Metabolites with Antioxidant Activity from Marine Macroalgae
Abstract
:1. Introduction
2. Brief Overview of the Methods Employed for the Evaluation of Antioxidant Activity
3. Phenolic Compounds
4. Terpenoids
5. Meroterpenoids
6. Nitrogenous Compounds
7. Carbohydrates and Polysaccharides
8. Miscellaneous Compounds
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Leto, T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 2008, 15, 164–187. [Google Scholar] [CrossRef] [Green Version]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Phys. India 2004, 52, 794–804. [Google Scholar]
- Gammone, M.; Riccioni, G.; D’Orazio, N. Marine carotenoids against oxidative stress: Effects on human health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Rosenfeld, M.E.; Yla, H.S.; Gurtner, G.C.; Socher, S.S.; Butler, S.W.; Carew, T.E.; Parthasarathy, S.; Steinberg, D.; Witztum, J.L. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA 1989, 86, 1372–1376. [Google Scholar] [CrossRef] [Green Version]
- Golbidi, S.; Ebadi, S.A.; Laher, I. Antioxidants in the treatment of diabetes. Curr. Diabetes Rev. 2011, 7, 106–125. [Google Scholar] [CrossRef]
- Bodamyali, T.; Kanczler, J.M.; Millar, T.M.; Stevens, C.R.; Blake, D.R. Free radicals in rheumatoid arthritis: Mediators and modulators. Oxid. Stress Dis. 2004, 10, 591–610. [Google Scholar]
- Cuzzocrea, S.; Riley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001, 53, 135–159. [Google Scholar] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [Green Version]
- Traysman, R.J.; Kirsch, J.R.; Koehler, R.C. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J. Appl. Physiol. 1991, 71, 1185–1195. [Google Scholar] [CrossRef]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Sulthana, S.M.; Kumar, S.N.; Sridhar, M.G.; Bhat, B.V.; Rao, K.R. Levels of non enzymatic antioxidants in Down syndrome. Indian J. Pediatr. 2012, 79, 1473–1476. [Google Scholar] [CrossRef]
- Chen, H.; Yu, M.; Li, M.; Zhao, R.; Zhu, Q.; Zhou, W.; Lu, M.; Lu, Y.; Zheng, T.; Jiang, J.; et al. Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase-1 (GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Mol. Cell. Biochem. 2012, 363, 85–91. [Google Scholar] [CrossRef]
- Young, I.; Woodside, J. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing by products-a review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Ahmad, B.; Shah, M.; Choi, S. Oceans as a source of immunotherapy. Mar. Drugs 2019, 17, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current status and future prospects of marine natural products (MNPs) as antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Ding, T.; Li, J. Medicinal purposes: Bioactive metabolites from marine-derived organisms. Mini-Rev. Med. Chem. 2019, 19, 138–164. [Google Scholar] [CrossRef]
- MarinLit. A Database of the Marine Natural Products Literature. Available online: http://pubs.rsc:marinlit/ (accessed on 31 March 2021).
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, R.; Dubinsky, Z.; Kishimoto, E.; Masaki, K.; Masuda, Y.; Takeuchi, T. A comparison of screening methods for antioxidant activity in seaweeds. J. Appl. Phycol. 1997, 9, 29–35. [Google Scholar] [CrossRef]
- Athiperumalsami, T.; Rajeswari, V.D.; Poorna, S.H.; Kumar, V.; Jesudass, L.L. Antioxidant activity of seagrasses and seaweeds. Bot. Mar. 2010, 53, 251–257. [Google Scholar] [CrossRef]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula. J. Appl. Phycol. 2007, 19, 449–458. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C. Marine algal antioxidants. Antioxidants 2020, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.P.S.; Kim, M.; Son, K.-T.; Jeong, Y.; Jeon, Y.-J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 26.1–26.28. [Google Scholar] [CrossRef]
- Jiao, G.-L.; Yu, G.L.; Zhao, X.-L.; Zhang, J.-Z.; Ewart, H.S. Natural polymers with antioxidant properties: Poly-/oligosaccharides of marine origin. In Antioxidant Polymers: Synthesis, Properties, and Applications, 1st ed.; Cirillo, G., Lemma, F., Eds.; Wiley, Scrivener Publishing LLC: Beverly, MA, USA, 2012; pp. 179–202. [Google Scholar]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Measurement of oxygen radical absorbance capacity in biological samples. Meth. Enzymol. 1999, 299, 50–62. [Google Scholar] [CrossRef]
- Wayner, D.D.M.; Burton, G.W.; Ingold, K.U.; Locke, S. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985, 187, 33–37. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Zhou, G.; Lu, X.; Xu, Z.; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodophyta) in aging mice. Pharmacol. Res. 2003, 48, 151–155. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Dontha, S. A review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Miller, H.E. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Saran, M. Inhibition of bleaching of the carotenoid crocin, a rapid test for quantifying antioxidant activity. Biochim. Biophys. Acta 1984, 796, 312–319. [Google Scholar] [CrossRef]
- Winston, G.W.; Regoli, F.; Dugas, A.J., Jr.; Fong, J.H.; Blanchard, K.A. A rapid gas chromatographic assay for determining oxyradical scavenging capacity of antioxidants and biological fluids. Free Radic. Biol. Med. 1998, 24, 480–493. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M.N. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. The autoxidation of vitamin E and related chainbreaking antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Okawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Popov, I.; Lewin, G. Antioxidative homeostasis: Characterization by means of chemiluminescent technique. Methods Enzymol. 1999, 300, 437–456. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. A novel total antioxidant capacity index for dietary polyphenols, vitamins c and e, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Usuguchi, J.; Nakatani, N. Constituents of Zingiberaceae. I. Diarylheptanoids from the rhizomes of ginger (Zingiber officinale roscoe). Chem. Pharm. Bull. 1991, 39, 120–122. [Google Scholar] [CrossRef] [Green Version]
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The nitric oxide scavenging property of Ginkgo biloba extract EGB 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef]
- Verde, V.; Fogliano, V.; Ritieni, A.; Maiani, G.; Morisco, F.; Caporaso, N. Use of N,N-dimethyl-p-phenylenediamine to evaluate the oxidative status of human plasma. Free Radic. Res. 2002, 36, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Maitra, I.; Marcocci, L.; Droy-Lefaix, M.T.; Packer, L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem. Pharmacol. 1995, 49, 1649–1655. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions-antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Korycka-Dahl, M.; Richardson, M. Photogeneration of superoxide anion in serum of bovine milk and in model systems containing riboflavin and aminoacids. J. Dairy Sci. 1978, 61, 400–407. [Google Scholar] [CrossRef]
- Yagi, K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976, 15, 212–216. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Measurement of total vitamin C activity in citrus products by HPLC. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 2367–2387. [Google Scholar] [CrossRef]
- Gonzalez, E.; Vaillant, F.; Rojas, G.; Pérez, A. Novel semiautomated method for assessing in vitro cellular antioxidant activity using the light scattering properties of human erythrocytes. J. Agric. Food Chem. 2010, 58, 1455–1461. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (Ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Noro, T.; Oda, Y.; Miyase, T.; Ueno, A.; Fukushima, S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem. Pharm. Bull. 1983, 31, 3984–3987. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.J.; Li, X.M.; Wang, B.G. Highly brominated mono- and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef]
- Fang, Z.; Jeong, S.Y.; Jung, H.A.; Choi, J.S.; Min, B.S.; Woo, M.H. Anticholinesterase and antioxidant constituents from Gloiopeltis furcata. Chem. Pharm. Bull. 2010, 58, 1239. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. Isolation, characterization, and antioxidant activity of bromophenols of the marine red alga Rhodomela confervoides. J. Agric. Food Chem. 2011, 59, 9916–9921. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, T.K.; Kang, R.S.; Shin, H.J.; Lee, H.S. The in vitro antioxidant activities of the bromophenols from the red alga Tichocarpus crinitus and phenolic derivatives. J. Korean Magn. Reson. Soc. 2007, 11, 56–63. [Google Scholar]
- Park, H.J.; Kim, H.R.; Choi, J.S. Antioxidant effect of 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether (TDB) from the red alga, Symphyocladia latiuscula. J. Fish. Sci. Technol. 2009, 12, 86–89. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706. [Google Scholar] [CrossRef]
- Rezai, M.; Bayrak, Ç.; Taslimi, P.; Gülҫin, I.; Menzek, A. The first synthesis and antioxidant and anticholinergic activities of 1-(4,5-dihydroxybenzyl)pyrrolidin-2-one derivative bromophenols including natural products. Turk. J. Chem. 2018, 42, 808–825. [Google Scholar]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef]
- Xu, X.L.; Yin, L.Y.; Gao, J.H.; Chen, J.H.; Li, J.X.; Song, F.H. Two new bromophenols with radical scavenging activity from marine red alga Symphyocladia latiuscula. Mar. Drugs 2013, 11, 842–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Wang, Y.F.; Li, X.M.; Wang, W.J.; Ai, X.Z.; Li, X.; Yang, S.Q.; Gloer, J.B.; Wang, B.G.; Xu, T. Isolation, synthesis, and radical-scavenging activity of rhodomelin A, a ureidobromophenol from the marine red alga Rhodomela confervoides. Org. Lett. 2018, 20, 417–420. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Ji, N.Y.; Wang, B.G. Bromophenols from the marine red alga Polysiphonia urceolata with DPPH radical scavenging activity. J. Nat. Prod. 2008, 71, 28–30. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Fernando, P.D.S.M.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Marine compound 3-bromo-4,5-dihydroxybenzaldehyde protects skin cells against oxidative damage via the Nrf2/HO-1 pathway. Mar. Drugs 2019, 17, 234. [Google Scholar] [CrossRef] [Green Version]
- Olsen, E.K.; Hansen, E.; Isaksson, J.; Andersen, J.H. Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa. Mar. Drugs 2013, 11, 2769–2784. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Hyun, C.L.; Kang, H.K.; Yoo, E.S.; Young, S.K.; Lee, N.H.; et al. Protective effect of 3,4-dihydroxybenzoic acid isolated from Cladophora wrightiana Harvey against ultraviolet B radiation-induced cell damage in human HaCaT keratinocytes. Appl. Biochem. Biotechnol. 2014, 172, 2582–2592. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Ji, N.Y.; Wang, B.G. Natural bromophenols from the marine red alga Polysiphonia urceolata (Rhodomelaceae): Structural elucidation and DPPH radical-scavenging activity. Bioorg. Med. Chem. 2007, 15, 6627–6631. [Google Scholar] [CrossRef]
- Islam, M.R.; Mikami, D.; Kurihara, H. Two new algal bromophenols from Odonthalia corymbifera. Tetrahedron Lett. 2017, 58, 4119–4121. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Ye, B.R.; Kim, E.A.; Kim, J.; Kim, M.S.; Lee, W.W.; Ahn, G.N.; Kang, N.; Jung, W.K.; Heo, S.J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, X.M.; Ji, N.Y.; Gloer, J.B.; Wang, B.G. Urceolatin, a structurally unique bromophenol from Polysiphonia urceolata. Org. Lett. 2008, 7, 1429–1432. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef]
