Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) Counteracts UV-B Radiation-Induced ROS Formation in Corneal Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Histological Analysis
2.4. Immunohistochemistry (IHC) Analysis
2.5. Cell Cultures
2.6. Immunofluorescence Analysis
2.7. Cell Viability Assay
2.8. Fluorescence Microscopic Analysis of Cell Death
2.9. Detection of ROS
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
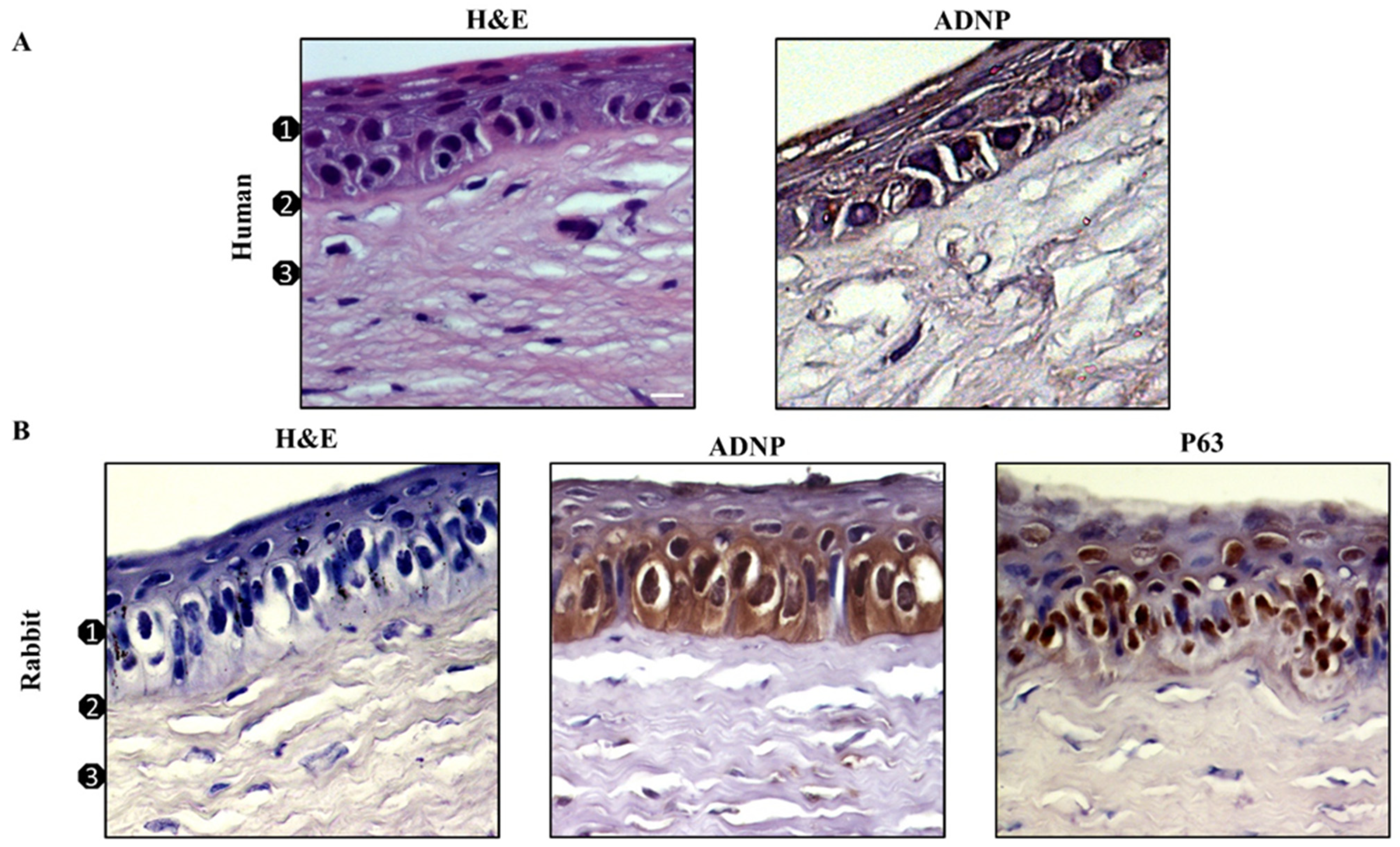
3.1. ADNP Expression in Human and Rabbit Corneal Epithelium
3.2. NAP Treatment Reduced UVB-Irradiation-Induced Apoptosis on Corneal Epithelial Cells
3.3. NAP Treatment Reduced ROS Formation Induced by UV-B Irradiation of Corneal Epithelial Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eagle, R.C. Eye Pathology: An Atlas and Text, 3rd ed.; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2016. [Google Scholar]
- Dua, H.S.; Shanmuganathan, V.A.; Powell-Richards, A.O.; Tighe, P.J.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar] [CrossRef]
- Downes, J.E.; Swann, P.G.; Holmes, R.S. Differential corneal sensitivity to ultraviolet light among inbred strains of mice. Correlation of ultraviolet B sensitivity with aldehyde dehydrogenase deficiency. Cornea 1994, 13, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kolozsvári, L.; Nógrádi, A.; Hopp, B.; Bor, Z. UV absorbance of the human cornea in the 240- to 400-nm range. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2165–2168. [Google Scholar] [PubMed]
- Cullen, A.P. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Int. J. Toxicol. 2002, 21, 455–464. [Google Scholar] [CrossRef]
- Glupker, C.D.; Boersma, P.M.; Schotanus, M.P.; Haarsma, L.D.; Ubels, J.L. Apoptosis of Corneal Epithelial Cells Caused by Ultraviolet B-induced Loss of K(+) is Inhibited by Ba(2.). Ocul. Surf. 2016, 14, 401–409. [Google Scholar] [CrossRef][Green Version]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Amenta, A.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. Protective effect of PACAP against ultraviolet B radiation-induced human corneal endothelial cell injury. Neuropeptides 2020, 79, 101978. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D’Amico, A.G.; et al. Protective Effects of PACAP in Peripheral Organs. Front. Endocrinol. (Lausanne) 2020, 11, 377. [Google Scholar] [CrossRef]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012, 166, 4–17. [Google Scholar] [CrossRef]
- Dejda, A.; Jolivel, V.; Bourgault, S.; Seaborn, T.; Fournier, A.; Vaudry, H.; Vaudry, D. Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: A better understanding towards therapeutic applications in neurodegenerative diseases. J. Mol. Neurosci. 2008, 36, 26–37. [Google Scholar] [CrossRef]
- Canonico, P.L.; Copani, A.; D’Agata, V.; Musco, S.; Petralia, S.; Travali, S.; Stivala, F.; Cavallaro, S. Activation of pituitary adenylate cyclase-activating polypeptide receptors prevents apoptotic cell death in cultured cerebellar granule cells. Ann. N.Y. Acad. Sci. 1996, 26, 470–472. [Google Scholar] [CrossRef]
