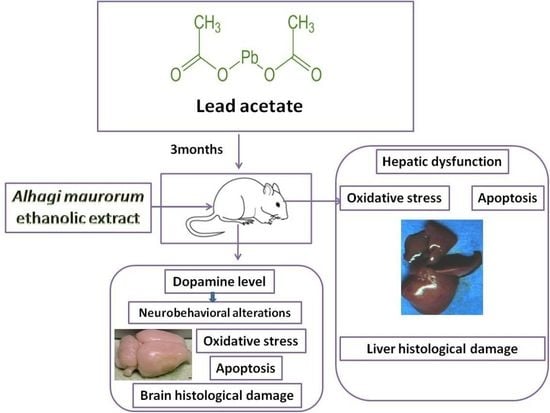
Alhagi maurorum Ethanolic Extract Rescues Hepato-Neurotoxicity and Neurobehavioral Alterations Induced by Lead in Rats via Abrogating Oxidative Stress and the Caspase-3-Dependent Apoptotic Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Tested Compounds
2.2. A. maurorum Ethanolic Extract Preparation
2.3. Gas Chromatography/Mass Spectrometry Analysis (GC–MS) of A. maurorum Ethanolic Extract
2.4. Experimental Animals
2.5. Experimental Design
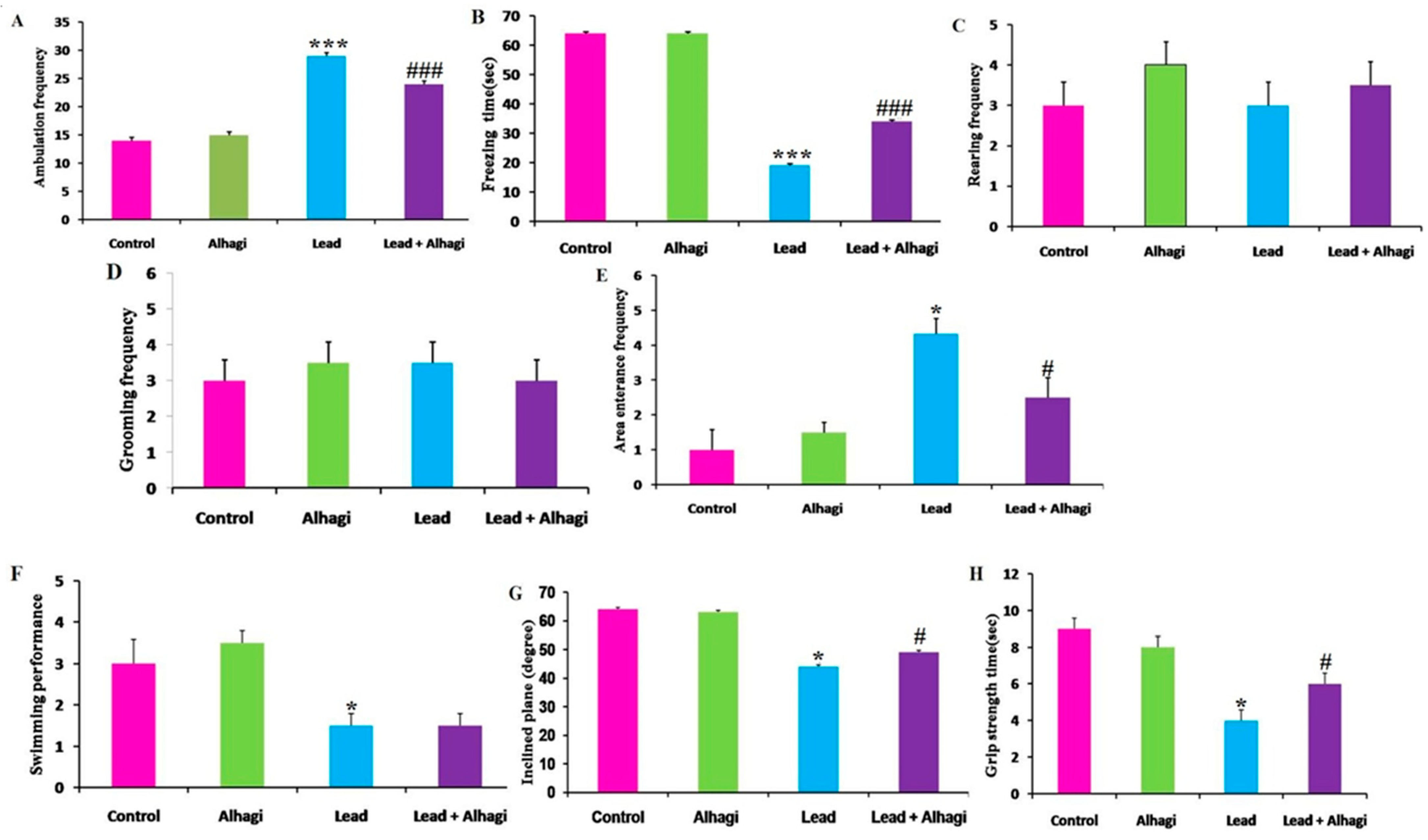
2.6. Behavioral Response Assessment
2.6.1. Open Field Test
2.6.2. Swimming Performance Test
2.6.3. Inclined Plane Test
2.6.4. Grip Strength Test
2.7. Sample Collection
2.8. Biochemical Assays
2.8.1. Evaluation of Liver Function Markers
2.8.2. Assessment of Hepatic and Brain Oxidant/Antioxidant Status
2.8.3. Determination of Dopamine in the Brain
2.9. Analysis of Apoptosis- and Oxidative-Stress-Related Gene Expression
2.10. Histopathological Investigations
2.11. Immunohistochemical Investigation of Bcl-2
