Standardized Edible Bird’s Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin
Abstract
:1. Introduction
2. Materials and Methods
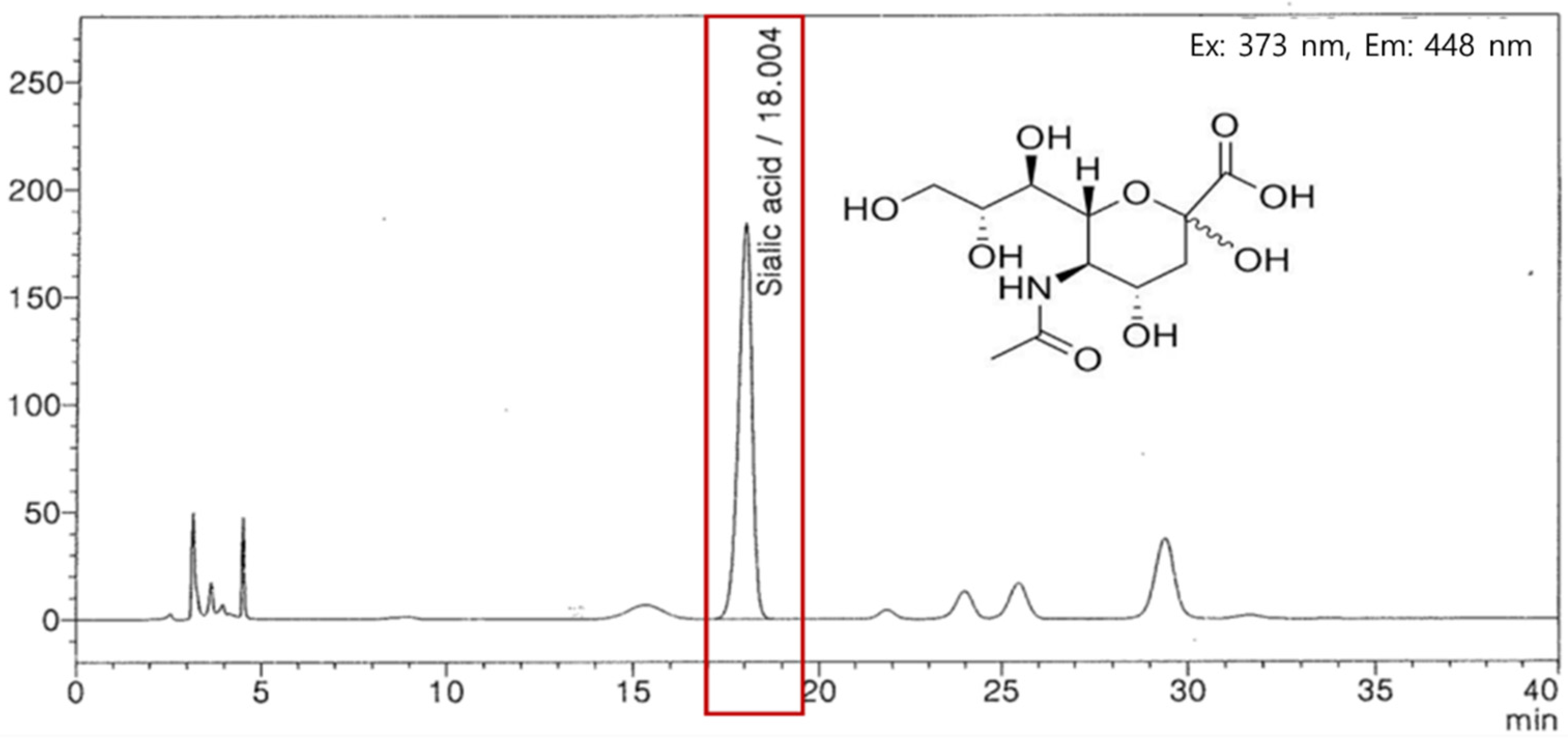
2.1. Edible Bird’s Nest Extract Preparation
2.2. Animals and UVB Irradiation
2.3. Measurement of Transepidermal Water Loss
2.4. Histological Observation
2.5. Measurement of Antioxidant Enzyme Activity in the Dorsal Skin
2.6. Cell Culture and Treatments
2.7. Protein Extraction and Western Blot Analysis
2.8. Isolation of Total RNA and Reverse Transcription-PCR
2.9. Measurement of Hyaluronic Acid and Sphingomyelin Levels
2.10. Measurement of Intracellular Melanin and Glutathione (GSH) Contents
2.11. Measurement of Tyrosinase Activity, Nitric Oxide, and cAMP Levels
2.12. Statistical Analysis
3. Results
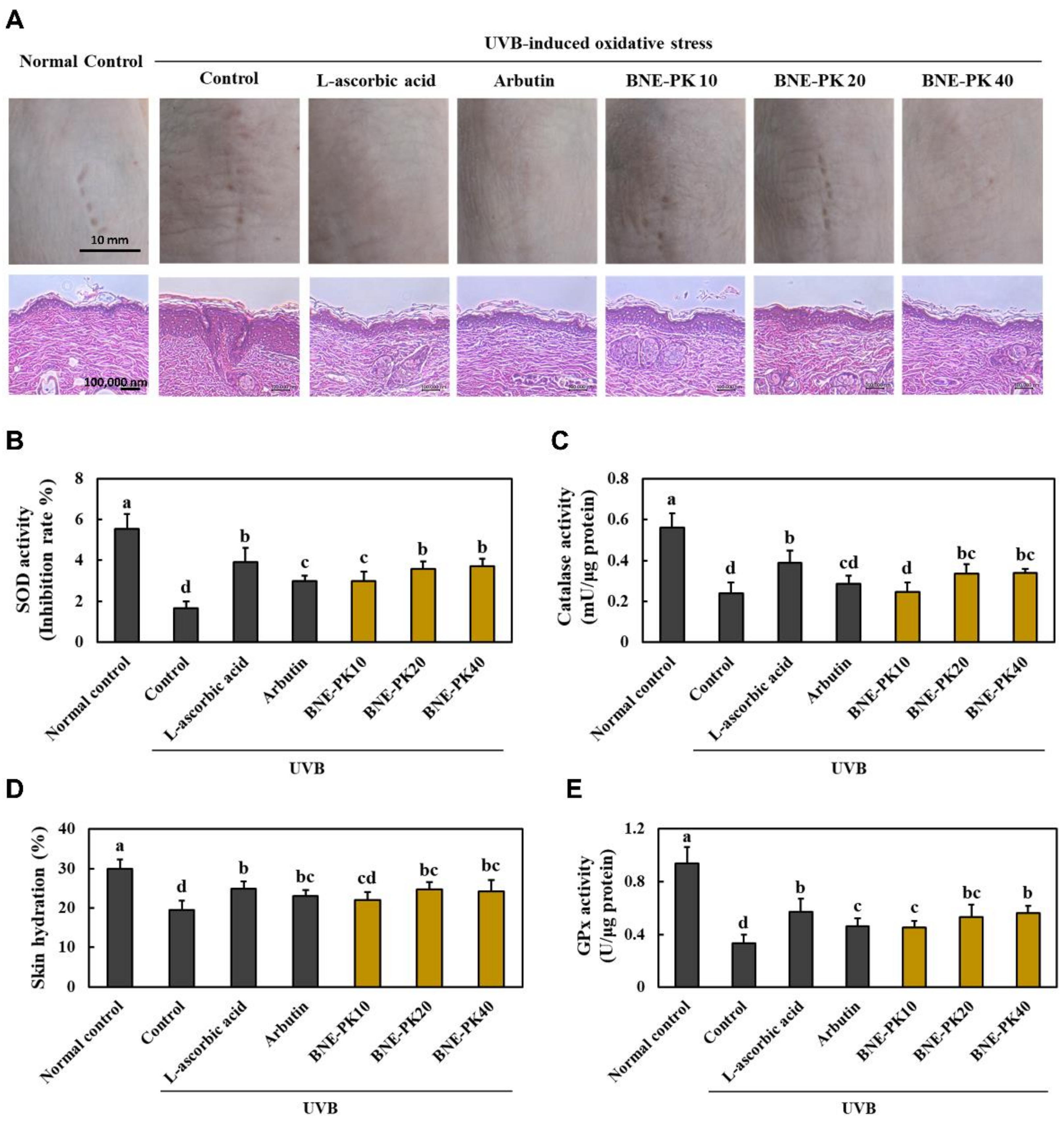
3.1. BNE-PK Suppressed Morphological and Histopathological Changes and Oxidative Stress in UVB-Irradiated Hairless Mice
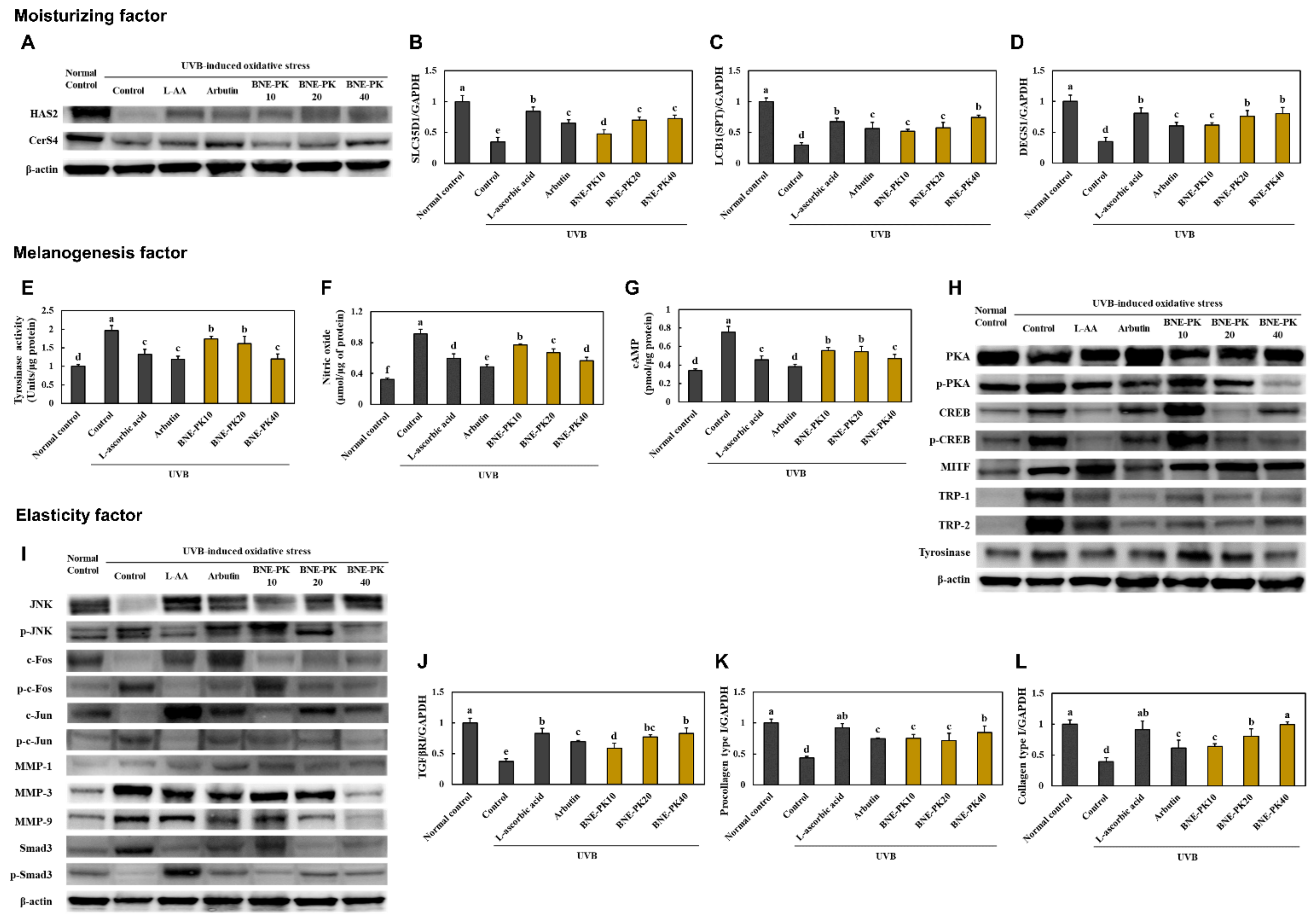
3.2. BNE-PK Increased Moisturizing and Elasticity Factors Activation and Decreased Melanogenesis Factor Activation in UVB-Irradiated Hairless Mice
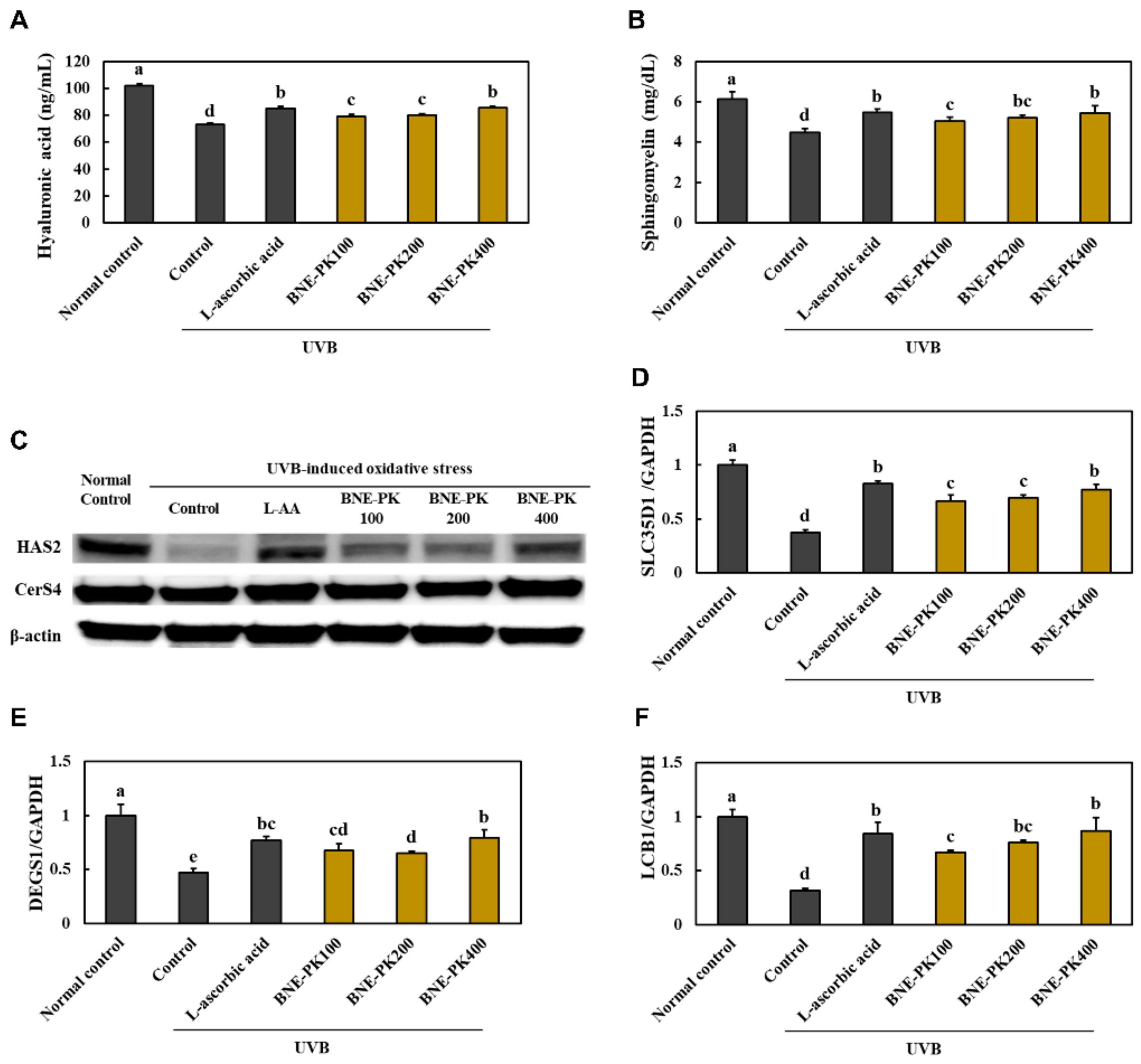