- Maugeri, G.; Longo, A.; D’Amico, A.G.; Rasà, D.M.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; D’Agata, V. Trophic effect of PACAP on human corneal endothelium. Peptides 2018, 99, 20–26. [Google Scholar] [CrossRef]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 69, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Federico, C.; Saccone, S.; Morello, G.; La Cognata, V.; Cavallaro, S.; D’Agata, V. Molecular mechanisms involved in the protective effect of pituitary adenylate cyclase-activating polypeptide in an in vitro model of amyotrophic lateral sclerosis. J. Cell. Physiol. 2019, 234, 5203–5214. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Morello, G.; Reglodi, D.; Cavallaro, S.; D’Agata, V. Differential Vulnerability of Oculomotor versus Hypoglossal Nucleus during ALS: Involvement of PACAP. Front. Neurosci. 2020, 14, 805. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of Pacap on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Saccone, S.; Federico, C.; Cavallaro, S.; Reglodi, D.; D’Agata, V. PACAP Modulates the Autophagy Process in an in Vitro Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2943. [Google Scholar] [CrossRef]
- Bassan, M.; Zamostiano, R.; Davidson, A.; Pinhasov, A.; Giladi, E.; Perl, O.; Bassan, H.; Blat, C.; Gibney, G.; Glazner, G.; et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999, 72, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Zamostiano, R.; Pinhasov, A.; Gelber, E.; Steingart, R.A.; Seroussi, E.; Giladi, E.; Bassan, M.; Wollman, Y.; Eyre, H.J.; Mulley, J.C.; et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001, 276, 708–714. [Google Scholar] [CrossRef]
- Leker, R.R.; Teichner, A.; Grigoriadis, N.; Ovadia, H.; Brenneman, D.E.; Fridkin, M.; Giladi, E.; Romano, J.; Gozes, I. NAP, a femtomolar-acting peptide, protects the brain against ischemic injury by reducing apoptotic death. Stroke 2002, 33, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Beni-Adani, L.; Gozes, I.; Cohen, Y.; Assaf, Y.; Steingart, R.A.; Brenneman, D.E.; Eizenberg, O.; Trembolver, V.; Shohami, E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 2001, 296, 57–63. [Google Scholar]
- Gozes, I.; Steingart, R.A.; Spier, A.D. NAP mechanisms of neuroprotection. J. Mol. Neurosci. 2004, 24, 67–72. [Google Scholar] [CrossRef]
- Idan-Feldman, A.; Schirer, Y.; Polyzoidou, E.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.C.; Gozes, I. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol. Dis. 2011, 44, 327–339. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Nap Interferes with Hypoxia-Inducible Factors and VEGF Expression in Retina of Diabetic Rats. J. Mol. Neurosci. 2017, 61, 256–266. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. NAP counteracts hyperglycemia/hypoxia induced retinal pigment epithelial barrier breakdown through modulation of HIFs and VEGF expression. J. Cell. Physiol. 2018, 233, 1120–1128. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.; Federico, C.; Saccone, S.; Lazzara, F.; Fidilio, A.; Drago, F.; Bucolo, C.; D’Agata, V. NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell. Physiol. 2019, 234, 5230–5240. [Google Scholar] [CrossRef]