- Karthik, P.; Manigandan, V.; Sheeba, R.; Saravanan, R.; Rajesh, P.R. Structural characterization and comparative biomedical properties of phloroglucinol from Indian brown seaweeds. J. Appl. Phycol. 2016, 28, 3561–3573. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.-J.; Hong, S.H.; Kim, G.-Y.; Kim, S.; Kim, H.-S.; Kim, B.W.; Jeon, Y.-J.; Choi, Y.H. Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Qian, Z.J.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Antioxidant Effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Andriani, Y.; Syamsumir, D.F.; Yee, T.C.; Harisson, F.S.; Herng, G.M.; Abdullah, S.A.; Orosco, C.A.; Ali, A.M.; Latip, J.; Kikuzaki, H.; et al. Biological activities of isolated compounds from three edible Malaysian red seaweeds, Gracilaria changii, G. manilaensis and Gracilaria sp. Nat. Prod. Commun. 2016, 11, 1117–1120. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Lee, I.K.; Cho, G.Y.; Oh, K.H.; Lim, Y.W.; Yun, B.S. Sargassumol, a novel antioxidant from the brown alga Sargassum micracanthum. J. Antibiot. 2012, 65, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.C.; Lee, I.K.; Kang, K.A.; Piao, M.J.; Ryu, M.J.; Kim, J.M.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava up-regulates the oxidant sensitive 8-oxoguanine DNA glycosylase 1. Mar. Drugs 2014, 12, 5357–5371. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.C.; Kim, K.N.; Lakmal, H.H.C.; Kim, E.A.; Wijesinghe, W.A.J.P.; Yang, X.; Heo, S.J.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea prevents and protects against high glucose-induced oxidative damage in vitro and in vivo. Environ. Toxicol. Pharmacol. 2014, 38, 607–615. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, S.M.; Ko, S.C.; Kang, M.C.; Jeon, Y.J. Octaphlorethol A, a novel phenolic compound isolated from Ishige foliacea, protects against streptozotocin-induced pancreatic cell damage by reducing oxidative stress and apoptosis. Food Chem. Toxicol. 2013, 643–649. [Google Scholar] [CrossRef]
- Jun, Y.J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.H.; Kim, H.R. Eckol enhances heme oxygenase-1 expression through activation of Nrf2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.S.; Chung, H.Y.; Jung, J.H.; Son, B.W.; Choi, J.S. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem. Pharm. Bull. 2003, 51, 1012–1014. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Lee, Y.; Kim, S.Y.; Kim, H.S.; Joo, H.G.; Park, J.W.; et al. Eckol isolated from Ecklonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett. 2005, 579, 6295–6304. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava extract and dieckol attenuate cellular lipid peroxidation in keratinocytes exposed to PM10. J. Evid. Based Complementary Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Heo, S.J.; Cha, S.H.; Kim, K.N.; Lee, S.H.; Ahn, G.; Kang, D.H.; Oh, C.; Choi, Y.U.; Affan, A.; Kim, D.; et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Hyun, Y.J.; Kang, K.A.; Fernando, P.D.S.M.; Cho, S.J.; Ahn, M.J.; Hyun, J.W. Diphlorethohydroxycarmalol attenuates fine particulate matter-induced subcellular skin dysfunction. Mar. Drugs 2019, 17, 95. [Google Scholar] [CrossRef] [Green Version]
- Cha, S.H.; Heo, S.J.; Jeon, Y.J.; Park, S.M. Dieckol, an edible seaweed polyphenol, retards rotenone-induced neurotoxicity and α-synuclein aggregation in human dopaminergic neuronal cells. RSC Adv. 2016, 6, 110040–110046. [Google Scholar] [CrossRef]
- Yoon, J.S.; Yadunandam, A.K.; Kim, S.J.; Woo, H.C.; Kim, H.R.; Kim, G.D. Dieckol, isolated from Ecklonia stolonifera, induces apoptosis in human hepatocellular carcinoma Hep3B cells. J. Nat. Med. 2013, 67, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Cérantola, S.; Breton, F.; Gall, A.E.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Ham, Y.M.; Baik, J.S.; Hyun, J.W.; Lee, N.H. Isolation of a new phlorotannin, fucodiphlorethol G, from a brown alga Ecklonia cava. Bull. Korean Chem. Soc. 2007, 28, 1595–1597. [Google Scholar]
- Kim, K.C.; Piao, M.J.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kumara, M.H.S.R.; Han, X.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Fucodiphlorethol G purified from Ecklonia cava suppresses ultraviolet B radiation-induced oxidative stress and cellular damage. Biomol. Ther. 2014, 22, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.; Carmeli, S.; Klimo, K.; Gerhaeuser, C.; Koenig, G.M. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2010, 71, 221–229. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.Y.; Oh, J.Y.; Kim, E.A.; Kim, C.Y.; Jeon, Y.J. Evaluation of phlorofucofuroeckol-A isolated from Ecklonia cava (Phaeophyta) on anti-lipid peroxidation in vitro and in vivo. Algae 2015, 30, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.H.; Suh, H.J.; Lee, I.K.; Yun, B.S.; Kim, T.W.; Hwang, D.I.; Kim, Y.J.; Kim, M.J.; Kwon, O.O.; Kim, C.G.; et al. Determination of singlet oxygen quenching and antioxidant activity of Bieckols isolated from the brown alga Eisenia bicyclis. Eur. Food Res. Technol. 2013, 237, 501–508. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, B.W.; Lee, S.Y. Isolation of 6,6’-bieckol from Grateloupia elliptica and its antioxidative and anti-cholinesterase activity. Ocean Polar Res. 2017, 39, 45–49. [Google Scholar] [CrossRef]
- Park, M.H.; Heo, S.J.; Park, P.J.; Moon, S.H.; Sung, S.H.; Jeon, B.T.; Lee, S.H. 6,6′-Bieckol isolated from Ecklonia cava protects oxidative stress through inhibiting expression of ROS and proinflammatory enzymes in high-glucose-induced human umbilical vein endothelial cells. Appl. Biochem. Biotechnol. 2014, 174, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Haulader, S.; Karki, S.; Jung, H.J.; Kim, H.R.; Jung, H.A. Acetyl- and butyryl-cholinesterase inhibitory activities of the edible brown alga Eisenia bicyclis. Arch. Pharm. Res. 2015, 38, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Heo, S.J.; Kim, K.N.; Lee, S.H.; Jeon, Y.J. Isolation and identification of new compound, 2,7″-phloroglucinol-6,6′-bieckol from brown algae, Ecklonia cava and its antioxidant effect. J. Funct. Foods 2012, 4, 158–166. [Google Scholar] [CrossRef]
- Zeng, L.M.; Wang, C.J.; Yu, S.J.; Li, D.; Owen, N.L.; Lu, Y.; Lu, N.; Zheng, Q.T. Flavonoids from the red alga Acanthophora spicifera. Chin. J. Chem. 2001, 19, 1097–1100. [Google Scholar] [CrossRef]
- Ragan, M.A.; Glombitza, K.W. Phlorotannins, brown algal polyphenols. In Progress in Phycological Research; Round, F.E., Chapman, D.J., Eds.; Biopress Ltd.: Bristol, UK, 1986; pp. 129–241. [Google Scholar]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Gross, H.; König, G.M. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochem. Rev. 2006, 5, 115–141. [Google Scholar] [CrossRef]
- Shapumba, C.W.; Knott, M.; Kapewangolo, P. Antioxidant activity of a halogenated monoterpene isolated from a Namibian marine algal Plocamium species. J. Food Sci. Technol. 2017, 54, 3370–3373. [Google Scholar] [CrossRef]
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010, 122, 31–41. [Google Scholar] [CrossRef]
- Guajardo, E.; Correa, J.A.; Contreras-Porcia, L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 2016, 243, 767–781. [Google Scholar] [CrossRef]
- Ge, N.; Liang, H.; Zhao, Y.Y.; Liu, Y.; Gong, A.J.; Zhang, W.L. Aplysin protects against alcohol-induced liver injury via alleviating oxidative damage and modulating endogenous apoptosis-related genes expression in rats. J. Food. Sci. 2018, 83, 2612–2621. [Google Scholar] [CrossRef]
- He, J.; Liang, H.; Li, Y.; Shi, D.Y.; Ma, A.G. Antioxidant effect of Aplysin on aged mice exposed to D-galatose. Chin. J. Public Health 2009, 25, 1122–1123. [Google Scholar]
- Gressler, V.; Stein, É.M.; Dörr, F.; Fujii, M.T.; Colepicolo, P.; Pinto, E. Sesquiterpenes from the essential oil of Laurencia dendroidea (Ceramiales, Rhodophyta): Isolation, biological activities and distribution among seaweeds. Rev. Bras. Farmacogn. 2011, 21, 248–254. [Google Scholar] [CrossRef]
- Maneesh, A.; Chakraborty, K. Previously undescribed frido oleanenes and oxygenated labdanes from the brown seaweed Sargassum wightii and their protein tyrosine phosphatase-1B inhibitory activity. Phytochemistry 2017, 144, 19–32. [Google Scholar] [CrossRef]
- Alarif, W.M.; Basaif, S.A.; Badria, F.A.; Ayyadd, S.E.N. Two new cytotoxic C-29 steroids from the Red Sea brown alga Cystoseira trinodis. Chem. Nat. Compd. 2015, 51, 697–702. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, Y.R.; Byeon, J.S.; Choung, S.Y.; Sohn, H.S.; Jung, H.A. Protective effect of fucosterol isolated from the edible brown algae, Ecklonia stolonifera and Eisenia bicyclis, on tert-butyl hydroperoxide- and tacrine-induced HepG2 cell injury. J. Pharm. Pharmacol. 2015, 67, 1170–1178. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Jeon, Y.J. Squalene isolated from marine macroalgae Caulerpa racemosa and its potent antioxidant and anti-inflammatory activities. J. Food Biochem. 2018, 42, e12628. [Google Scholar] [CrossRef]
- Chakraborty, K.; Antony, T. First report of antioxidative abeo-oleanenes from red seaweed Gracilaria salicornia as dual inhibitors of starch digestive enzymes. Med. Chem. Res. 2019, 28, 696–710. [Google Scholar] [CrossRef]
- Renju, G.L.; Kurup, G.M.; Kumari, C.H.S. Effect of lycopene from Chlorella marina on high cholesterolinduced oxidative damage and inflammation in rats. Inflammopharmacology 2014, 22, 45–54. [Google Scholar] [CrossRef]
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Lee, H.; Kwon, Y.-G.; Chung, C.-K.; Kim, Y.-M. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulatedmacrophages by suppressing redox-based NF-κB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef]
- de Sousa, C.B.; Gangadhar, K.N.; Macridachis, J.; Pavão, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J.H.G. Cystoseira algae (Fucaceae): Update on their chemical entities and biological activities. Tetrahedron Asymmetry 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kim, O.K.; Koshimizu, K.; Ohigashi, H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leukocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar] [CrossRef]
- Niu, T.; Xuan, R.; Jiang, L.; Wu, W.; Zhen, Z.; Song, Y.; Hong, L.; Zheng, K.; Zhang, J.; Xu, Q.; et al. Astaxanthin induces the Nrf2/HO-1 antioxidant pathway in human umbilical vein endothelial cells by generating trace amounts of ROS. J. Agric. Food Chem. 2018, 66, 1551–1559. [Google Scholar] [CrossRef]
- Saw, C.L.; Yang, A.Y.; Guo, Y.; Kong, A.N. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef]
- Tripathi, D.N.; Jena, G.B. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res. 2010, 696, 69–80. [Google Scholar] [CrossRef]