3.3. Effect of BNE-PK Treatment on Moisturizing Factors in UVB-Irradiated HaCaT Cells
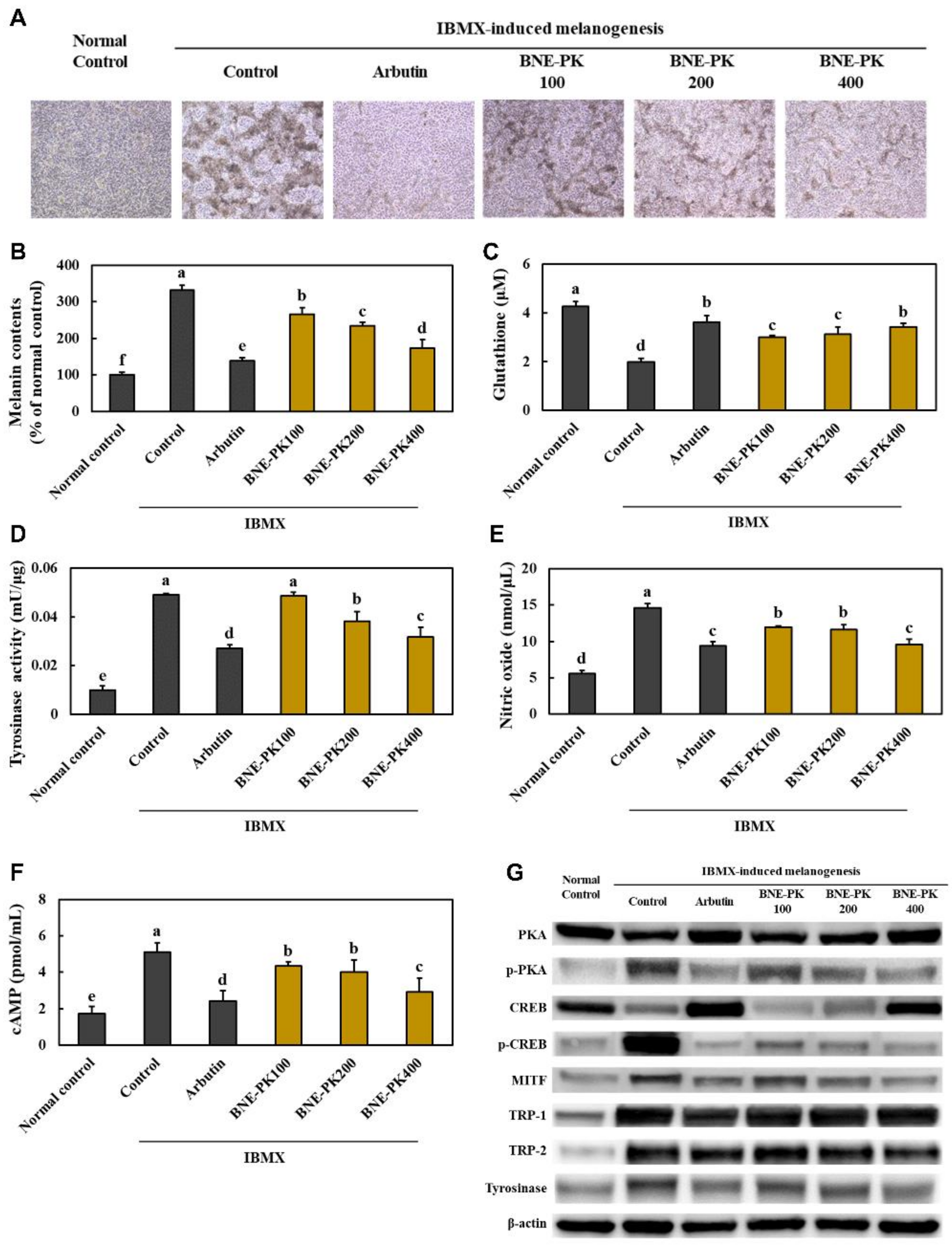
3.4. Effect of BNE-PK Treatment on Melanogenesis Factors in IBMX-Treated B16F10 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chuong, C.M.; Nickoloff, B.J.; Elias, P.M.; Goldsmith, L.A.; Macher, E.; Maderson, P.A.; Sundberg, J.P.; Tagami, H.; Plonka, P.M.; Thestrup-Pederson, K.; et al. What is the ’true’ function of skin? Exp. Dermatol. 2002, 11, 159–187. [Google Scholar]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B.; et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation-a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chen, H.C.; Chiu, H.H.; Chen, C.W.; Wang, S.M.; Wen, K.C. Neonauclea reticulata (Havil.) Merr stimulates skin regeneration after UVB exposure via ROS scavenging and modulation of the MAPK/MMPs/Collagen pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 324864. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.I.; Kim, K.S.; Ahn, H.J.; Kang, I.H.; Shin, M.K. Reduced matrix metalloproteinase and collagen transcription mediated by the TGF-beta/Smad pathway in passaged normal human dermal fibroblasts. J. Cosmet. Dermatol. 2020, 19, 1211–1218. [Google Scholar] [CrossRef]
