidative stress, nitric oxide, and diabetes. Rev. Diabet. Stud. 2010, 7, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szaleczky, E.; Prechl, J.; Feher, J.; Somogyi, A. Alterations in enzymatic antioxidant defence in diabetes mellitus−A rational approach. Postgrad. Med. J. 1999, 75, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, S.; Riedel, H.D.; Stremmel, W. Direct evidence for catalase as the predominant H2O2 -removing enzyme in human erythrocytes. Blood 1997, 90, 4973–4978. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef] [PubMed]
- Marouani, N.; Chahed, A.; Abderrazek, H.; Mohamed Hédi, H. Both aluminum and polyphenols in green tea decoction (Camellia sinensis) affect iron status and hematological parameters in rats. Eur. J. Nutr. 2007, 46, 453–459. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Berná, G.; Otaolaurruchi, E.; Troncoso, A.M.; Martín, F.; García-Parrilla, M.C. Changes in antioxidant endogenous enzymes (activity and gene expression levels) after repeated red wine intake. J. Agric. Food Chem. 2009, 57, 6578–6583. [Google Scholar] [CrossRef]
- Noguer, M.A.; Cerezo, A.B.; Donoso Navarro, E.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012, 65, 609–614. [Google Scholar] [CrossRef]
- Conway, B.N.; Miller, R.G.; Orchard, T.J. Are Hemoglobin Levels Elevated in Type 1 Diabetes? Diabetes Care 2010, 33, 341–343. [Google Scholar] [CrossRef] [Green Version]
- Koponen, A.S.; Peltonen, J.E.; Päivinen, M.K.; Aho, J.M.; Hägglund, H.J.; Uusitalo, A.L.; Lindholm, H.J.; Tikkanen, H.O. Low total haemoglobin mass, blood volume and aerobic capacity in men with type 1 diabetes. Eur. J. Appl. Physiol. 2013, 113, 1181–1188. [Google Scholar] [CrossRef]












| Sample | The Volume Fraction of Ethyl Alcohol, % | Mass Content of Sugars, g/L | Mass Content of Titratable Acids, g/L | Mass Content of Total Extract Determination, g/L | The Efficiency of Phenolic Compounds Extraction, % | Mass Concentration of Dyes Compounds, mg/L | The Efficiency of Dyes Compounds Extraction, % |
|---|---|---|---|---|---|---|---|
| Dry red wine, variety Odessa Black 2017 | 14 | 1.6–2.0 | 4.8 | 25.0 | 83.0 | 1543.0 | 89.0 |
| Peak. No. | tR (HPLC) (min) | Compound | UV λmax (nm) | [M − H]−/[M + H]+ (m/z) | Other Ions (m/z) | Content (mg per 100 mL) | |
|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||
| Anthocyanins [M + H]+ | |||||||
| 1 | 6.70 | Dpglc | 522 | 465.1026 | 303.0530 | 5.99 ± 0.16 | 6.58 ± 0.13 |
| 2 | 8.05 | Cy glc | 514 | 449.1092 | 287.0562 | 1.35 ± 0.12 | 1.66 ± 0.20 |
| 3 | 8.94 | Pt glc | 523 | 479.1210 | 317.0676 | 6.80 ± 0.06 | 6.99 ± 0.20 |
| 4 | 10.35 | Pnglc | 516 | 463.1239 | 301.0727 | 13.92 ± 0.15 | 14.91 ± 0.47 |
| 5 | 11.02 | Mv glc | 526 | 493.1339 | 331.0825 | 39.28 ± 0.30 | 42.18 ± 1.97 |
| 6 | 11.45 | Vit A-Pnglc | 503 | 531.1199 | 369.0592 | 2.13 ± 0.13 | 1.93 ± 0.57 |
| 7 | 12.08 | Dp acetyl glc | 510 | 507.1139 | 303.0494 | 6.64 ± 0.05 | 6.98 ± 0.40 |
| 8 | 12.08 | Vit A- Mv glc | 561.1240 | 399.0722 | |||
| 9 | 13.03 | Vit A-Mv acetyl glc | 507 | 603.1283 | 399.0681 | Tr | Tr |