- Wen, X.; Huang, A.; Hu, J.; Zhong, Z.; Liu, Y.; Li, Z.; Pan, X.; Liu, Z. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: Involvement of the Akt/GSK-3β pathway. Neuroscience 2015, 303, 558–568. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.-S.; Wang, H.-D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.-L.; Wang, X.-L. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.J.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a Potential neuroprotective agent for neurological diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.-L.; Han, X.-D.; Li, Y.; Chu, X.-F.; Miao, W.-M.; Zhang, J.-L.; Fan, S.-J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Zhao, Y.; Zhang, X.; Lin, X. Astaxanthin inhibits acetaldehyde-induced cytotoxicity in SH-SY5Y cells by modulating Akt/CREB and p38MAPK/ERK signaling pathways. Mar. Drugs 2016, 14, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taira, J.; Sonamoto, M.; Uehara, M. Dual biological functions of a cytoprotective effect and apoptosis induction by bioavailable marine carotenoid fucoxanthinol through modulation of the nrf2 activation in Raw264.7 macrophage cells. Mar. Drugs 2017, 15, 305. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Lee, S.H.; Lee, W.W.; Kang, N.; Kim, E.A.; Kim, S.Y.; Lee, D.H.; Kim, D.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Ishige okamurae against high-glucose induced oxidative stress in human umbilical vein endothelial cells and zebrafish model. J. Funct. Foods 2014, 11, 304–312. [Google Scholar] [CrossRef]
- Ragubeer, N.; Limson, J.L.; Beukes, D.R. Electrochemistry-guided isolation of antioxidant metabolites from Sargassum elegans. Food Chem. 2012, 131, 286–290. [Google Scholar] [CrossRef]
- Raguraman, V.; Abraham, L.S.; Mubarak Ali, D.; Narendrakumar, G.; Thirugnanasambandam, R.; Kirubagaran, R.; Thajuddin, N. Unraveling rapid extraction of fucoxanthin from Padina tetrastromatica: Purification, characterization and biomedical application. Process Biochem. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Inter. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Sellimi, S.; Ksouda, G.; Benslima, A.; Nasri, R.; Rinaudo, M.; Nasri, M.; Hajji, M. Enhancing colour and oxidative stabilities of reduced-nitrite turkey meat sausages during refrigerated storage using fucoxanthin purified from the Tunisian seaweed Cystoseira barbata. Food Chem. Toxicol. 2017, 107, 620–629. [Google Scholar] [CrossRef]
- Yu, J.; Lin, J.J.; Yu, R.; He, S.; Wang, Q.W.; Cui, W.; Zhang, J.R. Fucoxanthin prevents H2O2-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr. Res. 2017, 61, 1304678. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fang, H.; Xie, Q.; Sun, J.; Liu, R.; Hong, Z.; Yi, R.; Wu, H. Comparative evaluation of the radical-scavenging activities of fucoxanthin and its stereoisomers. Molecules 2014, 19, 2100–2113. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Huang, L.; Gao, B.; Zhang, C. Optimum production conditions, purification, identification, and antioxidant activity of violaxanthin from microalga Eustigmatos cf. polyphem (Eustigmatophyceae). Mar. Drugs 2018, 16, 190. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.H.; Lee, J.H.; Chand, H.S.; Lee, J.S.; Lin, Y.; Weathington, N.; Mallampalli, R.; Jeon, Y.J.; Nyunoya, T. Apo-9ʹ-fucoxanthinone extracted from Undariopsis peteseniana protects oxidative stress-mediated apoptosis in cigarette smoke-exposed human airway epithelial cells. Mar. Drugs 2016, 14, 140. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Wang, L.; Fernando, I.P.S.; Je, J.-G.; Ko, S.-C.; Kang, M.C.; Lee, J.M.; Yim, M.-J.; Jeon, Y.-J.; Lee, D.-S. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.K.A.; Kim, H.-Y.; Rho, J.-R.; Jee, Y.; Ahn, G.; Jeon, Y.-J. Sargassum horneri and isolated 6-hydroxy-4,4,7a-trimethyl-5,6,7,7atetrahydrobenzofuran- 2(4H)-one (HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Kim, H.A.; Miller, A.A.; Drummond, G.R.; Thrift, A.G.; Arumugam, T.V.; Phan, T.G.; Srikanth, V.K.; Sobey, C.G. Vascular cognitive impairment and Alzheimer’s disease: Role of cerebral hypoperfusion and oxidative stress. Naunyn Schmiedebergs Arch. Pharmakol. 2012, 385, 953–959. [Google Scholar] [CrossRef]
- Padurariu, M.; Ciobica, A.; Lefter, R.; Lacramioara Serban, I.; Stefanescu, C.; Chirita, R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr. Danub. 2013, 25, 401–409. [Google Scholar]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective effects of Astaxanthin: Therapeutic targets and clinical perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-J.; Pu, J.-L.; Krafft, P.R.; Zhang, J.-M.; Chen, S. The molecular mechanisms between autophagy and apoptosis: Potential role in central nervous system disorders. Cell. Mol. Neurobiol. 2015, 35, 85–99. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int. 1997, 42, 361–370. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Fisch, M.K.; Böhm, V.; Wright, A.D.; König, G.M. Antioxidative meroterpenoids from the brown alga Cystoseira crinita. J. Nat. Prod. 2003, 66, 968–975. [Google Scholar] [CrossRef]
- De los Reyes, C.; Zbakh, H.; Motilva, V.; Zubía, E. Antioxidant and anti-inflammatory meroterpenoids from the brown alga Cystoseira usneoides. J. Nat. Prod. 2013, 76, 621–629. [Google Scholar] [CrossRef]
- Kumagai, M.; Nishikawa, K.; Matsuura, H.; Umezawa, T.; Matsuda, F.; Okino, T. Antioxidants from the brown alga Dictyopteris undulata. Molecules 2018, 23, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Comm. 2015, 457, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, K.; Antony, T.; Joy, M. Prospective natural anti-inflammatory drimanes attenuating pro-inflammatory 5-lipoxygenase from marine macroalga Gracilaria salicornia. Algal Res. 2019, 40, 101472. [Google Scholar] [CrossRef]
- Antony, T.; Chakraborty, K. First report of antioxidative 2H-chromenyl derivatives from the intertidal red seaweed Gracilaria salicornia as potential antiinflammatory agents. Nat. Prod. Res. 2020, 34, 3470–3482. [Google Scholar] [CrossRef]
- Iwashima, M.; Mori, J.; Ting, X.; Matsunaga, T.; Hayashi, K.; Shinoda, D.; Satio, H.; Sankawa, U.; Hayashi, T. Antioxidant and antiviral activities of plastoquinones from the brown alga Sargassum micracanthum, and a new chromene derivative converted from the plastoquinones. Biol. Pharm. Bull. 2005, 28, 374–377. [Google Scholar] [CrossRef] [Green Version]
- Mori, J.; Iwashima, M.; Wakasugi, H.; Saito, H.; Matsunaga, T.; Ogasawara, M.; Takahashi, S.; Suzuki, H.; Hayashi, T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2005, 53, 1159–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, V.L.M.; Seca, A.M.L.; Barreto, M.C.; Neto, A.I.; Kijjoa, A.; Silva, A.M.S. Cytotoxic meroterpenoids from the macro alga Cystoseira abies-marina. Phytochem. Lett. 2013, 6, 593–597. [Google Scholar] [CrossRef]
- De los Reyes, C.; Ortega, M.J.; Zbakh, H.; Motilva, V.; Zubía, E. Cystoseira usneoides: A brown alga rich in antioxidant and antiinflammatory meroditerpenoids. J. Nat. Prod. 2016, 79, 395–405. [Google Scholar] [CrossRef]
- Jung, M.; Jang, K.H.; Kim, B.; Lee, B.H.; Choi, B.W.; Oh, K.B.; Shin, J. Meroditerpenoids from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2008, 71, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Lee, H.J.; Park, K.E.; Kim, Y.A.; Ahn, J.W.; Yoo, J.S.; Lee, B.J. Peroxynitrite scavenging constituents from the brown alga Sargassum thunbergii. Biotechnol. Bioprocess Eng. 2004, 9, 212–216. [Google Scholar] [CrossRef]
- Seo, Y.; Park, K.E.; Nam, T.J. Isolation of a new chromene from the brown alga Sargassum thunbergii. Bull. Korean Chem. Soc. 2007, 28, 1831–1833. [Google Scholar]
- Ham, Y.M.; Kim, K.N.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Chemical constituents from Sargassum micracanthum and antioxidant activity. Int. J. Pharmacol. 2010, 6, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.H.; Lee, B.H.; Choi, B.W.; Lee, H.S.; Shin, J. Chromenes from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2005, 68, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Seo, Y. Chromanols from Sargassum siliquastrum and their antioxidant activity in HT 1080 cells. Chem. Pharm. Bull. 2011, 59, 757–761. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.J.; Kim, K.N.; Heo, S.J.; Han, S.C.; Kim, J.; Ko, Y.J.; Kang, H.K.; Yoo, E.S. Sargachromanol G inhibits osteoclastogenesis by suppressing the activation NF-kappa B and MAPKs in RANKL-induced RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2013, 434, 892–897. [Google Scholar] [CrossRef]
- Mori, J.; Iwashima, A.; Takeuchi, M.; Saito, H. A synthetic study on antiviral and antioxidative chromene derivative. Chem. Pharm. Bull. 2006, 54, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.J.; Yoo, J.S.; Ahn, J.W.; Lee, B.J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Yoshiki, M.; Tsuge, K.; Tsuruta, Y.; Yoshimura, T.; Koganemaru, K.; Sumi, T.; Matsui, T.; Matsumoto, K. Production of new antioxidant compound from mycosporine-like amino acid, porphyra-334 by heat treatment. Food Chem. 2009, 113, 1127–1132. [Google Scholar] [CrossRef]
- Tamura, Y.; Takenaka, S.; Sugiyama, S.; Nakayama, R. Occurrence of anserine as an antioxidative dipeptide in a red alga, Porphyra yezoensis. Biosci. Biotechnol. Biochem. 1998, 62, 561–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, P.; Li, G.; Wang, C.; Wu, J.; Sun, Z.; Martin, G.E.; Wang, X.; Reibarkh, M.; Saurí, J.; Gustafson, K.-R. Characterization by empirical and computational methods of dictyospiromide, an intriguing antioxidant alkaloid from the marine alga Dictyota coriacea. Org. Lett. 2019, 21, 7577–7581. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant activity of the phycoerythrobilin compound formed from a dried Korean purple laver (Porphyra sp.) during in vitro digestion. Food Sci. Technol. Res. 2010, 16, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.L.; Lee, H.-S.; Kang, I.-J.; Won, M.-H.; You, S.G. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr. Sci. 2013, 4, 35234. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Cahyana, A.H.; Shuto, Y.; Kinoshita, Y. Pyropheophytin a as an antioxidative substance from the marine alga, arame (Eisenia bicyclis). Biosci. Biotechnol. Biochem. 1992, 56, 1533–1535. [Google Scholar] [CrossRef]
- Kitano, Y.; Murazumi, K.; Duan, J.; Kurose, K.; Kobayashi, S.; Sugawara, T.; Hirata, T. Effect of dietary porphyran from the red alga, Porphyra yezoensis, on glucose metabolism in diabetic KK-Ay mice. J. Nutr. Sci. Vitaminol. 2012, 58, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Li, Y.; Lee, S.H.; Qian, Z.J.; Kim, S.K. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and D-isofloridoside from marine red alga Laurencia undulata. J. Agric. Food Chem. 2010, 58, 578–586. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, X.; Jiang, Y.; Hu, X.; Mou, H.; Li, M.; Guan, H. In vitro antioxidative activities of three marine oligosaccharides. Nat. Prod. Res. 2007, 21, 646–654. [Google Scholar] [CrossRef]
- Rocha de Souza, M.C.; Marques, C.T.; Dore, C.M.G.; Ferreira da Silva, F.R.; Rocha, H.A.O.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Ammar, H.H.; Lajili, S.; Said, R.B.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira. Daru 2015, 23, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Xue, C.H.; Li, B.F. Study of antioxidant activities of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2008, 20, 431–436. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Chemical composition and antioxidant activity of sulphated polysaccharides extracted from Fucus vesiculosus using different hydrothermal processes. Chem. Pap. 2014, 68, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoterapia 2012, 83, 1628–1635. [Google Scholar] [CrossRef]