- Rashed, A.A.; Ahmad, H.; Abdul Khalid, S.K.; Rathi, D.G. The potential use of sialic acid from edible bird′s nest to attenuate mitochondrial dysfunction by in vitro study. Front. Pharmacol. 2021, 12, 633303. [Google Scholar] [CrossRef]
- Murugan, D.D.; Md Zain, Z.; Choy, K.W.; Zamakshshari, N.H.; Choong, M.J.; Lim, Y.M.; Mustafa, M.R. Edible bird’s nest protects against hyperglycemia-induced oxidative stress and endothelial dysfunction. Front. Pharmacol. 2020, 10, 1624. [Google Scholar] [CrossRef] [Green Version]
- Careena, S.; Sani, D.; Tan, S.N.; Lim, C.W.; Hassan, S.; Norhafizah, M.; Kirby, B.P.; Ideris, A.; Stanslas, J.; Bin Basri, H.; et al. Effect of edible bird′s nest extract on lipopolysaccharide-induced impairment of learning and memory in wistar rats. Evid.-Based Complement. Altern. Med. 2018, 2018, 9318789. [Google Scholar] [CrossRef] [Green Version]
- Makrantonaki, E.; Zouboulis, C.C. Molecular mechanisms of skin aging: State of the art. Ann. N. Y. Acad. Sci. 2007, 1119, 40–50. [Google Scholar] [CrossRef]
- Zhang, X.; Rosenstein, B.S.; Wang, Y.; Lebwohl, M.; Wei, H. Identification of possible reactive oxygen species involved in ultraviolet radiationinduced oxidative DNA damage. Free Radic. Biol. Med. 1997, 23, 980–985. [Google Scholar] [CrossRef]
