Cyclic Hydroxylamines as Monitors of Peroxynitrite and Superoxide-Revisited
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Rapid-Mixing Stopped-Flow
2.2.2. Electron Paramagnetic Resonance (EPR)
2.2.3. Continuous Radiolysis
2.2.4. Determination of the Rate Constant of Superoxide Reaction with Hydroxylamine
3. Results
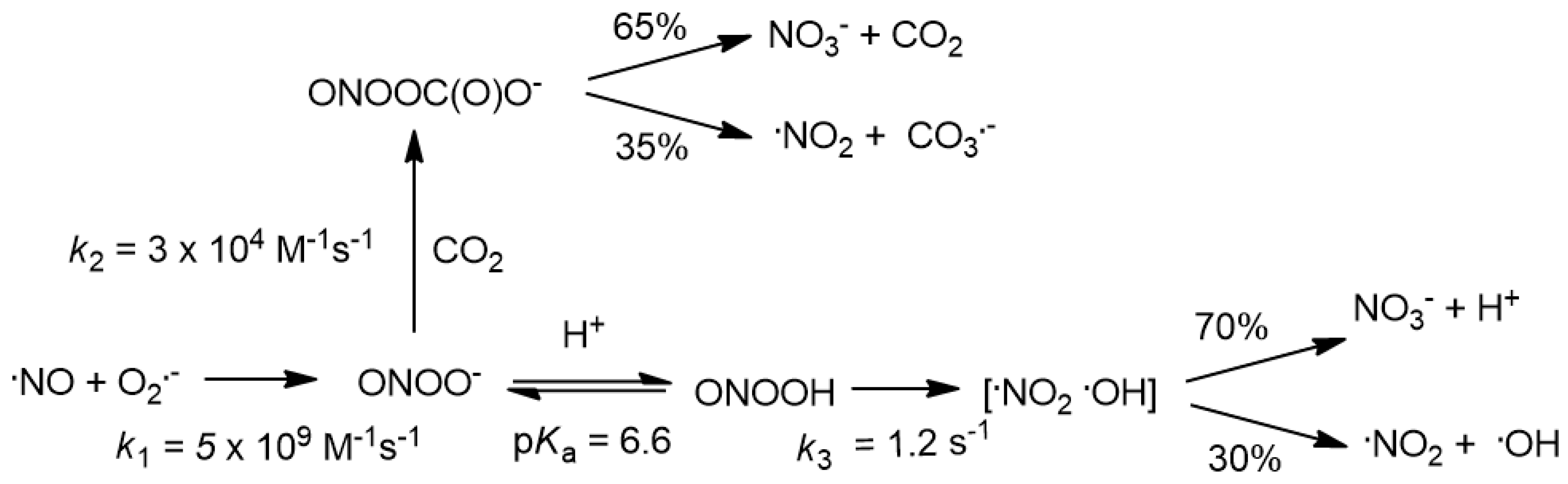
3.1. Reaction of Peroxynitrite with Hydroxylamines
3.1.1. Reaction of RN+HOH with ONOOH
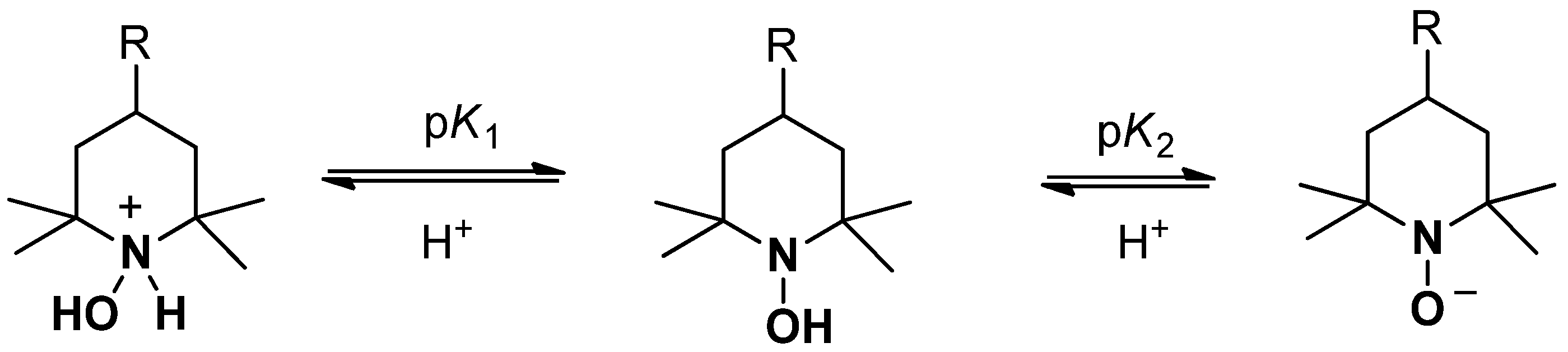
3.1.2. Reaction of RNO-H with ONOO−
3.1.3. Reaction of RNO-H/RN+HOH with ONOOH/ONOO−
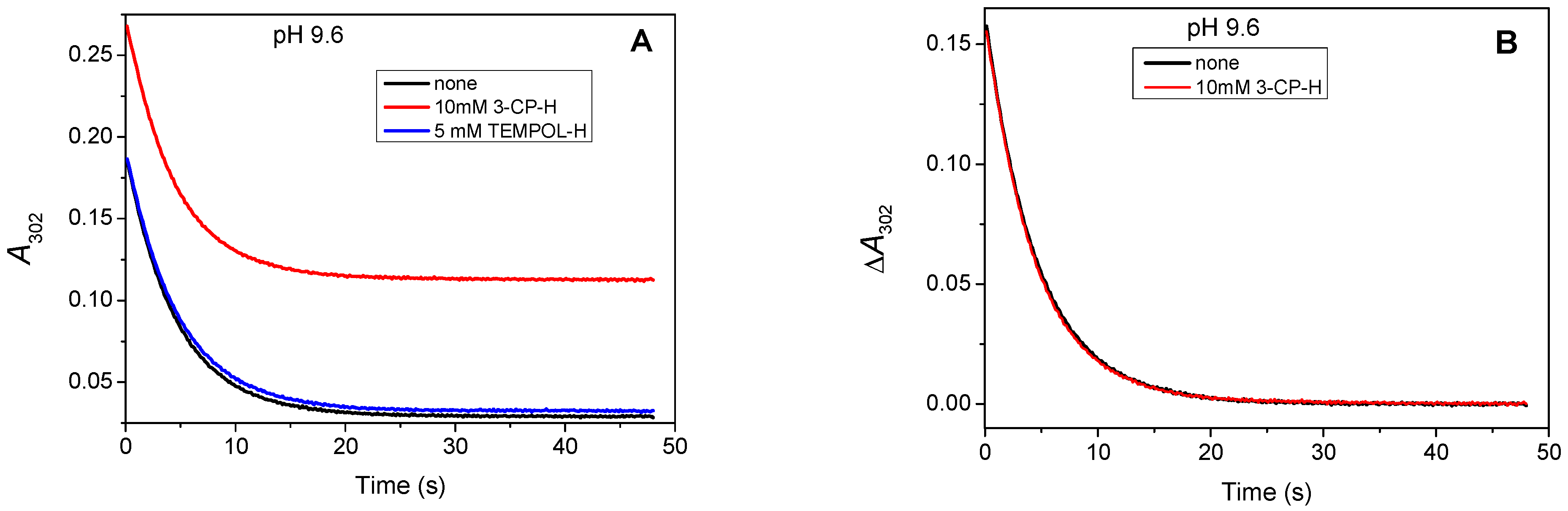
3.1.4. EPR Measurements
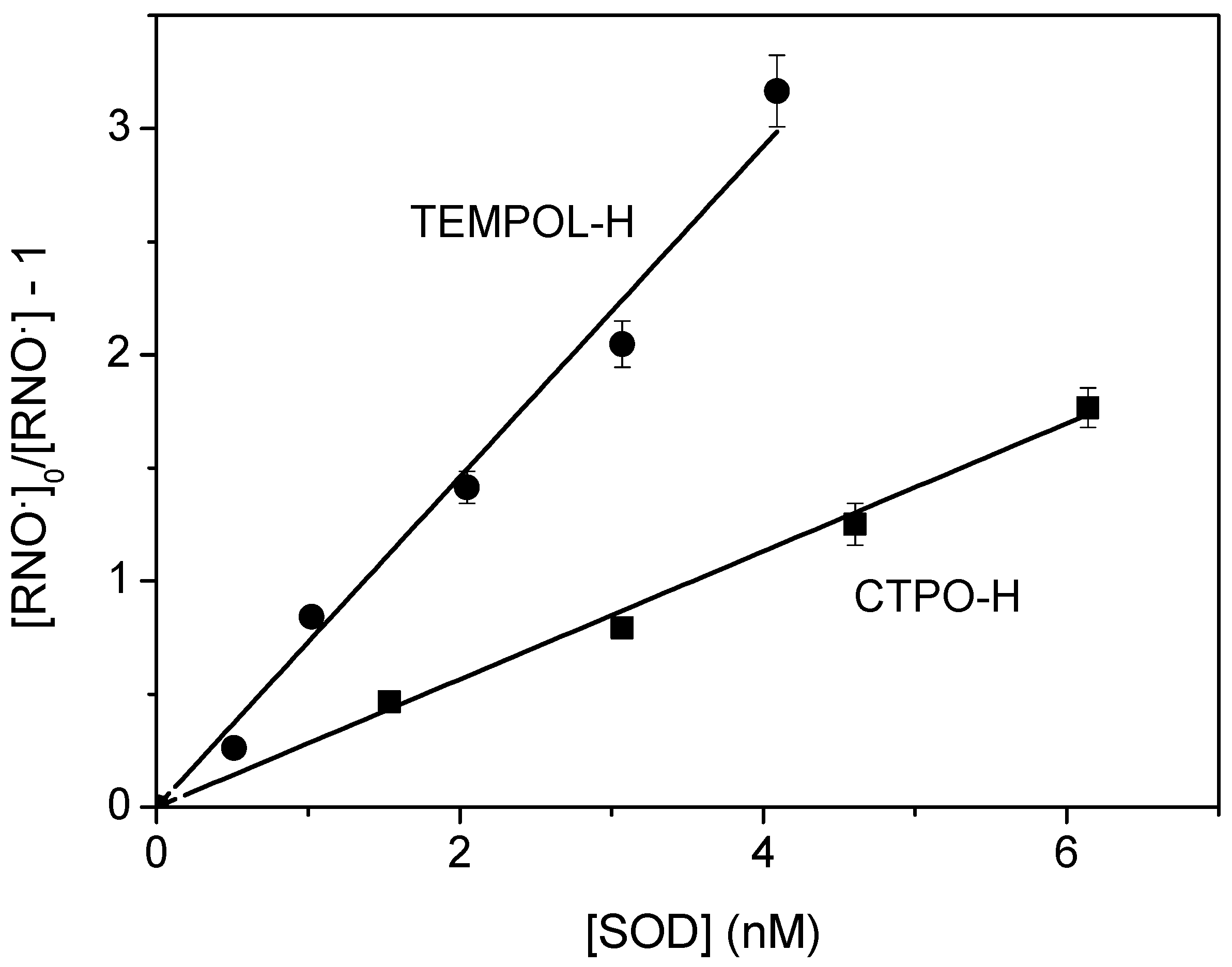
3.2. Reaction of Superoxide with Hydroxylamines
4. Discussion
4.1. Peroxynitrite Reaction with Hydroxylamines
4.2. Superoxide Reaction with Hydroxylamines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2− radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron paramagnetic resonance measurements of reactive oxygen species by cyclic hydroxylamine spin probes. Antioxid. Redox Signal. 2018, 28, 1433–1443. [Google Scholar] [CrossRef]
- Goldstein, S.; Lind, J.; Merenyi, G. Chemistry of peroxynitrites as compared to peroxynitrates. Chem. Rev. 2005, 105, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.B.; Ye, Y.; Rosen, H.; Beckman, J.S. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch. Biochem. Biophys. 1998, 356, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Skatchkov, M.; Bassenge, E. Quantification of peroxynitrite, superoxide, and peroxyl radicals by a new spin trap hydroxylamine 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine. Biochem. Biophys. Res. Commun. 1997, 230, 54–57. [Google Scholar] [CrossRef]