- Ryu, M.J.; Chung, H.S. Fucoidan reduces oxidative stress by regulating the gene expression of HO1 and SOD1 through the Nrf2/ERK signaling pathway in HaCaT cells. Mol. Med. Rep. 2016, 14, 3255–3260. [Google Scholar] [CrossRef] [Green Version]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and In vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Shi, X.; Song, H.; Zhang, J. In vitro antioxidant activities of acetylated, phosphorylated and benzoylated derivatives of porphyran extracted from Porphyra haitanensis. Carbohydr. Polym. 2009, 78, 449–453. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Qi, H.M.; Zhang, Q.B.; Zhao, T.T.; Hu, R.G.; Zhang, K.; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg. Med. Chem. Lett. 2006, 16, 2441–2445. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Yu, P.Z.; Li, Z.E.; Zhang, H.; Xu, Z.; Li, P.C. Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. Appl. Phycol. 2003, 15, 305–310. [Google Scholar] [CrossRef]
- Sun, L.Q.; Wang, L.; Li, J.; Liu, H.H. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Wei, C.-C.; Yen, P.-L.; Chang, S.-T.; Cheng, P.-L.; Lo, Y.-C.; Liao, V.H.-C. Antioxidative activities of both oleic acid and Camellia tenuifolia seed oil are regulated by the transcription factor DAF-16/FOXO in Caenorhabditis elegans. PLoS ONE 2016, 11, e0157195. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.X.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free radical mediated oxidative systems. J. Microbiol. Biotechnol. 2009, 19, 1319–1327. [Google Scholar] [CrossRef] [Green Version]
- Makkar, F.; Chakraborty, K. Antioxidant and anti-inflammatory oxygenated meroterpenoids from the thalli of red seaweed Kappaphycus alvarezii. Med. Chem. Res. 2018, 27, 2016–2026. [Google Scholar] [CrossRef]
- Chakraborty, K.; Raola, V.K. In vitro bioactive analysis and antioxidant activity of two species of seaweeds from the Gulf of Mannar. Nat. Prod. Res. 2018, 32, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Maneesh, A.; Chakraborty, K. Previously undescribed antioxidative O-heterocyclic angiotensin converting enzyme inhibitors from the intertidal seaweed Sargassum wightii as potential antihypertensives. Food Res. Inter. 2018, 113, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2018, 32, 2756–2765. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Unprecedented antioxidative cyclic ether from the red seaweed Kappaphycus alvarezii with anticyclooxygenase and lipoxidase activities. Nat. Prod. Res. 2017, 31, 1131–1141. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Panchagnula, G.K.; Gottumukkala, A.L.; Subbaraju, G.V. Synthesis, structural revision, and biological activities of 4−-chloroaurone, a metabolite of marine brown alga Spatoglossum variabile. Tetrahedron 2007, 63, 6909–6914. [Google Scholar] [CrossRef]
- Maneesh, A.; Chakraborty, K. Unprecedented antioxidative and anti-inflammatory aryl polyketides from the brown seaweed Sargassum wightii. Food Res. Inter. 2017, 100, 640–649. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Joy, M.; Raola, V.K. Characterization of substituted aryl meroterpenoids from red seaweed Hypnea musciformis as potential antioxidants. Food Chem. 2016, 212, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Dhara, S. First report of substituted 2H-pyranoids from brown seaweed Turbinaria conoides with antioxidant and anti-inflammatory activities. Nat. Prod. Res. 2020, 34, 3451–3461. [Google Scholar] [CrossRef]
- Iwasaki, S.; Widjaja-Adhi, M.; Koide, A.; Kaga, T.; Nakano, S.; Beppu, F.; Hosokawa, M.; Miyashita, K. In vivo antioxidant activity of fucoxanthin on obese/diabetes KK-Ay mice. Food Nutr. Sci. 2012, 3, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Ha, A.W.; Na, S.J.; Kim, W.K. Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet. Nutr. Res. Pract. 2013, 7, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Hydrogen atom transfer (HAT)-based assays | 2,2-azino-bis (3-ethyl benzothiazoline-6-sulfonic acid) diammonium salt (ABTS+) radical scavenging [51] β-carotene bleaching [52] crocin bleaching [53] hydrogen peroxide (H2O2) scavenging [54] hydroxyl radical averting capacity (HORAC) [55] hydroxyl scavenging [56] inhibited oxygen uptake (IOU) [57] lipid peroxidation inhibition capacity (LPIC) [58] oxygen radical absorbance capacity (ORAC) [40] photochemiluminescence (PCL) [59] total radical trapping antioxidant parameter (TRAP) [41] |
| Electron transfer (ET)-based assays | 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging [46,47] cupric reducing antioxidant capacity (CUPRAC) [60] ferric reducing antioxidant power (FRAP) [44,45] ferric thiocyanate (FTC) [61] nitric oxide radical scavenging [62] N,N-dimethyl-p-phenylene diamine (DMPD) radical scavenging [63] peroxyl radical scavenging [64] potassium ferricyanide reducing power (PFRAP) [65] superoxide anion radical scavenging [66] thiobarbituric acid reactive substances (TBARS) [67] total phenolics content (TPC) using Folin-Ciocalteu reagent [42] trolox equivalence antioxidant capacity (TEAC) using ABTS [43] |
| Other in vitro methods | ascorbic acid content [68] cellular antioxidant activity (CAA) [69] metal chelating activity [70] scavenging of phosphomolybdenum [71] scavenging of xanthine oxidase [72] |
| Compound | Isolation Source | Assay/Activity | Reference |
|---|---|---|---|
| 1 | Symphyocladia latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 14.0 μM | [74] |
| 2 | Gloiopeltis furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 = 86.2 μΜ ONOO− scavenging: 4.58 ± 0.01 μM | [75] |
| 3 | Rhodomela confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 1.60 ± 0.04 μM DPPH scavenging: IC50 = 50.6 ± 0.2 μM | [76] |
| 4 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 1.56 ± 0.02 μM DPPH scavenging: IC50 = 42.3 ± 0.2 μM; 67% | [76,77] |
| 5 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | bleomycin-dependent DNA damage deoxyribose assay | [78] |
| 6 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 1.62 ± 0.03 μM DPPH scavenging: IC50 = 40.5 ± 0.2 μM; 30% | [76,77] |
| 7 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 15.5 μM | [74] |
| 8 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 7.5 µM | [79] |
| 9 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 1.36 ± 0.01 μM DPPH scavenging: IC50 = 38.4 ± 0.2 μM | [76] |
| 10 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 2.11 ± 0.04 μM DPPH scavenging: IC50 = 7.43 ± 0.10 μM | [76] |
| 11 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 1.87 ± 0.02 μM DPPH scavenging: IC50 = 20.5 ± 0.1 μM | [76] |
| 12 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 71.0 μM DPPH scavenging: IC50 = 14.4; 18.5 μM CUPRAC Fe2+ chelation: IC50 = 44.7 μM FRAP AChE inhibition: IC50 = 13.85 nM BChE inhibition: IC50 = 38.22 nM | [74,80] |
| 13 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 8.07 μM; TEAC = 2.68 mM DPPH scavenging: IC50 = 12.4; 15.9 μM CUPRAC Fe2+ chelation: IC50 = 65.2 μM FRAP AChE inhibition: IC50 = 17.10 nM BChE inhibition: IC50 = 40.57 nM | [80,81] |
| 14 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 8.1 μM; TEAC = 2.21 mM DPPH scavenging: IC50 = 14.6; 18.5 μM CUPRAC Fe2+ chelation: IC50 = 54.6 μM FRAP AChE inhibition: IC50 = 29.88 nM BChE inhibition: IC50 = 46.51 nM | [80,81] |
| 15 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.31 mM DPPH scavenging: IC50 = 5.43 μM | [81] |
| 16 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.14 mM DPPH scavenging: IC50 = 5.70 μM | [81] |
| 17 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 27.9 μM | [82] |
| 18 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 4.37 ± 0.24 mM DPPH scavenging: IC50 = 3.82 ± 0.01 μM | [83] |
| 19 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.06 ± 0.08 mM DPPH scavenging: IC50 = 9.52 ± 0.04 μM | [76] |
| 20 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 24.0 μM | [74] |
| 21 | Polysiphonia morrowii, Polysiphonia urceolata, R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 20.3 μM cytoprotective effect against cellular oxidative stress HO-1 activity and expression in keratinocytes Nrf2 expression Nrf2 nuclear translocation | [84,85] |
| 22 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 1.32 ± 0.02 mM DPPH scavenging: IC50 = 58.2 ± 0.4 μM | [76] |
| 23 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 35.8 μM | [84] |
| 24 | R. confervoides, Vertebrata lanosa (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 1.09 ± 0.01 mM CAA CLPAA DPPH scavenging: IC50 = 32.0 ± 0.1 μM ORAC | [76,86] |
| 25 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 24.7 μM | [74] |
| 26 | Cladophora wrightiana (Chlorophyta, Ulvophyceae, Cladophorales) | DPPH scavenging: 69% at 160 μM OH scavenging O2− scavenging protective effect against UVB-induced apoptosis and DNA damage in HaCaT cells scavenging activity against H2O2- or UVB-generated intracellular ROS in HaCaT cells | [87] |
| 27 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 1.86 ± 0.02 mM DPPH scavenging: IC50 = 50.3 ± 0.3 μM | [76] |
| 28 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.11 mM DPPH scavenging: IC50 = 23.6 μM | [81] |
| 29 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 1.98 ± 0.01 mM DPPH scavenging: IC50 = 30.9 ± 0.1 μM | [76] |
| 30 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.35 ± 0.02 mM DPPH scavenging: IC50 = 26.3 ± 0.2 μM | [76] |
| 31 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.87 ± 0.11 mM DPPH scavenging: IC50 = 19.8 ± 0.1 μM | [76] |
| 32 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.07 ± 0.12 mM DPPH scavenging: IC50 = 30.2 ± 0.2 μM | [76] |
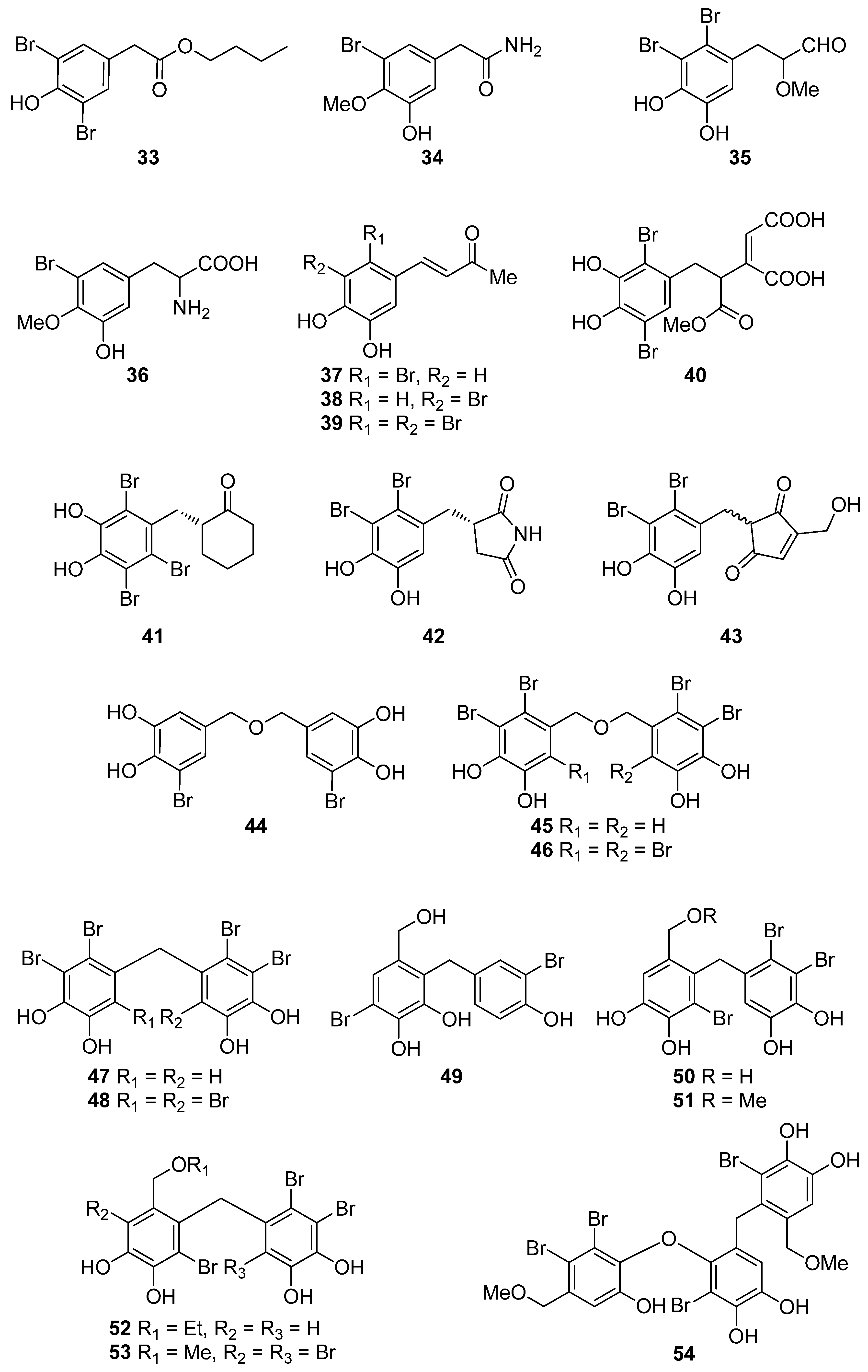
| 33 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 16.1 ± 0.1 μM | [88] |
| 34 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.36 mM DPPH scavenging: IC50 = 20.8 μM | [81] |
| 35 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.11 ± 0.11 mM DPPH scavenging: IC50 = 18.6 ± 0.1 μM | [76] |
| 36 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 1.63 ± 0.01 mM DPPH scavenging: IC50 = 50.9 ± 0.3 μM | [76] |
| 37 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.68 ± 0.12 mM DPPH scavenging: IC50 = 8.72 ± 0.05 μM | [76] |
| 38 | P. urceolata, R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.10 ± 0.13 mM DPPH scavenging: IC50 = 9.40 ± 0.05; 9.67 ± 0.04 μM | [76,88] |
| 39 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.45 ± 0.12 mM DPPH scavenging: IC50 = 7.62 ± 0.01 μM | [76] |
| 40 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 43.8 μM | [82] |
| 41 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 8.5 µM | [79] |
| 42 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.87 mM DPPH scavenging: IC50 = 5.22 μM | [81] |
| 43 | Odonthalia corymbifera (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 17.3 ± 0.1 μM Cu2+-chelation: IC50 = 61.9 ± 0.1 μM CUPRAC: ECA0.50 = 13.6 ± 0.1 μM DPPH scavenging: IC50 = 24.7 ± 0.0 μM FRAP: ECA0.50 = 11.1 ± 0.1 μM tyrosinase inhibition: IC50 = 17.3 ± 0.1 μM | [89] |
| 44 | P. morrowii (Rhodophyta, Florideophyceae, Ceramiales) | LPS-induced ROS generation and ROS-mediated ERK signaling in RAW 264.7 macrophages | [90] |
| 45 | R. confervoides, V. lanosa (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.05 mM CAA CLPAA DPPH scavenging: IC50 = 17.6 μM ORAC | [81,86] |