| 10 | 13.27 | Cy acetyl glc | 512 | 491.1145 | 287.0562 | 1.09 ± 0.01 | 1.23 ± 0.07 |
| 11 | 14.23 | Pt acetyl glc | 527 | 521.1305 | 317.0676 | 1.67 ± 0.52 | 1.50 ± 0.56 |
| 12 | 15.85 | Pn acetyl glc | 516 | 505.1350 | 301.0727 | 3.09 ± 0.06 | 3.60 ± 0.29 |
| 13 | 16.30 | Mv acetyl glc | 529 | 535.1441 | 331.0825 | 8.41 ± 0.19 | 8.99 ± 0.85 |
| 14 | 17.53 | Vit A-Pnp-coumglc | 508 | 677.1500 | 369.0592 | 0.44 ± 0.14 | 0.41 ± 0.02 |
| 15 | 17.96 | Vit A-Mv p-coumglc | 512 | 707.1570 | 399.0722 | 0.39 ± 0.17 | 0.34 ± 0.02 |
| 16 | 18.33 | Cy p-coumglc | 506 | 595.2069 | 287.0562 | 0.23 ± 0.22 | 0.19 ± 0.01 |
| 17 | 18.82 | Pt p-coumglc | 527 | 625.1439 | 317.0676 | 0.10 ± 0.02 | 0.12 ± 0.06 |
| 18 | 19.57 | Pnp-coumglc | 522 | 609.1618 | 301.0727 | 1.73 ± 0.07 | 1.97 ± 0.03 |
| 19 | 19.80 | Mv p-coumglc | 528 | 639.1744 | 331.0788 | 3.66 ± 0.04 | 4.00 ± 0.15 |
| 20 | 21.23 | Mv 3-glc-4-vin ph adduct | 502 | 609.1618 | 447.1086 | 0.52 ± 0.00 | 0.55 ± 0.01 |
| Total | 97.44 | 104.13 | |||||
| Phenolic acid [M − H]− | |||||||
| 21 | 1.54 | Gallic acid | 272 | 169.0116 | 29.10 ± 1.90 | 34.90 ± 1.77 | |
| 22 | 2.76 | Protocatechuic acid | 260/295 | 153.0164 | 3.99 ± 0.18 | 4.91 ± 0.48 | |
| 23 | 3.91 | Caftaric acid | 326 | 311.0404 | 179.0342 | 66.85 ± 3.83 | 69.37 ± 1.71 |
| 24 | 5.86 | Coutaric acid | 312 | 295.0457 | 163.0379 | 41.97 ± 2.15 | 44.12 ± 2.46 |
| Total | 141.91 | 153.30 | |||||
| Compound | EC50 (µg/mL) |
|---|---|
| PC concentrate | 357.59 ± 75.23 |
| Trolox | 229.83 ± 6.12 |
| Quercetin | 77.78 ± 1.51 |
| Compound | IC50 (µg/mL) |
|---|---|
| PC concentrate | 108.38 ± 11.19 |
| L(+) ascorbic acid | 22.80 ± 2.19 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabadashka, M.; Hertsyk, D.; Strugała-Danak, P.; Dudek, A.; Kanyuka, O.; Kucharska, A.Z.; Kaprelyants, L.; Sybirna, N. Anti-Diabetic and Antioxidant Activities of Red Wine Concentrate Enriched with Polyphenol Compounds under Experimental Diabetes in Rats. Antioxidants 2021, 10, 1399. https://doi.org/10.3390/antiox10091399
Sabadashka M, Hertsyk D, Strugała-Danak P, Dudek A, Kanyuka O, Kucharska AZ, Kaprelyants L, Sybirna N. Anti-Diabetic and Antioxidant Activities of Red Wine Concentrate Enriched with Polyphenol Compounds under Experimental Diabetes in Rats. Antioxidants. 2021; 10(9):1399. https://doi.org/10.3390/antiox10091399
Chicago/Turabian StyleSabadashka, Mariya, Dariya Hertsyk, Paulina Strugała-Danak, Anita Dudek, Olena Kanyuka, Alicja Z. Kucharska, Leonid Kaprelyants, and Nataliia Sybirna. 2021. "Anti-Diabetic and Antioxidant Activities of Red Wine Concentrate Enriched with Polyphenol Compounds under Experimental Diabetes in Rats" Antioxidants 10, no. 9: 1399. https://doi.org/10.3390/antiox10091399
APA StyleSabadashka, M., Hertsyk, D., Strugała-Danak, P., Dudek, A., Kanyuka, O., Kucharska, A. Z., Kaprelyants, L., & Sybirna, N. (2021). Anti-Diabetic and Antioxidant Activities of Red Wine Concentrate Enriched with Polyphenol Compounds under Experimental Diabetes in Rats. Antioxidants, 10(9), 1399. https://doi.org/10.3390/antiox10091399






