Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Samples Treatment
2.3. Hypotonic Swelling and Oxygraphic Assay
2.4. Samples Preparation for Two Dimensional Electrophoresis (2DE) and Redox Proteomic Analysis
2.5. Two-Dimensional Electrophoresis
2.6. Redox Proteomics
2.7. Statistical Analysis
3. Results
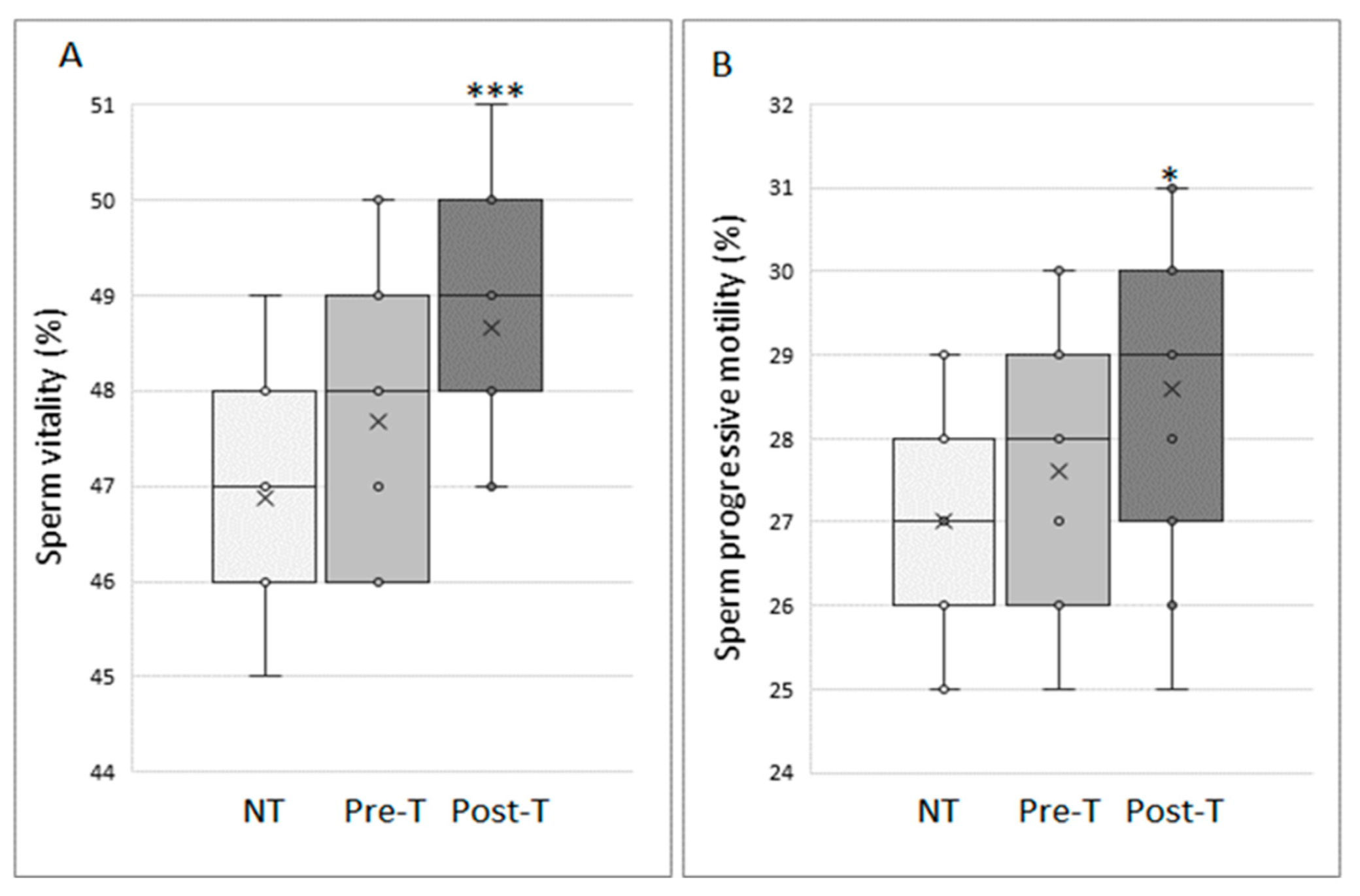
3.1. Myo-Inositol In Vitro Treatment Increases Vitality and Progressive Motility of Cryopreserved Sperm
3.2. Myo-Inositol In Vitro Treatment Increases the Oxygen Consumption Rate of Cryopreserved Sperm
3.3. Proteomic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saito, K.; Suzuki, K.; Iwasaki, A.; Yumura, Y.; Kubota, Y. Sperm Cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer 2005, 104, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, G.; Ling, K.L.E.; Hart, R.; Ralph, D.; Wafa, R.; Ashraf, A.; Jaman, N.; Mahmud, S.; Oyede, A.W. Semen quality and cryopreservation in adolescent cancer patients. Hum. Reprod. 2002, 17, 3157–3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Opuwari, C.S.; Henkel, R.R. An update on oxidative damage to spermatozoa and oocytes. Biomed. Res. Int. 2016, 2016, 9540142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Nallella, K.P.; Thomas, A.J.; Alvarez, J.G.; Sikka, S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006, 86, 878–885. [Google Scholar] [CrossRef]
- Connolly, N.M.C.; Theurey, P.; Adam-Vizi, V.; Bazan, N.G.; Bernardi, P.; Bolaños, J.P.; Culmsee, C.; Dawson, V.L.; Deshmukh, M.; Duchen, M.R.; et al. Guidelines on experimental nethods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ. 2018, 25, 542–572. [Google Scholar] [CrossRef] [Green Version]
- Bahmyari, R.; Zare, M.; Sharma, R.; Agarwal, A.; Halvaei, I. The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: A systematic review and meta-analysis. Andrologia 2020, 52, e13514. [Google Scholar] [CrossRef]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal. Res. 2011, 50, 310–318. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Diogo, P.; Dinis, M.T.; Herráez, M.P.; Sarasquete, C.; Cabrita, E. Incorporation of ascorbic acid and α-tocopherol to the extender media to enhance antioxidant system of cryopreserved sea bass sperm. Theriogenology 2012, 77, 1129–1136. [Google Scholar] [CrossRef]
- Asa, E.; Ahmadi, R.; Mahmoodi, M.; Mohammadniya, A. Supplementation of freezing media with alpha lipoic acid preserves the structural and functional characteristics of sperm against cryodamage in infertile men with asthenoteratozoospermia. Cryobiology 2020, 96, 166–174. [Google Scholar] [CrossRef]
- Governini, L.; Ponchia, R.; Artini, P.G.; Casarosa, E.; Marzi, I.; Capaldo, A.; Luddi, A.; Piomboni, P. Respiratory mitochondrial efficiency and DNA oxidation in human sperm after In Vitro Myo-Inositol treatment. J. Clin. Med. 2020, 9, E1638. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Jodar, M.; Selvaraju, S.; Sendler, E.; Diamond, M.P.; Krawetz, S.A. Reproductive medicine network the presence, role and clinical use of spermatozoal RNAs. Hum. Reprod. Update 2013, 19, 604–624. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Stendardi, A.; Focarelli, R.; Piomboni, P.; Palumberi, D.; Serafini, F.; Ferramosca, A.; Zara, V. Evaluation of mitochondrial respiratory efficiency during in vitro capacitation of human spermatozoa. Int. J. Androl. 2011, 34, 247–255. [Google Scholar] [CrossRef]