| 46 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 8.5 μM | [74] |
| 47 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.18 mM DPPH scavenging: IC50 = 16.9 μM; 27% | [77,81] |
| 48 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 8.1 μM | [74] |
| 49 | Avrainvillea sp. (Chlorophyta, Ulvophyceae, Bryopsidales) | DPPH scavenging: strong exogenous ROS scavenging in TPA-treated HL-60 cells (DCFH-DA): IC50 = 6.1 μM | [91] |
| 50 | R. confervoides, V. lanosa (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.16 mM CAA CLPAA DPPH scavenging: IC50 = 19.6 μM ORAC | [81,86] |
| 51 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.00 mM DPPH scavenging: IC50 = 14.3 μM | [81] |
| 52 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 2.78 mM DPPH scavenging: IC50 = 13.8 μM | [81] |
| 53 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: ΙC50 = 10.5 μM | [74] |
| 54 | O. corymbifera (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: IC50 = 6.7 ± 0.1 μM Cu2+-chelation: IC50 = 74.3 ± 0.1 μM CUPRAC: ECA0.50 = 7.8 ± 0.1 μM DPPH scavenging: IC50 = 13.5 ± 0.0 μM FRAP: ECA0.50 = 10.8 ± 0.1 μM tyrosinase inhibition: ΙC50 = 31.0 ± 0.1 μM | [90] |
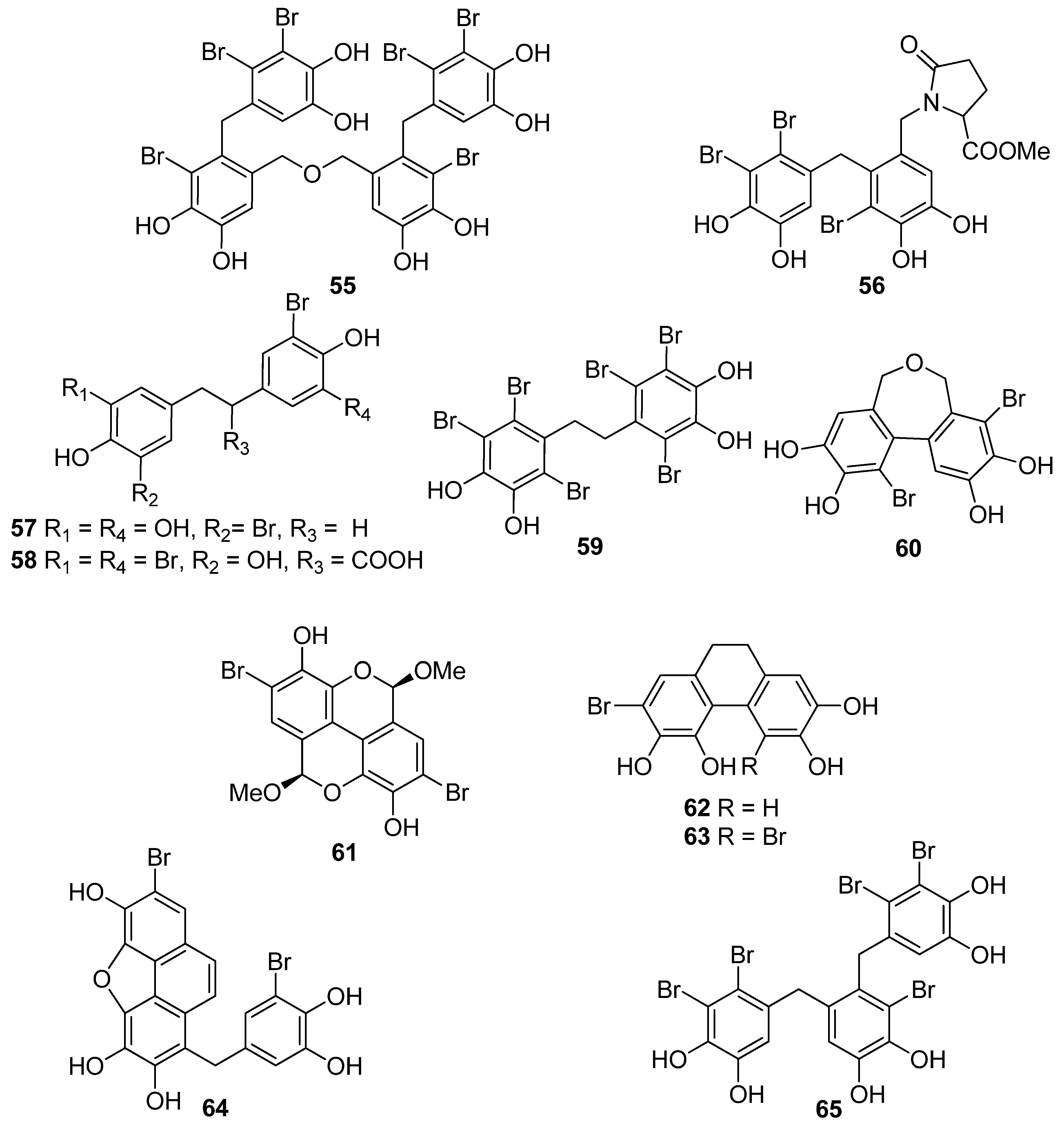
| 55 | V. lanosa (Rhodophyta, Florideophyceae, Ceramiales) | CAA CLPAA ORAC | [86] |
| 56 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.21 mM DPPH scavenging: IC50 = 13.6 μM | [81] |
| 57 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 19.6 ± 0.1 μM | [88] |
| 58 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 21.9 ± 0.1 μM | [88] |
| 59 | S. latiuscula (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 10.2 μM | [74] |
| 60 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 8.1 μM | [84] |
| 61 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 15.1 μM | [84] |
| 62 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 6.8 μM | [84] |
| 63 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 6.1 μM | [84] |
| 64 | P. urceolata (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: IC50 = 7.9 μM | [92] |
| 65 | R. confervoides (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: TEAC = 3.58 mM DPPH scavenging: IC50 = 8.90 μM | [81] |
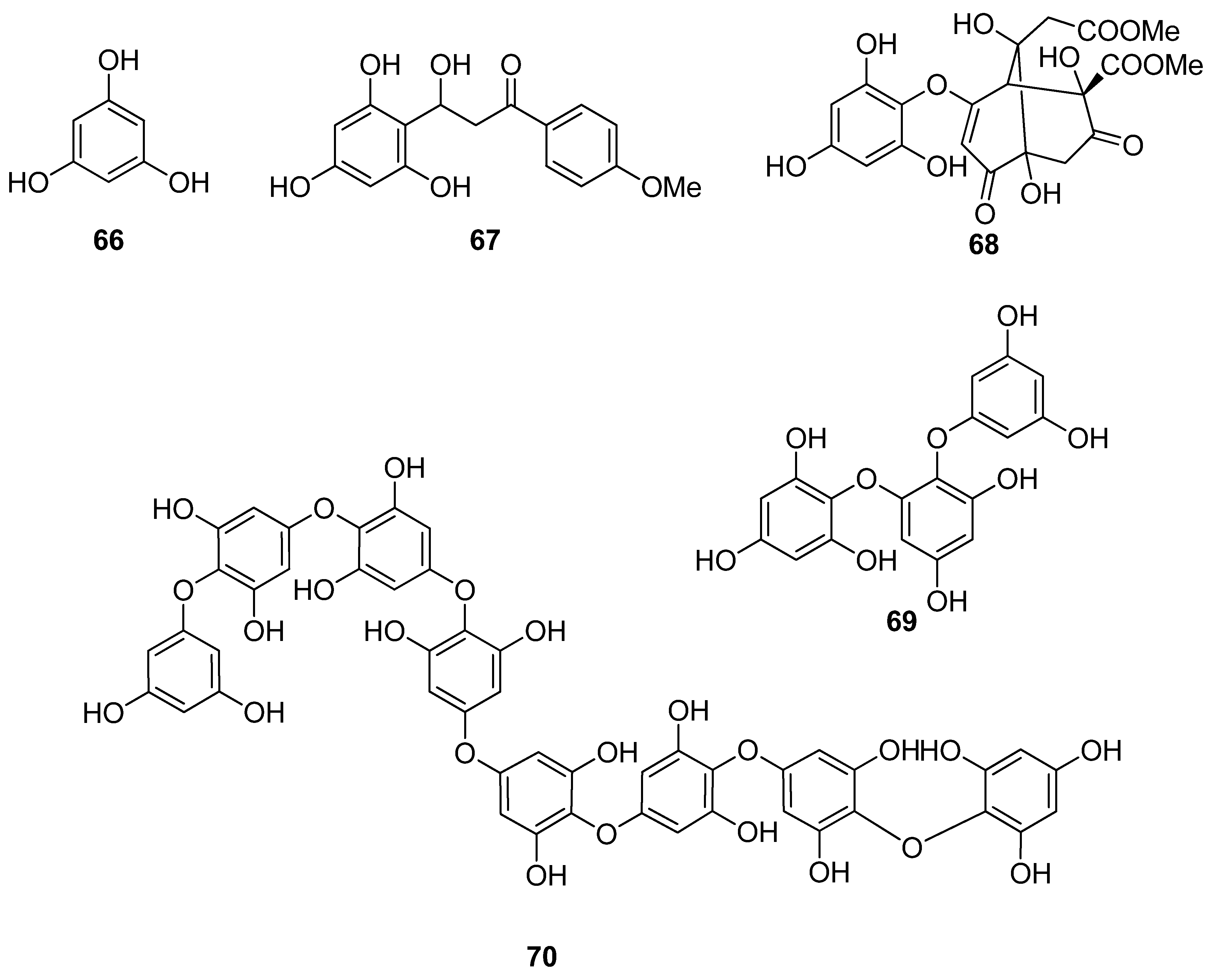
| 66 | Sargassum wightii, Sargassum tenerrimum, Turbinaria conoides (Ochrophyta, Phaeophyceae, Fucales) Ishige okamurae (Ochrophyta, Phaeophyceae, Ishigeales) Ecklonia cava (Ochrophyta, Phaeophyceae, Laminariales) | alkyl scavenging: IC50 = 103.5 ± 1.9 μM DPPH scavenging: 64.71–71.07% at 200 μg/mL H2O2 scavenging: 88.33–89.7% at 200 μg/mL OH scavenging: IC50 = 392.5 ± 2.8; 408.5 ± 3.7 μM O2− scavenging: IC50 = 115.2 ± 2.5; 124.7 ± 2.4 μM ROO scavenging: IC50 = 128.9 ± 2.2 μM metal chelating activity: 11.40–14.38% at 200 μg/mL H2O2-induced apoptosis, cytotoxicity, DNA damage, mitochondrial dysfunction and ROS generation in HaCaT keratinocytes intracellular ROS generation (DCFH-DA) in RAW 264.7 macrophages/V79-4 cells Nrf2/HO-1 signaling pathway in HaCaT keratinocytes | [93,94,95,96,97] |
| 67 | Gracilaria sp. (Rhodophyta, Florideophyceae, Gracilariales) | DPPH scavenging: 83.8 ± 2.6% XO inhibition: 64.7 ± 0.7% | [98] |
| 68 | Sargassum micracanthum (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 47 μM | [99] |
| 69 | E. cava (Ochrophyta, Phaeophyceae, Laminariales) | oxidative stress-induced DNA damage in V79-4 cells | [100] |
| 70 | Ishige foliacea (Ochrophyta, Phaeophyceae, Ishigeales) | enzyme activity (SOD, CAT, GPx) intracellular ROS generation and lipid peroxidation in HUVEC/pancreatic β cells oxidative stress-induced cell death in zebrafish embryo streptozotocin-induced pancreatic β cell damage in rat insulinoma cell line | [101,102] |
| 71 | E. cava, Ecklonia kurome, Ecklonia stolonifera, Eisenia bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | DPPH scavenging: IC50 = 11.5; 22.9 ± 0.52; 26 µM OH scavenging: IC50 = 51.8 ± 2.5 µM O2− scavenging: IC50 = 26.5 ± 1.25; 107 µM ROO scavenging: IC50 = 28.4 ± 1.5 µM inhibitory effect on total ROS: IC50 = 4.04 ± 0.04 µM cellular membrane protein oxidation in RAW 264.7 macrophages GSH levels in HepG2 cells/RAW 264.7 macrophages HO-1 expression H2O2-induced lipid peroxidation (TBARS) in V79-4 cells intracellular ROS generation (DCFH-DA) and oxidative stress induced cell damage in lung fibroblast cells MPO activity in HL60 cells Nrf2 nuclear translocation and activation PM10 (particulate matter of less than 10 mm) -induced lipid peroxidation and cytokine expression in human epidermal keratinocytes | [95,103,104,105,106,107,108] |
| 72 | E. stolonifera (Ochrophyta, Phaeophyceae, Laminariales) | DPPH scavenging: IC50 = 8.8 ± 0.4 μM intracellular ROS scavenging | [109] |
| 73 | I. okamurae (Ochrophyta, Phaeophyceae, Ishigeales) | alkyl scavenging: IC50 = 18.8 ± 1.2 μM DPPH scavenging: IC50 = 10.5 ± 0.5 μM OH scavenging: IC50 = 27.1 ± 0.9 μM O2− scavenging: IC50 = 16.7 ± 0.6 μM H2O2-induced oxidative stress-induced ROS generation (DCFH-DA) in murine hippocampal neuronal cells intracellular Ca2+ level lipid peroxidation assay (TBARS) membrane protein oxidation MPO activity PM2.5 (fine particulate matter with a diameter ≤2.5 μm) -induced ROS generation in human keratinocytes PM2.5-induced DNA damage, endoplasmic reticulum stress and autophagy, mitochondrial damage, apoptosis via MAPK signaling pathways | [97,110,111] |
| 74 | E. cava (Ochrophyta, Phaeophyceae, Laminariales) | DPPH scavenging: IC50 = 18.6 ± 1.0 μM OH scavenging: IC50 = 39.6± 2.1 μM O2− scavenging: IC50 = 21.9 ± 1.8 μM ROO scavenging: IC50 = 22.7 ± 1.5 μM cellular membrane protein oxidation in RAW 264.7 cells GSH levels in RAW 264.7 cells intracellular ROS generation (DCFH-DA) MPO activity in HL60 cells | [95] |
| 75 | E. cava, E. kurome, E. stolonifera, E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | DPPH scavenging: IC50 = 6.2 ± 0.4; 8.28 ± 0.45; 13 μM OH scavenging: IC50 = 28.6 ±2.5 μM O2− scavenging: IC50 = 7.6; 16.2 ±1.0 μM ROO scavenging: IC50 = 14.5 ±1.8 μM apoptosis in Hep3B cells cellular membrane protein oxidation in RAW 264.7 cells detection of apoptosis-related proteins GSH levels in RAW 264.7 cells intracellular ROS generation (DCFH-DA) in RAW 264.7 cells MPO activity in HL60 cells PM10 (particulate matter of less than 10 mm) -induced lipid peroxidation and cytokine expression in human epidermal keratinocytes rotenone-induced oxidative stress in SH-SY5Y cells | [95,107,108,109,112,113] |
| 76 | Fucus spiralis (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: Q50 = 0.090 ± 0.002 μmol | [114] |
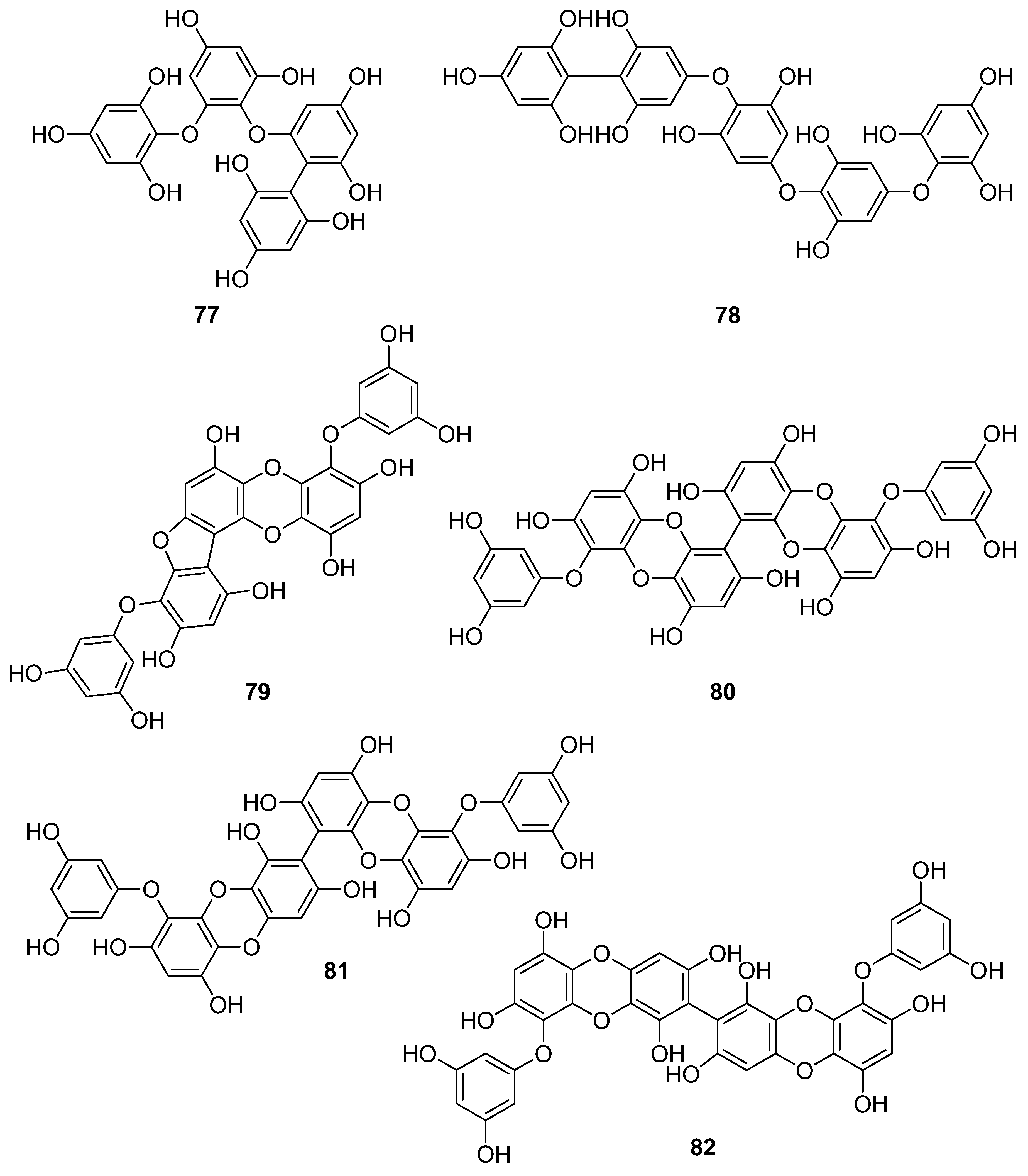
| 77 | E. cava (Ochrophyta, Phaeophyceae, Laminariales) | DPPH scavenging: IC50 = 0.60; 14.7 ± 1.2 μM OH scavenging: IC50 = 3.5 ± 1.55 μM O2− scavenging: IC50 = 18.6 ± 1.5 μM ROO scavenging: IC50 = 18.1 ± 1.0 μM cellular membrane protein oxidation in RAW 264.7 cells GSH levels in RAW 264.7 cells intracellular ROS generation (DCFH-DA) intracellular ROS detection in UVB-irradiated HaCaT keratinocytes MPO activity in HL60 cells | [95,115,116] |
| 78 | Fucus vesiculosus (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: ΙC50 = 16.1 ± 1.0 μM O2− scavenging: ΙC50 > 401.6 μM ORAC: 3.3 ± 0.3 units at 1 μg/mL | [117] |
| 79 | E. cava, E. kurome, E. stolonifera, E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | alkyl scavenging: IC50 = 3.9 μM DPPH scavenging: IC50 = 4.7 ± 0.3; 10.3; 12; 17.7 ± 0.8 μM OH scavenging: IC50 = 21.4; 39.2± 1.8 μM O2− scavenging: IC50 = 8.4 μM; IC50 = 21.6 ± 2.2 μM ROO scavenging: IC50 = 21.4 ± 2.1 μM total ROS generation: IC50 = 3.80 ± 0.09 μM intracellular ROS generation (DCFH-DA) in RAW 264.7 macrophages/Vero cells/zebrafish system | [95,105,108,109,118] |
| 80 | I. okamurae (Ochrophyta, Phaeophyceae, Ishigeales) E. cava, E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) Grateloupia elliptica (Rhodophyta, Florideophyceae, Halymeniales) | ABTS+ scavenging: IC50 = 37.1 ± 2.8 μΜ alkyl scavenging: IC50 = 17.3 ± 1.0 μM DPPH scavenging: IC50 = 8.69 ± 0.35; 9.1 ± 0.4; 28; 66.5 ± 0.5 μΜ OH scavenging: IC50 = 28.7 ± 1.1; 29.7 ± 1.5 μM O2− scavenging: IC50 = 15.4 ± 0.9; 15.9 ± 1.3 μM ROO scavenging: IC50 = 17.1 ± 2.2 μM singlet oxygen (1O2) quenching: QC50 = 30.7 ± 2.4 μM cellular membrane protein oxidation in RAW 264.7 macrophages GSH levels in RAW 264.7 macrophages high-glucose-induced oxidative stress intracellular ROS generation (DCFH-DA) in UVB-irradiated HaCaT keratinocytes MPO activity in HL60 cells | [95,97,119,120,121] |
| 81 | E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | ABTS+ scavenging: IC50 = 43.3 ± 2.3 μΜ DPPH scavenging: IC50 = 103.0 ± 3.5 μM singlet oxygen (1O2) quenching: QC50 = 35.7 ± 2.4 μM | [119] |
| 82 | E. cava, E. kurome, E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | ABTS+ scavenging: IC50 = 43.4 ± 2.0 μΜ DPPH scavenging: IC50 = 15.0; 95.9 ± 3.2 μΜ O2− scavenging: IC50 = 6.5 μM singlet oxygen (1O2) quenching: QC50 = 49.4 ± 1.7 μM H2O2-induced DNA damage intracellular ROS generation in Vero cells | [108,119] |
| 83 | E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | DPPH scavenging: IC50 = 0.86 ± 0.02 μM ONOO− scavenging: 1.80 ± 0.01 μM total ROS: 6.45 ± 0.04 μM | [122] |
| 84 | E. cava (Ochrophyta, Phaeophyceae, Laminariales) | alkyl scavenging: IC50 = 2.07 ± 1.00 μM DPPH scavenging: IC50 = 0.51 μM OH scavenging: IC50 = 75.6μM O2− scavenging: IC50 = 57.2 μM intracellular ROS generation (DCFH-DA) in H2O2-treated Vero cells | [123] |
| 85 | F. spiralis (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: Q50 = 0.087 ± 0.004 μmol | [114] |
| 86 | F. vesiculosus (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: ΙC50 = 19.3 ± 2.7 μM O2− scavenging: ΙC50 > 334.9 μM ORAC: 3.5 ± 0.2 units at 1 μg/mL | [117] |
| 87 | F. vesiculosus (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 15.8 ± 1.5 μM O2− scavenging: IC50 > 175.6 μM ORAC: 3.2 ± 0.2 units at 1 μg/mL | [117] |
| 88 | Acanthophora spicifera (Rhodophyta, Florideophyceae, Ceramiales) | lipid peroxidation and inhibition of the generation of MDA in rat liver: IC50 = 1.0 × 10−2 μM | [124] |
| 89 | A. spicifera (Rhodophyta, Florideophyceae, Ceramiales) | lipid peroxidation and inhibition of the generation of MDA in rat liver: IC50 = 1.5 × 10−2 μM | [124] |
| Compound | Isolation Source | Assay/Activity | Reference |
|---|---|---|---|
| 90 | Plocamium sp. (Rhodophyta, Florideophyceae, Plocamiales) | DPPH scavenging: IC50 = 0.05 ± 0.01 mM H2O2 scavenging: IC50 = 5.58 ± 1.11 mM NO scavenging: IC50 = 4.18 ± 0.22 mM reducing power (Fe3+ to Fe2+ reduction) | [128] |
| 91 | Ulva fasciata (Chlorophyta, Ulvophyceae, Ulvales) | ABTS+ scavenging: 66.8 ± 1.5% at 50 μM DPPH scavenging: IC50 = 13.74 ± 1.38 mM | [129] |
| 92 | Pyropia orbicularis (Rhodophyta, Bangiophyceae, Bangiales) | activation of antioxidant responses during desiccation | [130] |
| 93 | U. fasciata (Chlorophyta, Ulvophyceae, Ulvales) | ABTS+ scavenging DPPH scavenging: IC50 = 80.56 ± 2.43 mM | [129] |
| 94 | U. fasciata (Chlorophyta, Ulvophyceae, Ulvales) | ABTS+ scavenging DPPH scavenging: IC50 = 23.60 ± 1.15 mM | [129] |
| 95 | U. fasciata (Chlorophyta, Ulvophyceae, Ulvales) | ABTS+ scavenging DPPH scavenging: IC50 = 20.83 ± 0.92 mM | [129] |
| 96 | U. fasciata (Chlorophyta, Ulvophyceae, Ulvales) | ABTS+ scavenging: 78 ± 1.9% at 50 μM DPPH scavenging: IC50 = 10.24 ± 0.98 mM | [129] |