- Kovacs, D.; Raffa, S.; Flori, E.; Aspite, N.; Briganti, S.; Cardinali, G.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J. Dermatol. Sci. 2009, 54, 106–113. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin pigmentation and pigmentary disorders: Focus on epidermal/dermal cross-talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R.; Lee, K.J.; Lee, H.J.; Chun, P.; Byun, Y.; et al. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta 2013, 1830, 4752–4761. [Google Scholar] [CrossRef]
- Tuerxuntayi, A.; Liu, Y.Q.; Tulake, A.; Kabas, M.; Eblimit, A.; Aisa, H.A. Kaliziri extract upregulates tyrosinase, TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC Complement. Altern. Med. 2014, 14, 166. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Park, S.H.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Lee, H.S.; Cho, J.Y.; Lee, J. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-alpha/p38 MAPK-mediated pathways. Sci. Rep. 2018, 8, 14958. [Google Scholar]
- Gao, W.; Wang, Y.S.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.H. Rubus idaeus L. (red raspberry) blocks UVB-induced MMP production and promotes type I procollagen synthesis via inhibition of MAPK/AP-1, NF-kappa beta and stimulation of TGF-beta/Smad, Nrf2 in normal human dermal fibroblasts. J. Photochem. Photobiol. B 2018, 185, 241–253. [Google Scholar] [CrossRef]
- Fisher, G.J.; Shao, Y.; He, T.; Qin, Z.; Perry, D.; Voorhees, J.J.; Quan, T. Reduction of fibroblast size/mechanical force down-regulates TGF-beta type II receptor: Implications for human skin aging. Aging Cell 2016, 15, 67–76. [Google Scholar] [CrossRef]
- Wu, L.J.; He, X.Y.; Wang, W.X.; Liang, J.; Zhang, Y.D.; Liang, J.T.; Chen, D.Y. Dahuang Zhechong pills suppress silicosis fibrosis progression via p38 mapk/tgf-beta1/smad pathway in vitro. Evid.-Based Complement. Altern. Med. 2021, 2021, 6662261. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Namai, T.; Yoshino, F.; Lee, M.C.; Ishii, K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS Lett. 2007, 581, 2473–2477. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Cheng, L.J.; Shen, B.; Yuan, Z.L.; Feng, Y.Q.; Lu, S.H. Antihypertensive and antioxidant properties of sialic acid, the major component of edible bird’s nests. Curr. Top. Nutraceutical Res. 2019, 17, 376–380. [Google Scholar]
- Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012, 1253, 16–36. [Google Scholar] [CrossRef] [Green Version]
- Saengkrajang, W.; Matan, N.; Matan, N. Nutritional composition of the farmed edible bird′s nest (Collocalia fuciphaga) in Thailand. J. Food Compos. Anal. 2013, 31, 41–45. [Google Scholar] [CrossRef]
| Gene | Accession Number | Sequence |
|---|---|---|
| Procollagen Type I (M) | NM_008788.2 | F 5′-TTA CGT GGC AAG TGA GGG TTT-3′ |
| R 5′-TGT CCA GAT GCA CTT CTT GTT TG-3′ | ||
| Collagen Type I (M) | NM_007742.4 | F 5′-GAC CGT TCT ATT CCT CAG TGC AA-3′ |
| R 5′-CCC GGT GAC ACA CAA AGA CA-3′ | ||
| TGF-β RI (M) | AF271072.1 | F 5′-CAT CCT GAT GGC AAG AGC TAC A-3′ |
| R 5′-TAG TGG ATG CGG ACG TAA CCA-3′ | ||
| SLC35D1 (M) | AB117931.1 | F 5′-TTC CTC ATC GTG CTG GTC AA-3′ |
| R 5′-TTG GTG AGG GAA AAC CGT ATG-3′ | ||
| LCB1(SPT) (M) | AF003823.1 | F 5′-AGC GCC TGG CAA AGT TTA TG-3′ |
| R 5′-GTG GAG AAG CCG TAC GTG TAA AT-3′ | ||
| DEGS1 (M) | NM_007853.5 | F 5′-CCG GCG CAA GGA GAT CT-3′ |
| R 5′-TGT GGT CAG GTT TCA TCA AGG A-3′ | ||
| GAPDH (M) | NM_001289726.1 | F 5′-CAT GGC CTT CCG TGT TCC TA-3′ |
| R 5′-GCG GCA CGT CAG ATC CA-3′ | ||