- Ferramosca, A.; Focarelli, R.; Piomboni, P.; Coppola, L.; Zara, V. Oxygen uptake by mitochondria in demembranated human spermatozoa: A reliable tool for the evaluation of sperm respiratory efficiency. Int. J. Androl. 2008, 31, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Harlow, E.; Lane, D. Bradford Assay. CSH Protoc. 2006, 2006, 1121–1132. [Google Scholar] [CrossRef]
- Bjellqvist, B.; Pasquali, C.; Ravier, F.; Sanchez, J.C.; Hochstrasser, D. A nonlinear wide-range immobilized PH gradient for two-dimensional electrophoresis and its definition in a relevant PH scale. Electrophoresis 1993, 14, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Landi, C.; Bargagli, E.; Carleo, A.; Refini, R.M.; Bennett, D.; Bianchi, L.; Cillis, G.; Prasse, A.; Bini, L.; Rottoli, P. Bronchoalveolar lavage proteomic analysis in pulmonary fibrosis associated with systemic sclerosis: S100A6 and 14-3-3ε as potential biomarkers. Rheumatology 2019, 58, 165–178. [Google Scholar] [CrossRef]
- Landi, C.; Bargagli, E.; Magi, B.; Prasse, A.; Muller-Quernheim, J.; Bini, L.; Rottoli, P. Proteome analysis of bronchoalveolar lavage in pulmonary langerhans cell histiocytosis. J. Clin. Bioinform. 2011, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Landi, C.; Cameli, P.; Vantaggiato, L.; Bergantini, L.; d’Alessandro, M.; Peruzza, M.; Carleo, A.; Shaba, E.; Di Giuseppe, F.; Angelucci, S.; et al. Ceruloplasmin and oxidative stress in severe eosinophilic asthma patients treated with mepolizumab and benralizumab. Biochim. Biophys. Acta Proteins Proteom. 2020, 1869, 140563. [Google Scholar] [CrossRef]
- Landi, C.; Vantaggiato, L.; Shaba, E.; Cameli, P.; Carleo, A.; d’Alessandro, M.; Bergantini, L.; Bargagli, E.; Bini, L. Differential redox proteomic profiles of serum from severe asthma patients after one month of benralizumab and mepolizumab treatment. Pulm. Pharmacol. Ther. 2021, 70, 102060. [Google Scholar] [CrossRef]
- Towbin, H.; Ozbey, O.; Zingel, O. An immunoblotting method for high-resolution isoelectric focusing of protein isoforms on immobilized PH gradients. Electrophoresis 2001, 22, 1887–1893. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol. Reprod. Dev. 2000, 55, 282–288. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Vitale, S.G.; Laganà, A.S.; Cimino, L.; Calogero, A.E. Myo-inositol as a male fertility molecule: Speed them up! Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 30–35. [Google Scholar]
- Canepa, P.; Dal Lago, A.; De Leo, C.; Gallo, M.; Rizzo, C.; Licata, E.; Anserini, P.; Rago, R.; Scaruffi, P. Combined treatment with myo-inositol, alpha-lipoic acid, folic acid and vitamins significantly improves sperm parameters of sub-fertile men: A multi-centric study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7078–7085. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Assaf, H.; Abd El Maged, W.M.; Elsuity, M.; Fawzy, M. Increased cryo-survival rate in ejaculated human sperm from infertile men following pre-freeze in vitro myo-inositol supplementation. Clin. Exp. Reprod. Med. 2018, 45, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Abdolsamadi, M.; Mohammadi, F.; Nashtaei, M.S.; Teimouri, M.; Sardar, R.; Dayani, M.; Haghighi, M.; Ghasemi, S.; Vatannejad, A.; Zandieh, Z. Does myoinositol supplement improve sperm parameters and DNA Integrity in patients with oligoasthenoteratozoospermia after the freezing–thawing process? Cell Tissue Bank 2020, 21, 99–106. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef]