| 97 | Laurencia tristicha (Rhodophyta, Florideophyceae, Ceramiales) | alcohol-induced oxidative injury in rats enzyme activity (SOD, CAT, GPx) D-galactose-induced oxidation in mice endogenous apoptosis-related genes’ expression (BAX, cytochrome c, cytochrome P450, BCL-2, Caspase-9 and Caspase-3) GSH content lipid peroxidation | [131,132] |
| 98 | Laurencia dendroidea (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: 30.3% at 2.12 mM H2O2 scavenging | [133] |
| 99 | L. dendroidea (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: 27.5% at 2.12 mM H2O2 scavenging | [133] |
| 100 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging IC50 = 1.18 ± 0.07 mM DPPH scavenging: IC50 = 1.08 ± 0.07 mM | [134] |
| 101 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 0.72 ± 0.09 mM DPPH scavenging: IC50 = 0.75 ± 0.03 mM | [134] |
| 102 | Cystoseira trinodis (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 24.19 ± 1.15% inhibition at 2 mM | [135] |
| 103 | C. trinodis (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 27.50 ± 1.30% inhibition at 2 mM | [135] |
| 104 | C. trinodis (Ochrophyta, Phaeophyceae, Fucales) E. stolonifera, E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | ABTS+ scavenging: 24.05 ± 2.38% inhibition at 2 mM intracellular ROS generation (DCFH-DA) intracellular GSH levels in t-BHP- and tacrine-treated HepG2 cells t-BHP- and tacrine-induced oxidative stress in HepG2 cells | [135,136] |
| 105 | C. trinodis (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 26.37 ± 0.20% inhibition at 2 mM | [135] |
| 106 | C. trinodis (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 20.41 ± 0.13% inhibition at 2 mM | [135] |
| 107 | Caulerpa racemosa (Chlorophyta, Ulvophyceae, Bryopsidales) | Alkyl scavenging: IC50 = 0.66 ± 0.05 mM OH scavenging: IC50 = 0.29 ± 0.05 mM | [137] |
| 108 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging IC50 = 0.37 ± 0.02 mM DPPH scavenging: IC50 = 0.31 ± 0.02 mM | [134] |
| 109 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 0.37 ± 0.02 mM DPPH scavenging: IC50 = 0.34 ± 0.06 mM | [134] |
| 110 | Gracilaria salicornia (Rhodophyta, Florideophyceae, Gracilariales) | ABTS+ scavenging: IC50 = 1.09 mM DPPH scavenging: IC50 = 1.33 mM | [138] |
| 111 | G. salicornia (Rhodophyta, Florideophyceae, Gracilariales) | ABTS+ scavenging: IC50 = 1.24 mM DPPH scavenging: IC50 = 1.56 mM | [138] |
| 112 | from plants and microalgae, but also from macroalgae | enzyme activity (CAT, SOD, GPx and GSH reductase) GSH and TBARS levels in hepatic tissue of lycopene-treated rats | [139] |
| 113 | from plants and microalgae, but also from macroalgae | intracellular ROS generation in LPS-stimulated RAW 264.7 macrophages LPS- and IFN-γ-induced NO generation in RAW 264.7 macrophages TPA-induced O2− generation in differentiated human promyelocytic HL-60 cells | [140,141,142] |
| 114 | from plants and microalgae, but also from macroalgae | LPS- and IFN-γ-induced NO generation in RAW 264.7 macrophages TPA-induced O2− generation in differentiated human promyelocytic HL-60 cells | [141,142] |
| 115 | from plants and microalgae, but also from macroalgae | LPS- and IFN-γ-induced NO generation in RAW 264.7 macrophages TPA-induced O2− generation in differentiated human promyelocytic HL-60 cells | [141,142] |
| 116 | from plants and microalgae, but also from macroalgae | radical scavenging enzyme (SOD2, CAT, and GPx1) regulation in irradiated cells intracellular ROS generation (DCFH-DA) in acetaldehyde-treated SH-SY5Y cells LPS- and IFN-γ-induced NO generation in RAW 264.7 macrophages Nrf2/HO-1 antioxidant pathway Nrf2 dissociation and nuclear translocation Nrf2 expression regulation in irradiated cells Nrf2-regulated enzymes expression (HO-1, NQO-1, and GST-α1) PI3K/Akt and ERK signaling pathway regulation ROS-induced oxidative stress in a rat deep-burn model regulation of free radical production (XO/reduced form of Nox) Sp1/NR1 signaling pathway regulation TPA-induced O2− generation in differentiated human promyelocytic HL-60 cells Akt/CREB and p38 kinase/MAPK signaling pathway in acetaldehyde-treated SH-SY5Y cells | [141,142,143,144,145,146,147,148,149,150,151,152] |
| 117 | from plants and microalgae, but also from macroalgae | ROO scavenging (ORAC/ESR) caspase-3/7 activation Nrf2/ARE signaling in RAW 264.7 macrophages | [153] |
| 118 | from various species of Ochrophyta | ABTS+ scavenging: 72.06 ± 0.70% inhibition at 2 mM β-carotene bleaching: 95% inhibition at 150 μg/mL DPPH scavenging: IC50 = 19.6, 206.4 μM Fe2+ chelation: IC50 = 1.52 mM FRAP: 15.2 μg TE; 24.62 mg ascorbic acid eqs/g at 1.5 mM OH scavenging: IC50 = 51.6 μM O2− scavenging ROO scavenging (ORAC/ESR) caspase-3/7 activation high glucose-induced oxidative stress in HUVEC and zebrafish model H2O2-induced intracellular ROS and cytotoxicity in fibroblast cells H2O2-induced neuronal apoptosis in SH-SY5Y cells intracellular ROS generation in SH-SY5Y cells (DCFH-DA) LPS- and IFN-γ-induced NO generation and Nrf2/ARE signaling in RAW 264.7 macrophages oxidative DNA damage PI3-K/Akt cascade/ERK signaling square wave voltammetry TPA-induced O2− generation in differentiated HL-60 cells | [142,153,154,155,156,157,158,159,160,161,162] |
| 119 | Laminaria japonica (Ochrophyta, Phaeophyceae, Laminariales) | ABTS+ scavenging DPPH scavenging OH scavenging O2− scavenging | [162] |
| 120 | L. japonica (Ochrophyta, Phaeophyceae, Laminariales) | ABTS+ scavenging DPPH scavenging OH scavenging O2− scavenging | [162] |
| 121 | L. japonica (Ochrophyta, Phaeophyceae, Laminariales) | ABTS+ scavenging DPPH scavenging OH scavenging O2− scavenging | [162] |
| 122 | from plants and microalgae, but also from macroalgae | ABTS+ scavenging: IC50 = 25.4 μM DPPH scavenging: IC50 = 68.9 μM | [163] |
| 123 | Undariopsis peterseniana (Ochrophyta, Phaeophyceae, Laminariales) | oxidative stress-mediated apoptosis | [164] |
| 124 | Sargassum horneri (Ochrophyta, Phaeophyceae, Fucales) | alkyl scavenging (ESR): IC50: 0.22 ± 0.02 mM AAPH-induced intracellular ROS in Vero cells AAPH-induced lipid peroxidation in zebrafish models in vivo NF-κB, MAPK and oxidative stress regulation in RAW 264.7 macrophages Nrf2/HO-1 pathways regulation | [165,166] |
| Compound | Isolation Source | Assay/Activity | Reference |
|---|---|---|---|
| 125 | Cymopolia barbata (Chlorophyta, Ulvophyceae, Dasycladales) | DPPH scavenging: strong exogenous ROS scavenging in TPA-treated HL-60 cells (DCFH-DA): IC50 = 4.0 μM | [91] |
| 126 | C. barbata (Chlorophyta, Ulvophyceae, Dasycladales) | DPPH scavenging: strong exogenous ROS scavenging in TPA-treated HL-60 cells (DCFH-DA): IC50 >14.6 μM | [91] |
| 127 | Cystoseira crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging DPPH scavenging: 94.1% at 230 μM O2− generation (PCL assay) TBARS assay: 66.8% inhibition at 164 μM | [177] |
| 128 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging DPPH scavenging activity: 92.5% at 230 μM O2− generation (PCL assay) TBARS assay: 66.5% inhibition at 164 μM | [177] |
| 129 | Cystoseira usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 33.3 ± 2.3 μM; 0.78 TE | [178] |
| 130 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 51.6 ± 4.8 μM; 0.50 TE | [178] |
| 131 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 44.7 ± 1.1 μM; 0.58 TE | [178] |
| 132 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 55.9 ± 9.9 μM; 0.46 TE | [178] |
| 133 | Dictyopteris undulata (Ochrophyta, Phaeophyceae, Dictyotales) | DPPH scavenging: IC50 = 71 μM | [179] |
| 134 | D. undulata (Ochrophyta, Phaeophyceae, Dictyotales) | expression of phase-2 enzymes (i.e., NQO1, GSH S-transferase, HO-1 and PRDX4) Nrf2/ARE signaling pathway oxidative stress in HT22 hippocampal neuronal cells | [180] |
| 135 | D. undulata (Ochrophyta, Phaeophyceae, Dictyotales) | DPPH scavenging: IC50 = 121 μM | [179] |
| 136 | G. salicornia (Rhodophyta, Florideophyceae, Gracilariales) | ABTS+ scavenging: IC50 = 1.88 ± 0.02 mM DPPH scavenging: IC50 = 1.51 ± 0.01 mM | [181] |
| 137 | G. salicornia (Rhodophyta, Florideophyceae, Gracilariales) | ABTS+ scavenging: IC50 = 1.96 ± 0.01 mM DPPH scavenging: IC50 = 1.85 ± 0.02 mM | [181] |
| 138 | G. salicornia (Rhodophyta, Florideophyceae, Gracilariales) | ABTS+ scavenging: IC50 = 1.57 ± 0.02 mM DPPH scavenging: IC50 = 1.33 ± 0.01 mM | [181] |
| 139 | D. undulata (Ochrophyta, Phaeophyceae, Dictyotales) | DPPH scavenging: IC50 = 145 μM | [179] |
| 140 | G. salicornia (Rhodophyta, Florideophyceae, Gracilariales) | ABTS+ scavenging: IC50 = 1.50 mM DPPH scavenging: IC50 = 1.40 mM | [182] |
| 141 | G. salicornia (Rhodophyta, Florideophyceae, Gracilariales) | ABTS+ scavenging: IC50 = 1.33 mM DPPH scavenging: IC50 = 1.17 mM | [182] |
| 142 | S. micracanthum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 25.5 μM lipid peroxidation in rat liver: IC50 = 0.26 μM | [183] |
| 143 | S. micracanthum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 3.0% at 0.23 mM lipid peroxidation in rat liver: IC50 = 2.22 μM | [184] |
| 144 | Cystoseira abies-marina (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 29% at 1.06 mM | [185] |
| 145 | C. abies-marina (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 30% at 1.02 mM | [185] |
| 146 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging DPPH scavenging: 94.4% at 230 μM O2− radical generation (PCL assay) TBARS: 70.8% inhibition at 164 μM | [177] |
| 147 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.14 mM DPPH scavenging: 95.4% at 230 μM O2− radical generation (PCL assay): 1.35 TBARS: 71.8% inhibition at 164 μM | [177] |
| 148 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging DPPH scavenging: 96.1% at 230 μM O2− radical generation (PCL assay) TBARS: 68.9% inhibition at 164 μM | [177] |
| 149 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging DPPH scavenging: 95.5% at 230 μM O2− radical generation (PCL assay) TBARS: 70.3% inhibition at 164 μM | [177] |
| 150 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.37 mM DPPH scavenging: 95.5% at 230 μM O2− radical generation (PCL assay): 1.39 TBARS: 72.2% inhibition at 164 μM | [177] |
| 151 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.09 mM DPPH scavenging: 95.7% at 230 μM O2− radical generation (PCL assay): 0.72 TBARS: 71.1% inhibition at 164 μM | [177] |
| 152 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.09 mM DPPH scavenging: 96.4% at 230 μM O2− radical generation (PCL assay): 0.59 TBARS: 73.7% inhibition at 164 μM | [177] |
| 153 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.09 mM DPPH scavenging: 96.7% at 230 μM O2− radical generation (PCL assay): 0.51 TBARS: 73.4% inhibition at 164 μM | [177] |
| 154 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.08 mM DPPH scavenging: 65.4% at 230 μM O2− radical generation (PCL assay): 1.06 TBARS: 74.9% inhibition at 164 μM | [177] |
| 155 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.28 mM DPPH scavenging: 95.8% at 230 μM O2− radical generation (PCL assay): 0.79 TBARS: 74.6% inhibition at 164 μM | [177] |
| 156 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.77 TE | [186] |
| 157 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 24.5 ± 1.6 μM; 1.06 TE | [178] |
| 158 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.77 TE | [186] |