- Zheng, H.; Blat, D.; Fridkin, M. Novel neuroprotective neurotrophic NAP analogs targeting metal toxicity and oxidative stress: Potential candidates for the control of neurodegenerative diseases. J. Neural Transm. Suppl. 2006, 71, 163–172. [Google Scholar]
- Sethy, N.K.; Sharma, N.K.; Das, M.; Bhargava, K. Protein profiling reveals antioxidant and signaling activities of NAP (Davunetide) in rodent hippocampus exposed to hypobaric hypoxia. J. Mol. Neurosci. 2014, 54, 414–429. [Google Scholar] [CrossRef]
- Escher, U.; Giladi, E.; Dunay, I.R.; Bereswill, S.; Gozes, I.; Heimesaat, M.M. Anti-inflammatory Effects of the Octapeptide NAP in Human Microbiota-Associated Mice Suffering from Subacute Ileitis. Eur. J. Microbiol. Immunol. (Bp) 2018, 8, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Castrogiovanni, P.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. PACAP through EGFR transactivation preserves human corneal endothelial integrity. J. Cell. Biochem. 2019, 120, 10097–10105. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. PACAP and VIP regulate hypoxia-inducible factors in neuroblastoma cells exposed to hypoxia. Neuropeptides 2018, 69, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Saccone, S.; Federico, C.; Magro, G.; Cavallaro, S.; D’Agata, V. Caffeine Effect on HIFs/VEGF Pathway in Human Glioblastoma Cells Exposed to Hypoxia. Anti-Cancer Agents Med. Chem. 2018, 18, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Nicotine promotes blood retinal barrier damage in a model of human diabetic macular edema. Toxicol. In Vitro 2017, 44, 182–189. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Reitano, R.; Saccone, S.; Federico, C.; Parenti, R.; Magro, G.; D’Agata, V. Expression profile of Wilms Tumor 1 (WT1) isoforms in undifferentiated and all-trans retinoic acid differentiated neuroblastoma cells. Genes Cancer 2016, 7, 47–58. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef]
- Mandel, S.; Spivak-Pohis, I.; Gozes, I. ADNP Differential Nucleus/Cytoplasm Localization in Neurons Suggests Multiple Roles in Neuronal Differentiation and Maintenance. J. Mol. Neurosci. 2008, 35, 127–141. [Google Scholar] [CrossRef]
- Cullen, A.P.; Chou, B.R.; Hall, M.G.; Jany, S.E. Ultraviolet-B damages corneal endothelium. Am. J. Optom. Physiol. Opt. 1984, 61, 473–478. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ohgami, K.; Shiratori, K.; Jin, X.H.; Ilieva, I.; Koyama, Y.; Yazawa, K.; Yoshida, K.; Kase, S.; Ohno, S. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp. Eye Res. 2006, 82, 275–281. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef]