- Dikalov, S.; Skatchkov, M.; Bassenge, E. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem. Biophys. Res. Commun. 1997, 231, 701–704. [Google Scholar] [CrossRef]
- Zhang, R.; Goldstein, S.; Samuni, A. Kinetics of superoxide-induced exchange among nitroxide antioxidants and their oxidized and reduced forms. Free Radic. Biol. Med. 1999, 26, 1245–1252. [Google Scholar] [CrossRef]
- Dikalov, S.; Grigor’ev, I.A.; Voinov, M.; Bassenge, E. Detection of superoxide radicals and peroxynitrite by 1-hydroxy-4-phosphonooxy-2,2,6,6-tetramethylpiperidine: Quantification of extracellular superoxide radicals formation. Biochem. Biophys. Res. Commun. 1998, 248, 211–215. [Google Scholar] [CrossRef]
- Samuni, A.; Goldstein, S. Hydroxylamines inhibit tyrosine oxidation and nitration: The role of their respective nitroxide radicals. Free Radic. Biol. Med. 2020, 160, 837–844. [Google Scholar] [CrossRef]
- Goldstein, S.; Merenyi, G.; Russo, A.; Samuni, A. The role of oxoammonium cation in the SOD-mimic activity of cyclic nitroxides. J. Am. Chem. Soc. 2003, 125, 789–795. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A.; Hideg, K.; Merenyi, G. Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J. Phys. Chem. A 2006, 110, 3679–3685. [Google Scholar] [CrossRef]
- Saha, A.; Goldstein, S.; Cabelli, D.; Czapski, G. Determination of optimal conditions for synthesis of peroxynitrite by mixing acidified hydrogen peroxide with nitrite. Free Radic. Biol. Med. 1998, 24, 653–659. [Google Scholar] [CrossRef]