- Artini, P.G.; Casarosa, E.; Carletti, E.; Monteleone, P.; Di Noia, A.; Di Berardino, O.M. In vitro effect of myo-inositol on sperm motility in normal and oligoasthenospermia patients undergoing in vitro fertilization. Gynecol. Endocrinol. 2017, 33, 109–112. [Google Scholar] [CrossRef]
- Marchetti, C.; Jouy, N.; Leroy-Martin, B.; Defossez, A.; Formstecher, P.; Marchetti, P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum. Reprod. 2004, 19, 2267–2276. [Google Scholar] [CrossRef] [Green Version]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar]
- Marchetti, P.; Ballot, C.; Jouy, N.; Thomas, P.; Marchetti, C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia 2012, 44, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European association of urology working group on male infertility european association of urology guidelines on male infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef]
- Luddi, A.; Governini, L.; Capaldo, A.; Campanella, G.; De Leo, V.; Piomboni, P.; Morgante, G. Characterization of the age-dependent changes in antioxidant defenses and protein’s sulfhydryl/carbonyl stress in human follicular fluid. Antioxidants 2020, 9, 927. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Luddi, A.; Capaldo, A.; Focarelli, R.; Gori, M.; Morgante, G.; Piomboni, P.; De Leo, V. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod. Biol. Endocrinol. 2016, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.-M.; Marcocci, L.; Das, D.; Wang, X.; Luo, H.; Zungu-Edmondson, M.; Suzuki, Y.J. Mechanism of protein decarbonylation. Free Radic. Biol. Med. 2013, 65, 1126–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.M.; Marcocci, L.; Liu, L.; Suzuki, Y.J. Cell signaling by protein carbonylation and decarbonylation. Antioxid. Redox Signal. 2010, 12, 393–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponchia, R.; Bruno, A.; Renzi, A.; Landi, C.; Shaba, E.; Luongo, F.P.; Haxhiu, A.; Artini, P.G.; Luddi, A.; Governini, L.; et al. Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants 2022, 11, 10. https://doi.org/10.3390/antiox11010010
Ponchia R, Bruno A, Renzi A, Landi C, Shaba E, Luongo FP, Haxhiu A, Artini PG, Luddi A, Governini L, et al. Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants. 2022; 11(1):10. https://doi.org/10.3390/antiox11010010
Chicago/Turabian StylePonchia, Rosetta, Annunziata Bruno, Asia Renzi, Claudia Landi, Enxhi Shaba, Francesca Paola Luongo, Alesandro Haxhiu, Paolo Giovanni Artini, Alice Luddi, Laura Governini, and et al. 2022. "Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol" Antioxidants 11, no. 1: 10. https://doi.org/10.3390/antiox11010010
APA StylePonchia, R., Bruno, A., Renzi, A., Landi, C., Shaba, E., Luongo, F. P., Haxhiu, A., Artini, P. G., Luddi, A., Governini, L., & Piomboni, P. (2022). Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants, 11(1), 10. https://doi.org/10.3390/antiox11010010