| 159 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 26.3 ± 2.3 μM; 0.98 TE | [178] |
| 160 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.87 TE | [186] |
| 161 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 33.1 ± 5.1 μM; 0.78 TE | [178] |
| 162 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.67 TE | [186] |
| 163 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.81 TE | [186] |
| 164 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 43.1 ± 3.1 μM; 0.60 TE | [178] |
| 165 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.53 TE | [186] |
| 166 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.37 TE | [186] |
| 167 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.66 TE | [186] |
| 168 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.45 TE | [186] |
| 169 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.65 TE | [186] |
| 170 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.50 TE | [186] |
| 171 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: 0.62 TE | [186] |
| 172 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 24.4 ± 0.9 μM; 1.06 TE | [178] |
| 173 | C. usneoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 22.5 ± 2.1 μM; 1.15 TE | [178] |
| 174 | Sargassum siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.54 μM | [187] |
| 175 | Sargassum elegans, S. siliquastrum, Sargassum thunbergii (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.40; 46.9 μM ONOO scavenging: 78.03% at 23.4 μM ONOO− derived from SIN-1 scavenging: 100% at 23.4 μM electrochemistry-guided isolation of antioxidant metabolites (using square wave and cyclic voltammetry methods) | [157,187,188,189] |
| 176 | S. micracanthum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 52.6% inhibition at 143.6 μM lipid peroxidation in rat liver: IC50 = 63.6 μM | [184] |
| 177 | S. micracanthum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 32.3% inhibition at 144.0 μM lipid peroxidation in rat liver: IC50 = 1.66 μM | [184] |
| 178 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.27 μM | [187] |
| 179 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.25 μM | [187] |
| 180 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.68 μM | [187] |
| 181 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.64 μM | [187] |
| 182 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.62 μM | [187] |
| 183 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 0.21 μM | [187] |
| 184 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 23.3 μM | [187] |
| 185 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 26.1 μM | [187] |
| 186 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 25.4 μM | [187] |
| 187 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 37.9 μM | [187] |
| 188 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 35.4 μM | [187] |
| 189 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 18.7 μM | [187] |
| 190 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 25.9 μM | [187] |
| 191 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 30.4 μM | [187] |
| 192 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 47.9 μM | [187] |
| 193 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 26.3 μM | [187] |
| 194 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 25.1 μM | [187] |
| 195 | S. micracanthum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 933.3 μM lipid peroxidation in rat liver: IC50 = 2.33 μM | [183] |
| 196 | S. elegans (Ochrophyta, Phaeophyceae, Fucales) | electrochemistry-guided isolation of antioxidant metabolites (using square wave and cyclic voltammetry methods) | [157] |
| 197 | S. elegans, S. micracanthum, S. thunbergii (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 63.6; 100.2 μM; 69.82% at 250 μM ONOO scavenging: 64.18% at 23.6 μM ONOO− derived from SIN-1 scavenging activity: 75.39% at 23.6 μM electrochemistry-guided isolation of antioxidant metabolites (using square wave and cyclic voltammetry methods) | [157,188,189,190] |
| 198 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging DPPH scavenging: 29.0% at 230 μM O2− generation (PCL assay) TBARS: 43.3% inhibition at 164 μM | [177] |
| 199 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: TEAC = 0.30 mM DPPH scavenging: 38.6% at 230 μM O2− generation (PCL assay): 1.41 TBARS: 54.4% inhibition at 164 μM | [177] |
| 200 | C. barbata (Ochrophyta, Phaeophyceae, Fucales) | antioxidant activity against ROS and reactive nitrogen species | [141,183,184,189] |
| 201 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 90.0% at 0.29 mM | [191] |
| 202 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 87.4% at 0.29 mM | [191] |
| 203 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | H2O2-induced lipid peroxidation in HT 1080 cells intracellular GSH level in HT 1080 cells intracellular ROS generation (DCFH-DA) in HT 1080 cells | [192] |
| 204 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 90.5% at 0.24 mM | [191] |
| 205 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 89.6% at 0.23 mM H2O2-induced lipid peroxidation in HT 1080 cells intracellular GSH level in HT 1080 cells intracellular ROS generation (DCFH-DA) in HT 1080 cells: 67.2% decrease at 11.7 μM | [191,192] |
| 206 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 87.3% at 0.23 mM H2O2-induced lipid peroxidation in HT 1080 cells intracellular GSH level in HT 1080 cells intracellular ROS generation (DCFH-DA) in HT 1080 cells: 87.2% decrease at 11.7 μM | [191,192] |
| 207 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 88.2% at 0.23 mM | [191] |
| 208 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 90.4% at 0.23 mM expression of osteoclastic marker gene in RANKL-stimulated RAW264.7 cells (TRAP, CTSK, MMP9 and CTR) NF-κB activation in RANKL-stimulated RAW264.7 cells osteoclast differentiation in RANKL-stimulated RAW264.7 cells phosphorylation of MAPKs in RANKL-stimulated RAW264.7 cells | [191,193] |
| 209 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 89.2% at 0.23 mM H2O2-induced lipid peroxidation in HT 1080 cells intracellular GSH level in HT 1080 cells intracellular ROS generation (DCFH-DA assay) in HT 1080 cells | [191,192] |
| 210 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 87.8% at 0.23 mM | [191] |
| 211 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 90.4% at 0.23 mM | [191] |
| 212 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 89.1% at 0.23 mM | [191] |
| 213 | S. micracanthum (Ochrophyta, Phaeophyceae, Fucales) | NADPH-dependent lipid peroxidation in rat microsomes: IC50 = 0.65 μM | [194] |
| 214 | S. micracanthum, S. thunbergii (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 75.4 μM; 78.85% at 250 μM ONOO scavenging: 92.69% at 23.6 μM ONOO− derived from SIN-1 scavenging: 99.51% at 23.6 μM | [188,189,190] |
| 215 | S. thunbergii (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 82.9 μM | [189] |
| 216 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | H2O2-induced lipid peroxidation in HT 1080 cells: 43.2% at 112.0 μM intracellular GSH level in HT 1080 cells intracellular ROS generation (DCFH-DA) in HT 1080 cells | [192] |
| 217 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | H2O2-induced lipid peroxidation in HT 1080 cells: 38.9% at 112.0 μM intracellular GSH level in HT 1080 cells intracellular ROS generation (DCFH-DA) in HT 1080 cells | [192] |
| 218 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 88.8% at 0.24 mM | [191] |
| 219 | S. thunbergii (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 67.8 μM ONOO scavenging: 60.0% at 11.3 μM ONOO− derived from SIN-1 scavenging: 98.6% at 11.3 μM | [195] |
| 220 | S. thunbergii (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: IC50 = 70.0 μM ONOO scavenging: 57.1% at 11.3 μM ONOO− derived from SIN-1 scavenging: 90.6% at 11.3 μM | [195] |
| 221 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 90.1% at 0.24 mM | [191] |
| 222 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 88.7% at 0.23 mM | [191] |
| 223 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 89.2% at 0.24 mM | [191] |
| 224 | S. siliquastrum (Ochrophyta, Phaeophyceae, Fucales) | DPPH scavenging: 88.7% at 0.24 mM | [191] |
| Compound | Isolation Source | Assay/Activity | Reference |
|---|---|---|---|
| 225 | Porphyra yezoensis (Rhodophyta, Bangiophyceae, Bangiales) | DPPH scavenging: IC50 = 185.2 ± 3.2 μM ORAC: 51 ± 7% TE Nrf2-regulated antioxidant response in UVA-treated fibroblasts (1BR) | [196,197] |
| 226 | G. furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 = 399.0 ± 1.1 μM ORAC: 17 ± 7% TE Nrf2-regulated antioxidant response in UVA-treated fibroblasts (1BR) | [196] |
| 227 | P. yezoensis (Rhodophyta, Bangiophyceae, Bangiales) | DPPH scavenging: IC50 = 30.8 μM | [197] |
| 228 | P. yezoensis (Rhodophyta, Bangiophyceae, Bangiales) | TBARS: 85.2% inhibition FTC: 84.1% inhibition | [198] |
| 229 | P. yezoensis (Rhodophyta, Bangiophyceae, Bangiales) | TBARS: 94.4% inhibition FTC: 89.1% inhibition | [198] |
| 230 | Martensia fragilis (Rhodophyta, Florideophyceae, Ceramiales) | DPPH scavenging: moderate exogenous ROS scavenging in TPA-treated HL-60 cells (DCFH-DA): IC50 = 11 μM | [91] |
| 231 | Dictyota coriacea (Ochrophyta, Phaeophyceae, Dictyotales) | H2O2-induced oxidative damage and toxicity in neuron-like PC12 cell Nrf2/ARE signaling pathway | [199] |
| 232 | Porphyra dioica (Rhodophyta, Bangiophyceae, Bangiales) | ORAC: 3.79 ± 0.11 μmol TE/μM | [200] |
| 233 | P. dioica (Rhodophyta, Bangiophyceae, Bangiales) | ORAC: 3.14 ± 0.32 μmol TE/μM | [200] |
| 234 | P. dioica (Rhodophyta, Bangiophyceae, Bangiales) | ORAC: 0.09 ± 0.00 μmol TE/μM | [200] |
| 235 | P. dioica (Rhodophyta, Bangiophyceae, Bangiales) | ORAC: 2.85 ± 0.42 μmol TE/μM | [200] |
| 236 | P. dioica (Rhodophyta, Bangiophyceae, Bangiales) | ORAC: 2.50 ± 0.16 μmol TE/μM | [200] |
| 237 | P. dioica (Rhodophyta, Bangiophyceae, Bangiales) | ORAC: 4.27 ± 0.15 μmol TE/μM | [200] |
| 238 | P. dioica (Rhodophyta, Bangiophyceae, Bangiales) | ORAC: 0.92 ± 0.10 μmol TE/μM | [200] |
| 239 | Porphyra sp. (Rhodophyta, Bangiophyceae, Bangiales) | ROO scavenging (CBA): 0.048 ± 0.003 mmol TE/g | [201] |
| 240 | Enteromorpha prolifera (Chlorophyta, Ulvophyceae, Ulvales) | DPPH scavenging: 88.6 ± 1.3% at 168.7 μM reducing power: 60% at 843.6 μM ROO scavenging: 50% at 843.6 μM TPC: 21.4 ± 0.1 mg GAE/g | [202] |
| 241 | from plants and microalgae, but also from macroalgae | β-carotene bleaching: 49.63% at 56.0 μM DPPH scavenging: 13.89% at 56.0 μM Fe2+ chelation: 55% at 200 μM lipid peroxidation: 95% at 100 μM ROO scavenging capacity: 308 | [141,203,204,205] |
| 242 | E. bicyclis (Ochrophyta, Phaeophyceae, Laminariales) | FTC TBARS | [206] |
| Compound | Isolation Source | MW/Sulfate Content | Assay/Activity | Reference |
|---|---|---|---|---|
| 243 | from a plethora of macroalgae | - | free radicals (DPPH, OH, NO, O2,) scavenging enzyme activity (a-glucosidase, AChE, BChE) | [210] |
| 244 | Laurencia undulata (Rhodophyta, Florideophyceae, Ceramiales) | - | alkyl scavenging: IC50 = 43.7 μM DPPH scavenging: IC50 = 39.3 μM OH scavenging: IC50 = 27.4 μM O2− scavenging: IC50 = 39.4 μM gene expression levels of GSH and SOD intracellular ROS levels (DCFH-DA) in RAW264.7 cells membrane protein oxidation MPO activity protein expression of MMP2 and MMP9 | [211] |