- Shen, H.M.; Liu, Z.G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006, 40, 928–939. [Google Scholar] [CrossRef]
- Cejkova, J.; Stipek, S.; Crkovska, J.; Ardan, T.; Midelfart, A. Reactive oxygen species (ROS)-generating oxidases in the normal rabbit cornea and their involvement in the corneal damage evoked by UVB rays. Histo. Histopath. 2001, 16, 523–533. [Google Scholar]
- Cejkova, J.; Stipek, S.; Crkovska, J.; Ardan, T.; Platenik, J.; Cejka, C.; Midelfart, A. UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol. Rev. 2004, 53, 1–10. [Google Scholar]
- Shoham, A.; Hadziahmetovic, M.; Dunaief, J.L.; Mydlarski, M.B.; Schipper, H.M. Oxidative stress in diseases of the human cornea. Free Radic. Biol. Med. 2008, 45, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Shimmura, S.; Suematsu, M.; Shimoyama, M.; Tsubota, K.; Oguchi, Y.; Ishimura, Y. Subthreshold UV radiation-induced peroxide formation in cultured corneal epithelial cells: The protective effects of lactoferrin. Exp. Eye Res. 1996, 63, 519–526. [Google Scholar] [CrossRef]
- Cullen, A.P. Ultraviolet induced lysosome activity in corneal epithelium. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1980, 214, 107–118. [Google Scholar] [CrossRef]
- Koliopoulos, J.X.; Margaritis, L.H. Response of the cornea to far ultraviolet light: An ultrastructural study. Ann. Ophthalmol. 1979, 11, 765–769. [Google Scholar]
- Redmond, T.M.; Duke, E.J.; Coles, W.H.; Simson, J.A.; Crouch, R.K. Localization of corneal superoxide dismutase by biochemical and histocytochemical techniques. Exp. Eye Res. 1984, 38, 369–378. [Google Scholar] [CrossRef]
- Jehle, T.; Dimitriu, C.; Auer, S.; Knoth, R.; Vidal-Sanz, M.; Gozes, I.; Lagrze, W.A. The neuropeptide NAP provides neuroprotection against retinal ganglion cell damage after retinal ischemia and optic nerve crush. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1255–1263. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, H.; She, H.; Liu, H.; Sun, N. Expression of peptide NAP in rat retinal Müller cells prevents hypoxia-induced retinal injuries and promotes retinal neurons growth. Biomed. Pharmacother. 2010, 64, 417–423. [Google Scholar] [CrossRef]
- Arya, A.; Meena, R.; Sethy, N.K.; Das, M.; Sharma, M.; Bhargava, K. NAP (davunetide) protects primary hippocampus culture by modulating expression profile of antioxidant genes during limiting oxygen conditions. Free Radic. Res. 2015, 49, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A.; Ichijo, H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal. 2005, 7, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.H.; Luo, S.J.; Chen, C.L.; Cheng, J.H.; Hsieh, C.Y.; Wang, C.Y.; Huang, W.C.; Su, W.C.; Lin, C.F. Vinca alkaloids cause aberrant ROS-mediated JNK activation, Mcl-1 downregulation, DNA damage, mitochondrial dysfunction, and apoptosis in lung adenocarcinoma cells. Biochem. Pharmacol. 2012, 83, 1159–1171. [Google Scholar] [CrossRef]
- Tseng, S.C.G. Concept and application of limbal stem cells. Eye 1989, 3, 141–157. [Google Scholar] [CrossRef]