2.12. Statistical Analysis
3. Results
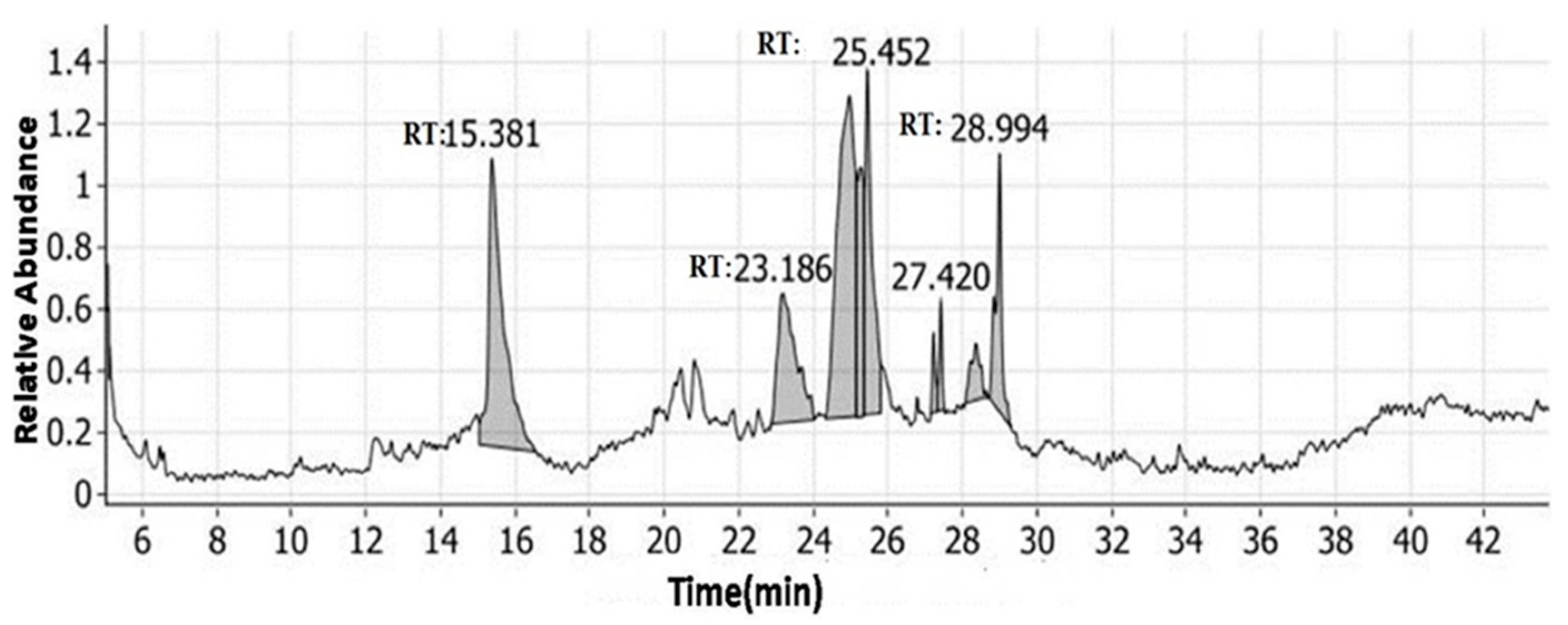
3.1. GC-MS Profile of A. maurorum Ethanolic Extract
3.2. Effect of the A. maurorum Extract on Body Weight Gain, and Relative Brain and Liver Weights in Lead-Exposed Rats
3.3. Effect of the A. maurorum Extract on the Neurobehavioral Responses of Lead-Exposed Rats
3.4. Effect of the A. maurorum Extract on Liver Function Markers in Lead-Treated Rats
3.5. Effect of the A. maurorum Extract on Hepatic and Brain Oxidative Stress Biomarkers in Lead-Exposed Rats
3.6. Effect of the A. maurorum Extract on the Brain Dopamine Level in Lead-Treated Rats
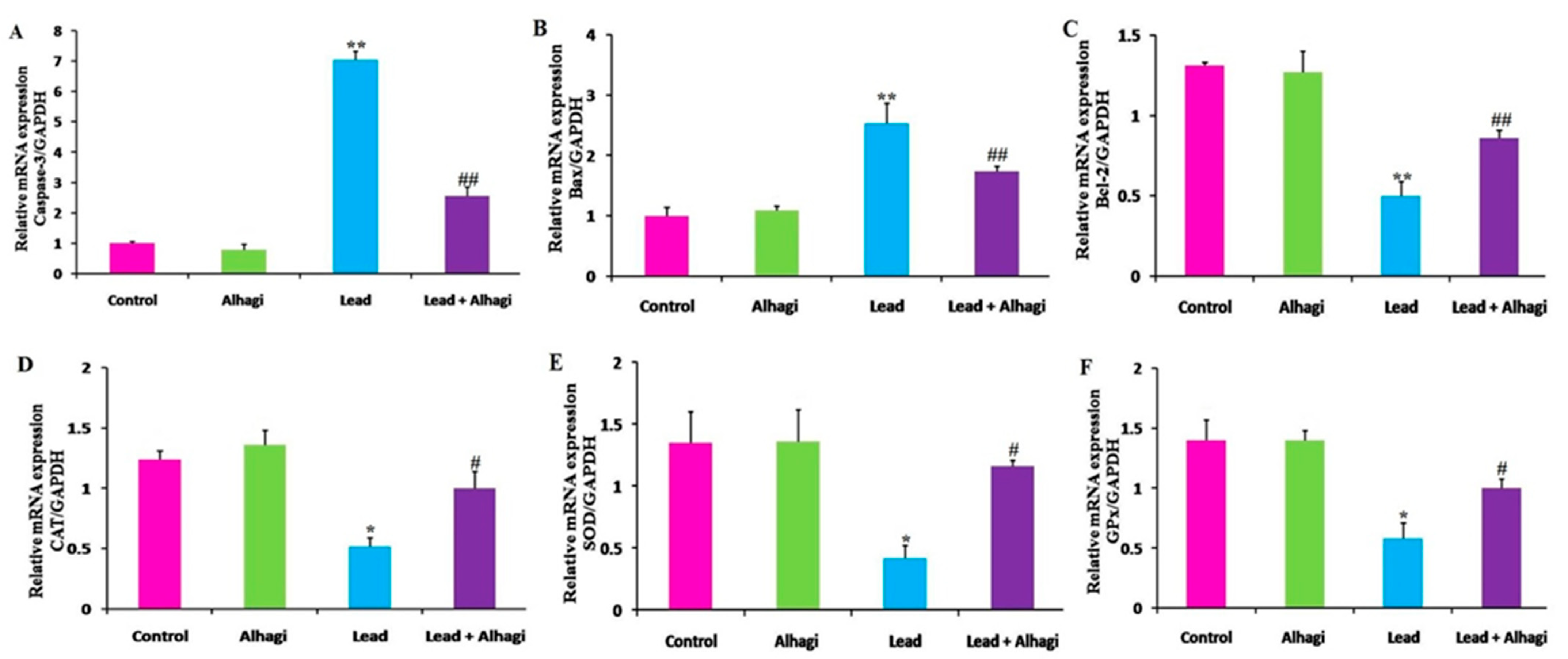
3.7. Effect of the A. maurorum Extract on the Hepatic mRNA Expression of Apoptotic Marker and Antioxidant Genes in Lead-Exposed Rats
3.8. Effect of the A. maurorum Extract on the mRNA Transcriptional Levels of Genes Related to Apoptosis and Oxidative Stress in the Brain of Lead-Exposed Rats
3.9. Histopathological and Immunohistochemical Investigations