| 245 | L. undulata (Rhodophyta, Florideophyceae, Ceramiales) | - | alkyl scavenging: IC50 = 32.3 μM DPPH scavenging: IC50 = 41.8μM OH scavenging: IC50 = 22.7 μM O2− scavenging: IC50 = 33.6 μM gene expression levels of GSH and SOD intracellular ROS levels (DCFH-DA) in RAW264.7 cells membrane protein oxidation MPO activity protein expression of MMP2 and MMP9 | [211] |
| 246 | enzymatically produced from commercially available polysaccharides | n.d. | OH scavenging O2− scavenging erythrocyte hemolysis inhibiting lipid peroxidation metal chelating activity | [212] |
| 247 | enzymatically produced from commercially available polysaccharides | n.d. | OH scavenging O2− scavenging erythrocyte hemolysis inhibiting lipid peroxidation metal chelating activity | [212] |
| 248 | F. vesiculosus (Ochrophyta, Phaeophyceae, Fucales) | 170 kDa/44.10 ± 0.16% | OH scavenging: IC50 = 0.157 ± 0.005 mg/mL O2− scavenging: IC50 = 0.058 ± 0.011 mg/mL liver microsomal lipid peroxidation: IC50 = 1.250 ± 0.174 mg/mL | [213] |
| 249 | Cystoseira sedoides (Ochrophyta, Phaeophyceae, Fucales) | 642 kDa/16.3% | DPPH scavenging: IC50 = 0.96 ± 0.01 mg/mL | [214] |
| 250 | Cystoseira compressa (Ochrophyta, Phaeophyceae, Fucales) | 545 kDa/16.6% | DPPH scavenging: IC50 = 0.84 ± 0.06 mg/mL | [214] |
| 251 | C. crinita (Ochrophyta, Phaeophyceae, Fucales) | 339 kDa/15.7% | DPPH scavenging: IC50 = 0.76 ± 0.04 mg/mL | [214] |
| 252 | Padina gymnospora (Ochrophyta, Phaeophyceae, Dictyotales) | 200 kDa/18.40 ± 0.28% | OH scavenging O2− scavenging: IC50 = 0.243 ± 0.014 mg/mL liver microsomal lipid peroxidation: IC50 = 2.753 ± 0.051 mg/mL | [213] |
| 253 | P. gymnospora (Ochrophyta, Phaeophyceae, Dictyotales) | 18 kDa/27.57 ± 0.17% | OH scavenging: IC50 = 0.353 ± 0.036 mg/mL O2− scavenging: IC50 = 0.243 ± 0.013 mg/mL liver microsomal lipid peroxidation: IC50 = 23.887 ± 5.975 mg/mL | [213] |
| 254 | L. japonica (Ochrophyta, Phaeophyceae, Laminariales) | 742 kDa/16.5% | OH scavenging: IC50 = 0.60 mg/mL O2− scavenging: IC50 = 0.43 mg/mL | [215] |
| 255 | L. japonica (Ochrophyta, Phaeophyceae, Laminariales) | 175.9 kDa/33.5% | OH scavenging: IC50 = 0.85 mg/mL O2− scavenging: IC50 = 0.53 mg/mL | [215] |
| 256 | Undaria pinnatifida (Ochrophyta, Phaeophyceae, Laminariales) | 10 kDa/n.d. | DPPH scavenging: 8.77 ± 1.24 TE (μg/mL) OH scavenging: 86.98 ± 1.16% | [216] |
| 257 | U. pinnatifida (Ochrophyta, Phaeophyceae, Laminariales) | 300 kDa/20.01 ± 0.82% | DPPH scavenging: 9.01 ± 1.93 TE (μg/mL) OH scavenging: 74.32 ± 1.41% | [216] |
| 258 | F. vesiculosus (Ochrophyta, Phaeophyceae, Fucales) | n.d./21.1 ± 1.7% | ABTS+ scavenging DPPH scavenging lipid oxidation differential pulse voltammetry | [217] |
| 259 | F. vesiculosus (Ochrophyta, Phaeophyceae, Fucales) | n.d./21.2 ± 0.8% | ABTS+ scavenging DPPH scavenging lipid oxidation differential pulse voltammetry | [217] |
| 260 | F. vesiculosus (Ochrophyta, Phaeophyceae, Fucales) | n.d./27.0% | DPPH scavenging: IC50 = 0.035 ± 0.002 mg/mL reducing power: RC0.5AU = 1.48 mg/mL | [218] |
| 261 | Sargassum binderi (Ochrophyta, Phaeophyceae, Fucales) | n.d./n.d. | DPPH scavenging: IC50 = 2.01 ± 0.29 mg/mL OH scavenging: 60.95 ± 0.69% O2− scavenging: 26.78 ± 1.90% reducing power: 0.60 ± 0.08 mg GAE/100 g | [219] |
| 262 | hydrolyzed from commercially available polysaccharides | 5–30 kDa/n.d. | LPS-induced ROS generation in RAW 264.7 macrophages | [220] |
| 263 | not specified | n.d./n.d. | HO-1, SOD1, Nrf2 and Keap1 expression in human keratinocytes | [221] |
| 264 | U. pinnatifida (Ochrophyta, Phaeophyceae, Laminariales) | n.d./n.d. | DPPH scavenging metal chelating activity NO scavenging OH scavenging reducing power arthritis-induced physical changes in rats | [222] |
| 265 | Eucheuma spinosa (Rhodophyta, Florideophyceae, Gigartinales) | n.d./27.60 ± 0.12% | OH scavenging: IC50 = 0.281 ± 0.072 mg/mL O2− scavenging: IC50 = 0.332 ± 0.080 mg/mL liver microsomal lipid peroxidation: IC50 = 0.830 ± 0.063 mg/mL | [213] |
| 266 | Eucheuma cottonii (Rhodophyta, Florideophyceae, Gigartinales) | n.d./17.90 ± 0.05% | OH scavenging: IC50 = 0.335 ± 0.016 mg/mL O2− scavenging: IC50 = 0.112 ± 0.003 mg/mL liver microsomal lipid peroxidation: IC50 = 0.323 ± 0.011 mg/mL | [213] |
| 267 | Gigartina acicularis, Gigartina pisillata (Rhodophyta, Florideophyceae, Gigartinales) | n.d./33.38 ± 0.06% | OH scavenging: IC50 = 0.357 ± 0.120 mg/mL O2− scavenging: IC50 = 0.046 ± 0.001 mg/mL liver microsomal lipid peroxidation: IC50 = 2.697 ± 0.267 mg/mL | [213] |
| 268 | Porphyra haitanensis (Rhodophyta, Bangiophyceae, Bangiales) | n.d./17.7% | OH scavenging: IC50 = 6.55 mg/mL O2− scavenging: ~60% at 2.5 μg/mL reducing power: 0.42 at 6.17 mg/mL | [223] |
| 269 | Ulva pertusa (Chlorophyta, Ulvophyceae, Ulvales) | n.d./19.5% | OH scavenging O2− scavenging: IC50 = 20.0 μg/mL metal chelating assay reducing power | [224] |
| 270 | U. pertusa (Chlorophyta, Ulvophyceae, Ulvales) | 151.7 kDa/n.d. | Fe2+ chelation OH scavenging: IC50 > 1 mg/mL O2− scavenging: IC50 = 22.1 μg/mL reducing power | [225] |
| 271 | U. pertusa (Chlorophyta, Ulvophyceae, Ulvales) | n.d./n.d. | Fe2+ chelation: 10% to 20% at 0.31–1.88 mg/mL OH scavenging: 3.3–37% at 0.25–1.52 mg/mL O2− scavenging: IC50 = 9.17 μg/mL reducing power | [226] |
| Compound | Isolation Source | Assay/Activity | Reference |
|---|---|---|---|
| 272 | G. furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 = 290.5 ± 1.5 μM ONOO− scavenging: IC50 = 8.45 ± 0.46 μM AChE inhibition: IC50 = 94.4 ± 1.7 μM BChE inhibition: IC50 = 242.0 ± 4.8 μM | [75] |
| 273 | G. furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 > 274.4 ONOO− scavenging: IC50 = 218.7 ± 1.5 μM AChE inhibition: IC50 = 31.2 ± 1.0 μM BChE inhibition: IC50 = 526.7 ± 6.1 μM | [75] |
| 274 | G. furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 > 195.0 μΜ ONOO− scavenging: IC50 = 28.5 ± 0.0 μM AChE inhibition: IC50 = 33.9 ± 0.9 μM BChE inhibition: IC50 > 390.0 μM | [75] |
| 275 | Cystoseira sp. (Ochrophyta, Phaeophyceae, Fucales) | guglone-induced oxidative stress and intracellular ROS measurement in Caenorhabditis elegans | [141,232] |
| 276 | G. furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 > 179.6 μΜ ONOO− scavenging: IC50 = 58.3 ± 0.3 μM AChE inhibition: IC50 = 44.9 ± 1.4 μM BChE inhibition: IC50 = 57.1 ± 2.7 μM | [75] |
| 277 | G. furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 > 165.3 μΜ ONOO− scavenging: IC50 = 52.4 ± 0.2 μM AChE inhibition: IC50 = 38.1 ± 1.4 μM BChE inhibition: IC50 = 21.7 ± 1.1 μM | [75] |
| 278 | L. undulata (Rhodophyta, Florideophyceae, Ceramiales) | alkyl scavenging: IC50 = 45.0 ± 1.6 µM DPPH scavenging: IC50 = 27.1 ± 1.1 µM OH scavenging: IC50 = 22.8 ± 0.8 µM O2− scavenging: IC50 = 33.5 ± 1.3 µM gene expression of enzymes GSH and SOD intracellular ROS levels (DCFH–DA) in RAW264.7 cells membrane protein oxidation MPO activity | [233] |
| 279 | G. furcata (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 > 220.9 μΜ ONOO− scavenging: IC50 = 206.6 ± 1.0 μM AChE inhibition: IC50 = 13.6 ± 0.5 μM BChE inhibition: IC50 = 420.1 ± 7.8 μM | [75] |
| 280 | Kappaphycus alvarezii (Rhodophyta, Florideophyceae, Gigartinales) | ABTS+ scavenging: IC50 = 3.63 ± 0.55 mM DPPH scavenging: IC50 = 3.53 ± 0.05 mM | [234] |
| 281 | K. alvarezii (Rhodophyta, Florideophyceae, Gigartinales) | ABTS+ scavenging: IC50 = 1.96 ± 0.51 mM DPPH scavenging: IC50 = 1.75 ± 0.20 mM | [234] |
| 282 | Jania rubens (Rhodophyta, Florideophyceae, Corallinales) | ABTS+ scavenging: IC50 = 1.48 mM DPPH scavenging: IC50 = 0.80 mM | [235] |
| 283 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 2.89 ± 0.04 mM DPPH scavenging: IC50 = 2.44 ± 0.11 mM Fe2+ chelation: IC50 = 3.64 ± 0.08 mM | [236] |
| 284 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 3.76 ± 0.08 mM DPPH scavenging: IC50 = 3.26 ± 0.04 mM Fe2+ chelation: IC50 = 4.65 ± 0.08 mM | [236] |
| 285 | K. alvarezii (Rhodophyta, Florideophyceae, Gigartinales) | ABTS+ scavenging: IC50 = 0.67 ± 0.25 mM DPPH scavenging: IC50 = 0.61 ± 0.06 mM | [234] |
| 286 | Gracilaria opuntia (Rhodophyta, Florideophyceae, Gracilariales | ABTS+ scavenging: IC50 = 0.50 mM DPPH scavenging: IC50 = 0.41 mM | [237] |
| 287 | C. trinodis (Ochrophyta, Phaeophyceae, Fucales) most probably as a contamination from Laurencia sp. (Rhodophyta, Florideophyceae, Ceramiales) | ABTS+ scavenging: 26.01 ± 0.01% | [135] |
| 288 | K. alvarezii (Rhodophyta, Florideophyceae, Gigartinales) | ABTS+ scavenging: IC50 = 1.30 ± 0.48 mM DPPH scavenging: IC50 = 0.97 ± 0.07 mM | [238] |
| 289 | K. alvarezii (Rhodophyta, Florideophyceae, Gigartinales) | ABTS+ scavenging: IC50 = 2.28 mM DPPH scavenging: IC50 = 2.02 mM | [235] |
| 290 | K. alvarezii (Rhodophyta, Florideophyceae, Gigartinales) | ABTS+ scavenging: IC50 = 1.42 mM DPPH scavenging: IC50 = 2.50 mM | [235] |
| 291 | Spatoglossum variabile (Ochrophyta, Phaeophyceae, Dictyotales) | O2− scavenging: IC50 = 22.2 μM | [239] |
| 292 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 1.28 ± 0.00 mM DPPH scavenging: IC50 = 1.05 ± 0.03 mM | [240] |
| 293 | Hypnea musciformis (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 = 231.2 ± 2.0 μM Fe2+ chelation: IC50 = 667.9 ± 0.8 μM lipid peroxidation (TBARS): 1.34 ± 0.01 MDAEQ/kg at 0.1 μg/mL | [241] |
| 294 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 1.81 ± 0.03 mM DPPH scavenging: IC50 = 1.2 ± 0.05 mM Fe2+ chelation: IC50 = 2.28 ± 0.03 mM | [236] |
| 295 | T. conoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 2.00 mM DPPH scavenging: IC50 = 1.71 mM | [242] |
| 296 | T. conoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 1.39 mM DPPH scavenging: IC50 = 1.29 mM | [242] |
| 297 | H. musciformis (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 = 25.0 ± 0.5 μM Fe2+ chelation: IC50 = 350.7 ± 0.5 μM lipid peroxidation (TBARS): 0.88 ± 0.01 MDAEQ/kg at 0.1 μg/mL | [241] |
| 298 | H. musciformis (Rhodophyta, Florideophyceae, Gigartinales) | DPPH scavenging: IC50 = 322.4 ± 1.1 μM Fe2+ chelation: IC50 = 5115.3 ± 2.1 μM lipid peroxidation (TBARS): 0.76 ± 0.01 MDAEQ/kg at 0.1 μg/mL | [241] |
| 299 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 0.81 ± 0.04 mM DPPH scavenging: IC50 = 0.64 ± 0.02 mM Fe2+ chelation: IC50 = 1.42 ± 0.02 mM | [236] |
| 300 | S. wightii (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 0.79 ± 0.03 mM DPPH scavenging: IC50 = 0.67 ± 0.03 mM | [240] |
| 301 | T. conoides (Ochrophyta, Phaeophyceae, Fucales) | ABTS+ scavenging: IC50 = 2.18 mM DPPH scavenging: IC50 = 1.95 mM | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tziveleka, L.-A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants 2021, 10, 1431. https://doi.org/10.3390/antiox10091431
Tziveleka L-A, Tammam MA, Tzakou O, Roussis V, Ioannou E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants. 2021; 10(9):1431. https://doi.org/10.3390/antiox10091431
Chicago/Turabian StyleTziveleka, Leto-Aikaterini, Mohamed A. Tammam, Olga Tzakou, Vassilios Roussis, and Efstathia Ioannou. 2021. "Metabolites with Antioxidant Activity from Marine Macroalgae" Antioxidants 10, no. 9: 1431. https://doi.org/10.3390/antiox10091431
APA StyleTziveleka, L.-A., Tammam, M. A., Tzakou, O., Roussis, V., & Ioannou, E. (2021). Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants, 10(9), 1431. https://doi.org/10.3390/antiox10091431