- Cappuyns, E.; Huyghebaert, J.; Vandeweyer, G.; Kooy, R.F. Mutations in ADNP affect expression and subcellular localization of the protein. Cell Cycle 2018, 17, 1068–1075. [Google Scholar] [CrossRef]
- Belokopytov, M.; Shulman, S.; Dubinsky, G.; Gozes, I.; Belkin, M.; Rosner, M. Ameliorative effect of NAP on laser-induced retinal damage. Acta Ophthalmol. 2011, 89, e126–e131. [Google Scholar] [CrossRef]
- Jeon, S.H.; Park, H.M.; Kim, S.J.; Lee, M.Y.; Kim, G.B.; Rahman, M.M.; Woo, J.N.; Kim, I.S.; Kim, J.S.; Kang, H.S. Taurine reduces FK506-induced generation of ROS and activation of JNK and Bax in Madin Darby canine kidney cells. Hum. Exp. Toxicol. 2010, 29, 627–633. [Google Scholar] [CrossRef]
- Scuderi, S.; D’Amico, A.G.; Castorina, A.; Federico, C.; Marrazzo, G.; Drago, F.; Bucolo, C.; D’Agata, V. Davunetide (NAP) protects the retina against early diabetic injury by reducing apoptotic death. J. Mol. Neurosci. 2014, 54, 395–404. [Google Scholar] [CrossRef]
- Park, G.B.; Choi, Y.; Kim, Y.S.; Lee, H.K.; Kim, D.; Hur, D.Y. ROS-mediated JNK/p38-MAPK activation regulates Bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. Int. J. Oncol. 2014, 44, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Qin, Q.; Fang, C.P.; Shen, W.; Sun, T.T.; Huang, Y.L.; Li, W.J.; Deng, A.M. UVB induces apoptosis via downregulation of CALML3-dependent JNK1/2 and ERK1/2 pathways in cataract. Int. J. Mol. Med. 2018, 41, 3041–3050. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, G.; D’Amico, A.G.; Giunta, S.; Giallongo, C.; Tibullo, D.; Bucolo, C.; Saccone, S.; Federico, C.; Scollo, D.; Longo, A.; et al. Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) Counteracts UV-B Radiation-Induced ROS Formation in Corneal Epithelium. Antioxidants 2022, 11, 128. https://doi.org/10.3390/antiox11010128
Maugeri G, D’Amico AG, Giunta S, Giallongo C, Tibullo D, Bucolo C, Saccone S, Federico C, Scollo D, Longo A, et al. Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) Counteracts UV-B Radiation-Induced ROS Formation in Corneal Epithelium. Antioxidants. 2022; 11(1):128. https://doi.org/10.3390/antiox11010128
Chicago/Turabian StyleMaugeri, Grazia, Agata Grazia D’Amico, Salvatore Giunta, Cesarina Giallongo, Daniele Tibullo, Claudio Bucolo, Salvatore Saccone, Concetta Federico, Davide Scollo, Antonio Longo, and et al. 2022. "Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) Counteracts UV-B Radiation-Induced ROS Formation in Corneal Epithelium" Antioxidants 11, no. 1: 128. https://doi.org/10.3390/antiox11010128
APA StyleMaugeri, G., D’Amico, A. G., Giunta, S., Giallongo, C., Tibullo, D., Bucolo, C., Saccone, S., Federico, C., Scollo, D., Longo, A., Avitabile, T., Musumeci, G., & D’Agata, V. (2022). Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) Counteracts UV-B Radiation-Induced ROS Formation in Corneal Epithelium. Antioxidants, 11(1), 128. https://doi.org/10.3390/antiox11010128