- Goldstein, S.; Michel, C.; Bors, W.; Saran, M.; Czapski, G. A critical reevaluation of some assay methods for superoxide dismutase activity. Free Radic. Biol. Med. 1988, 4, 295–303. [Google Scholar] [CrossRef]
- Goldstein, S.; Fridovich, I.; Czapski, G. Kinetic properties of Cu,Zn-superoxide dismutase as a function of metal content-Order restored. Free Radic. Biol. Med. 2006, 41, 937–941. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Loegager, T.; Sehested, K. Formation and decay of peroxynitrous acid: A pulse radiolysis study. J. Phys. Chem. 1993, 97, 6664–6669. [Google Scholar] [CrossRef]
- Kissner, R.; Nauser, T.; Bugnon, P.; Lye, P.G.; Koppenol, W.H. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem. Res. Toxicol. 1997, 10, 1285–1292. [Google Scholar] [CrossRef]
- Kato, Y.; Shimizu, Y.; Yijing, L.; Unoura, K.; Utsumi, H.; Ogata, T. Reversible half-wave potentials of reduction processes on nitroxide radicals. Electrochim. Acta 1995, 40, 2799–2802. [Google Scholar] [CrossRef]
- Israeli, A.; Patt, M.; Oron, M.; Samuni, A.; Kohen, R.; Goldstein, S. Kinetics and mechanism of the comproportionation reaction between oxoammonium cation and hydroxylamine derived from cyclic nitroxides. Free Radic. Biol. Med. 2005, 38, 317–324. [Google Scholar] [CrossRef]
- Mallard, W.G.; Ross, A.B.; Helman, W.P. NIST Standard Reference Database. 40, Version 3.0; NIST: Gaithersburg, MD, USA, 1998.