| SLC35D1 (H) | AB044343.1 | F 5′-TCC TGA TCG TGG TGG TGA ATA A-3′ |
| R 5′-ACA CAT AGT GAG GAG GGA AAT CTG T-3′ | ||
| LCB1(SPT) (H) | BC068537.1 | F 5′-CCA TGG AGT GGC CTG AAA GA-3′ |
| R 5′-CTG ACA CCA TTT GGT AAC AAT CCT A-3′ | ||
| DEGS1 (H) | NM_003676.4 | F 5′-GCT GAT GGC GTC GAT GTA GA-3′ |
| R 5′-TGA AAG CGG TAC AGA AGA ACC A-3′ | ||
| GAPDH (H) | NG_007073.2 | F 5′-CCC CAC ACA CAT GCA CTT ACC-3′ |
| R 5′-TTG CCA AGT TGC CTG TCC TT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, O.-K.; Kim, D.; Lee, M.; Park, S.-H.; Yamada, W.; Eun, S.; Lee, J. Standardized Edible Bird’s Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin. Antioxidants 2021, 10, 1452. https://doi.org/10.3390/antiox10091452
Kim O-K, Kim D, Lee M, Park S-H, Yamada W, Eun S, Lee J. Standardized Edible Bird’s Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin. Antioxidants. 2021; 10(9):1452. https://doi.org/10.3390/antiox10091452
Chicago/Turabian StyleKim, Ok-Kyung, Dakyung Kim, Minhee Lee, Seong-Hoo Park, Wakana Yamada, Sangwon Eun, and Jeongmin Lee. 2021. "Standardized Edible Bird’s Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin" Antioxidants 10, no. 9: 1452. https://doi.org/10.3390/antiox10091452