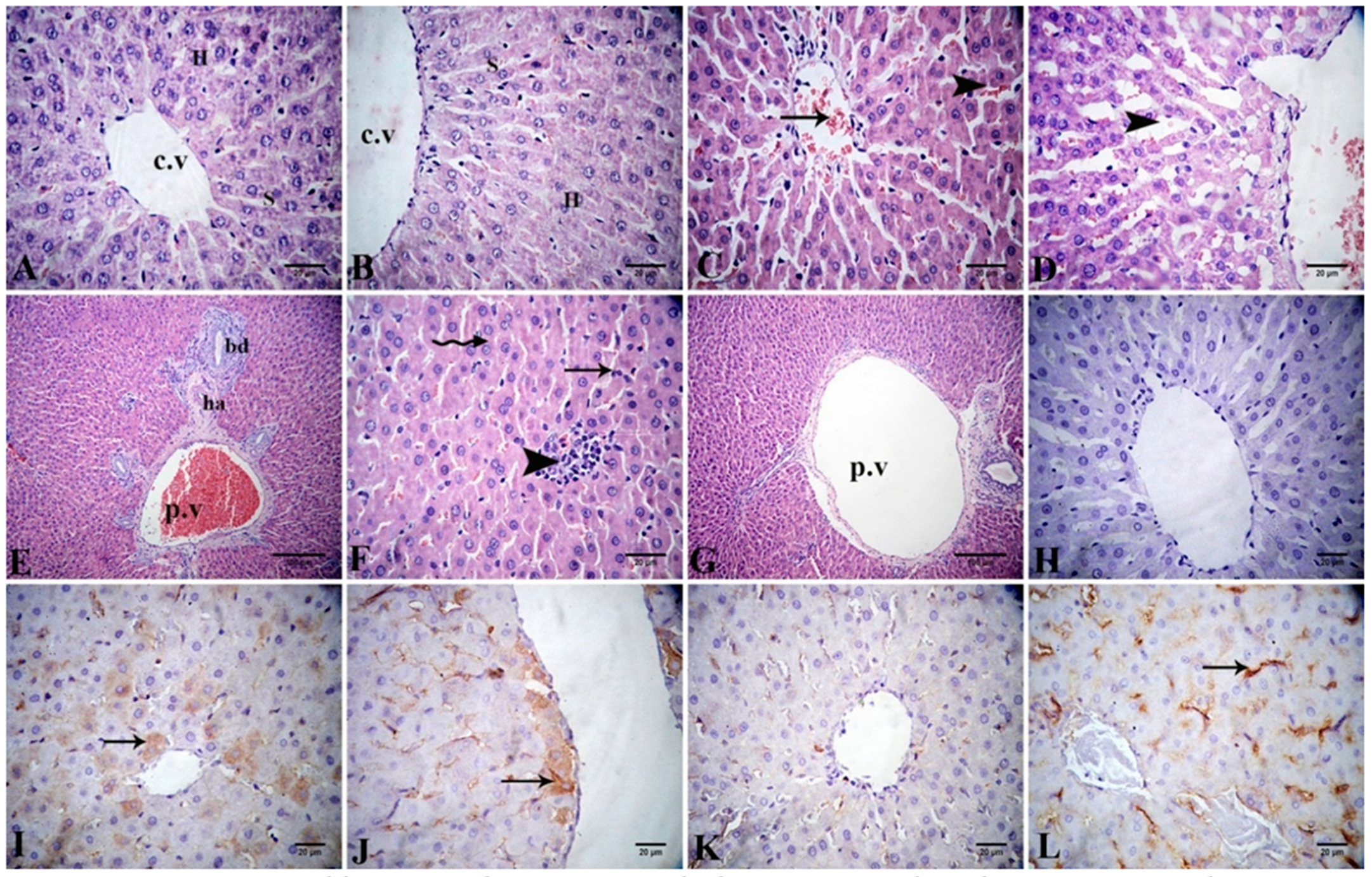
3.9.1. Histopathological and Immunohistochemical Findings in the Liver
3.9.2. Histopathological and Immunohistochemical Findings in the Brain
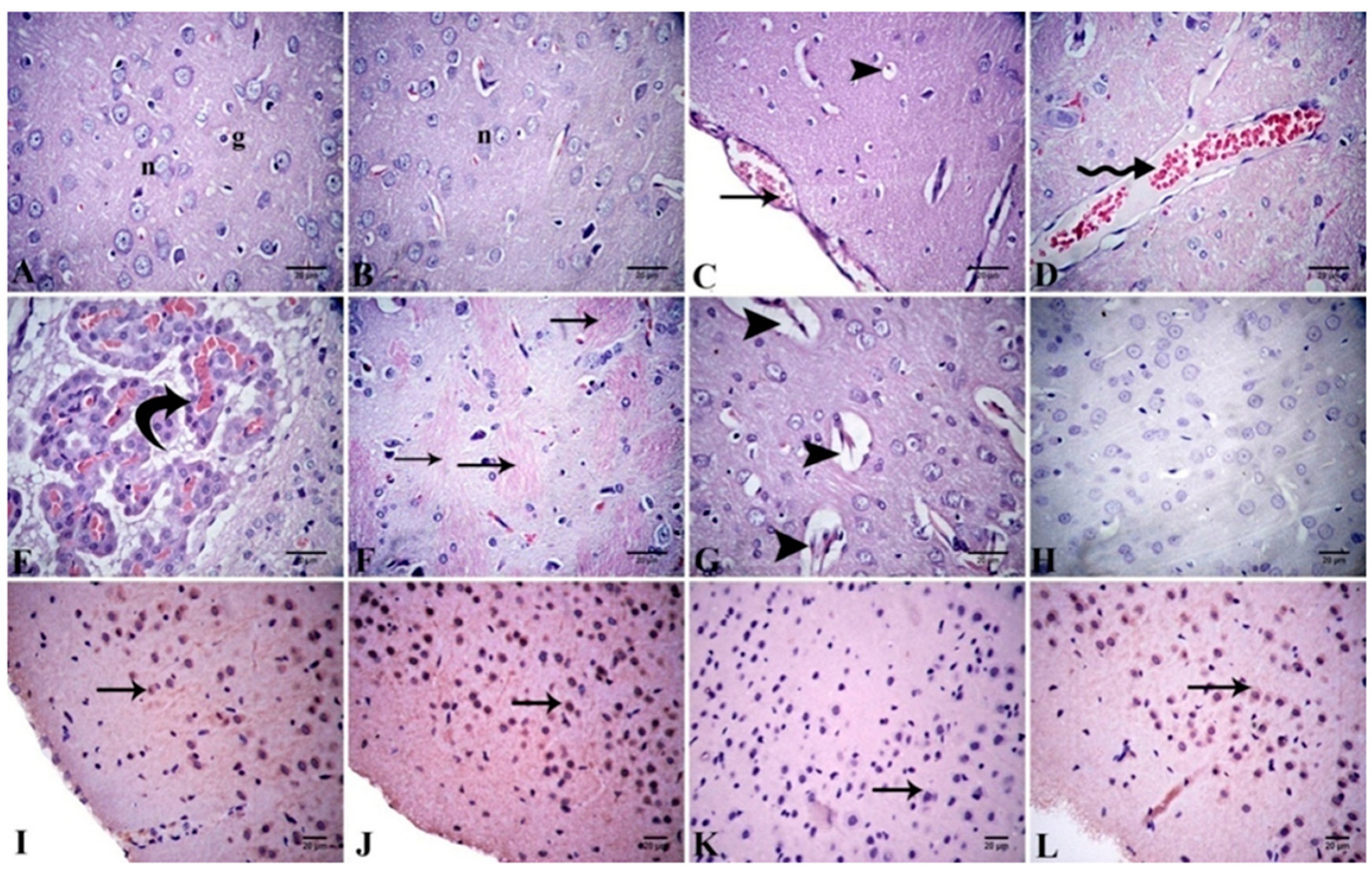
Cerebral Cortex
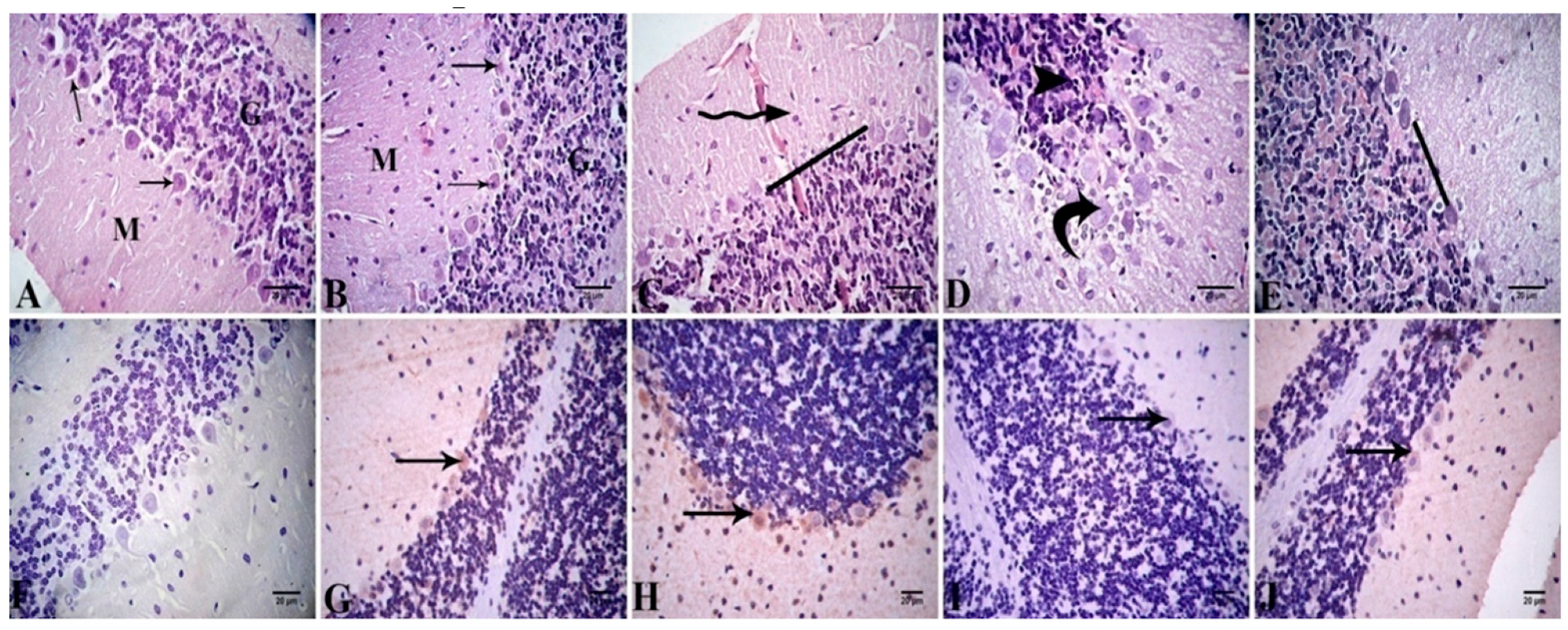
Cerebellar Cortex
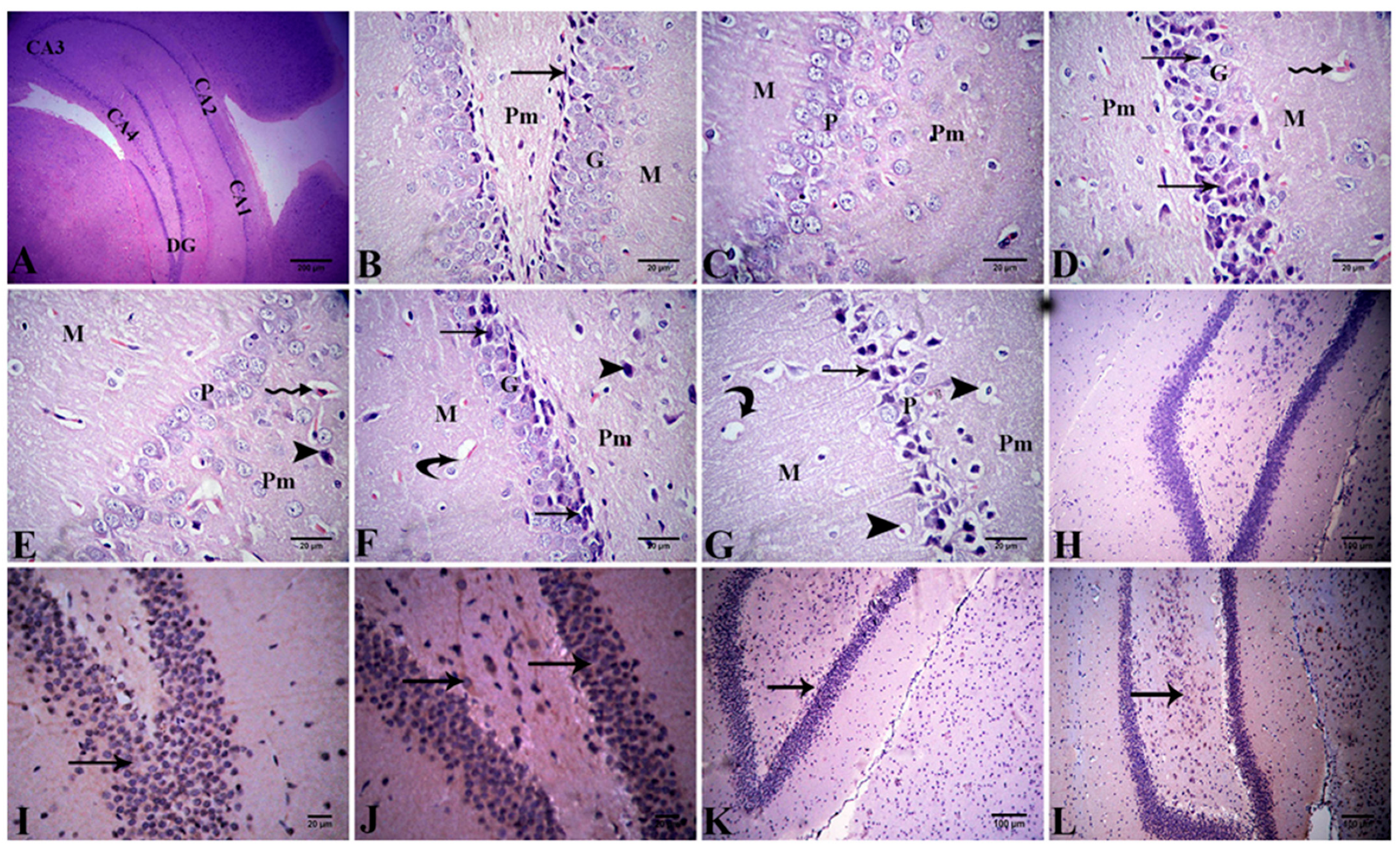
Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andjelkovic, M.; Buha Djordjevic, A.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Ramírez Ortega, D.; González Esquivel, D.F.; Blanco Ayala, T.; Pineda, B.; Gómez Manzo, S.; Marcial Quino, J.; Carrillo Mora, P.; Pérez de la Cruz, V. Cognitive impairment induced by lead exposure during lifespan: Mechanisms of lead neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Ericson, B.; Landrigan, P.; Taylor, M.P.; Frostad, J.; Caravanos, J.; Keith, J.; Fuller, R. The global burden of lead toxicity attributable to informal used lead-acid battery sites. Ann. Glob. Health 2016, 82, 686–699. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Mahgoub, H.A.; Ateya, A.I. Ameliorative effect of curcumin against lead acetate-induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 10950–10965. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, W.S.; Albasher, G. Moringa oleifera Lam. extract rescues lead-induced oxidative stress, inflammation, and apoptosis in the rat cerebral cortex. J. Food Biochem. 2021, 45, e13579. [Google Scholar] [CrossRef] [PubMed]
- BaSalamah, M.A.; Abdelghany, A.H.; El-Boshy, M.; Ahmad, J.; Idris, S.; Refaat, B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci. Rep. 2018, 8, 4853. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Khalifa, H.A.; Abdel-Motal, S.M.; Mohammed, H.H.; Elewa, Y.H.A.; Mahmoud, H.A. Spirulina platensis attenuates the associated neurobehavioral and inflammatory response impairments in rats exposed to lead acetate. Ecotoxicol. Environ. Saf. 2018, 157, 255–265. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Mashaly, S.; Newairy, A.A.; Abdou, H.M.; Eweda, S.M. Changes in oxidative stress and antioxidant enzyme activities in streptozotocin-induced diabetes mellitus in rats: Role of Alhagi maurorum extracts. Oxid. Med. Cell Longev. 2016, 2016, 5264064. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Rashed, K.; Said, A.; Bonesi, M.; Menichini, F.; Tundis, R. Anti proliferative and antioxidant properties of Alhagi maurorum Boiss (Leguminosae) aerial parts. Ind. Crops Prod. 2014, 53, 289–295. [Google Scholar] [CrossRef]
- Asghari, M.H.; Fallah, M.; Moloudizargari, M.; Mehdikhani, F.; Sepehrnia, P.; Moradi, B. A systematic and mechanistic review on the phytopharmacological properties of Alhagi Species. Anc. Sci. Life 2016, 36, 65. [Google Scholar]
- Shaker, E.; Mahmoud, H.; Mnaa, S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camelthorn). Food Chem. Toxicol. 2010, 48, 2785–2790. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, Y.; Rezatofighi, S.E.; Seyyed Nejad, S.M.; Roayaei Ardakani, M. Antiviral activity of Alhagi maurorum Medik’s methanolic extract on foot and mouth disease virus (FMDV) in cell cultures. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e59720. [Google Scholar] [CrossRef]