- Samuni, A.; Goldstein, S.; Russo, A.; Mitchell, J.B.; Krishna, M.C.; Neta, P. Kinetics and mechanism of hydroxyl radical and OH-adduct radical reactions with nitroxides and with their hydroxylamines. J. Am. Chem. Soc. 2002, 124, 8719–8724. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Samuni, A.; Russo, A. Reaction of cyclic nitroxides with nitrogen dioxide: The intermediacy of the oxoammonium cations. J. Am. Chem. Soc. 2003, 125, 8364–8370. [Google Scholar] [CrossRef] [PubMed]


| [RN+HOH]0, mM | [ONOOH]0, µM | [RNO•], µM | Yield % a | |
|---|---|---|---|---|
| TEMPOL-H2+ | 5 | 118 | 5.2 | 4.4 |
| 10 | 118 | 7.1 | 6.0 | |
| 50 | 118 | 9.7 | 8.2 | |
| TEMPONE-H2+ | 0.5 | 215 | 1.5 | 0.7 |
| 2.5 | 215 | 16.7 | 7.8 | |
| TEMPO-H2+ | 10 | 122 | 40 | 32.8 |
| 3-CP-H2+ | 10 | 132 | 63.6 | 48.2 |
| pH 6.4 | pH 7.4 | pH 7.8 | |
|---|---|---|---|
| 0.5 mM TEMPONE-H | 1.4 ± 0.1 | 2.1 ± 0.1 | 2.6 ± 0.1 |
| 0.5 mM TEMPOL-H | 1.1 ± 0.1 | 2.3 ± 0.1 | 3.0 ± 0.1 |
| 0.5 mM TEMPOL-H 20 μM TEMPOL | 1.3 ± 0.1 | ||
| 2 mM TEMPOL-H | 2.4 ± 0.1 | ||
| 0.5 mM 3-CP-H | 2.2 ± 0.1 | 2.9 ± 0.1 | 3.1 ± 0.1 |
| 2 mM 3-CP-H | 3.8 ± 0.2 | ||
| 2 mM 3-CP-H 50 μM 3-CP | 3.8 ± 0.2 | ||
| 0.5 mM 3-CTPO-H | 3.4 ± 0.1 | ||
| 1 mM 3-CTPO-H | 4.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuni, U.; Samuni, A.; Goldstein, S. Cyclic Hydroxylamines as Monitors of Peroxynitrite and Superoxide-Revisited. Antioxidants 2022, 11, 40. https://doi.org/10.3390/antiox11010040
Samuni U, Samuni A, Goldstein S. Cyclic Hydroxylamines as Monitors of Peroxynitrite and Superoxide-Revisited. Antioxidants. 2022; 11(1):40. https://doi.org/10.3390/antiox11010040
Chicago/Turabian StyleSamuni, Uri, Amram Samuni, and Sara Goldstein. 2022. "Cyclic Hydroxylamines as Monitors of Peroxynitrite and Superoxide-Revisited" Antioxidants 11, no. 1: 40. https://doi.org/10.3390/antiox11010040