- Behbahani, M. Evaluation of in vitro anticancer activity of Ocimum basilicum, Alhagi maurorum, Calendula officinalis and their parasite Cuscuta campestris. PLoS ONE 2014, 9, e116049. [Google Scholar] [CrossRef]
- Al-Saleem, M.M.S.; Al-Wahaib, L.H.; Abdel-Mageed, W.M.; Gouda, Y.G.; Sayed, H.M. Antioxidant flavonoids from Alhagi maurorum with hepatoprotective effect. Pharmacogn. Mag. 2019, 15, 592. [Google Scholar]
- Khalifa, H.A.; Shalaby, S.I.; Abdelaziz, A.S. Alhagi maurorum aqueous extract protects against norfloxacin-induced hepato-nephrotoxicity in rats. Chin. Herb. Med. 2020, 12, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Changizi-Ashtiyani, S.; Alizadeh, M.; Najafi, H.; Babaei, S.; Khazaei, M.; Jafari, M.; Hossaini, N.; Avan, A.; Bastani, B. Physalis alkekengi and Alhagi maurorum ameliorate the side effect of cisplatin-induced nephrotoxicity. Cancer Gene Ther. 2016, 23, 235–240. [Google Scholar] [CrossRef]
- Rehman, J.U.; Aktar, N.; Khan, M.Y.; Ahmad, K.; Ahmad, M.; Sultana, S.; Asif, H.M. Phytochemical screening and hepatoprotective effect of Alhagi maurorum Boiss (Leguminosae) against paracetamol-induced hepatotoxicity in rabbits. Trop. J. Pharm. Res. 2015, 14, 1029–1034. [Google Scholar] [CrossRef]
- El-Tantawy, W.H. Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J. Tradit. Complement. Med. 2016, 6, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Contó, M.B.; de Carvalho, J.G.; Benedito, M.A. Behavioral differences between subgroups of rats with high and low threshold to clonic convulsions induced by DMCM, a benzodiazepine inverse agonist. Pharmacol. Biochem. Behav. 2005, 82, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, F.; Yamaguchi, T.; Yamada, H.; Tamura, A. Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1998, 18, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.S.; Andersen, A.B.; Finger, S. Neurological correlates of unilateral and bilateral “strokes” of the middle cerebral artery in the rat. Physiol. Behav. 1991, 50, 263–269. [Google Scholar] [CrossRef]
- Nishikimi, M.; Roa, N.A.; Yogi, K. The occurrence of superoxide anion in the reaction of reduced Phenazine methosulphate and molecular oxygen. Biochem. Bioph. Res. Common. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Gross, R.T.; Bracci, R.; Rudolph, N.; Schroeder, E.; Kochen, J.A. Hydrogen peroxide toxicity and detoxification in the erythrocytes. Blood 1967, 29, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Arisha, A.H.; Ahmed, M.M.; Kamel, M.A.; Attia, Y.A.; Hussein, M.M.A. Morin ameliorates the testicular apoptosis, oxidative stress, and impact on blood-testis barrier induced by photo-extracellularly synthesized silver nanoparticles. Environ. Sci. Pollut. Res. Int. 2019, 26, 28749–28762. [Google Scholar] [CrossRef]
- Saber, T.M.; Mansour, M.F.; Abdelaziz, A.S.; Mohamed, R.M.S.; Fouad, R.A.; Arisha, A.H. Argan oil ameliorates sodium fluoride–induced renal damage via inhibiting oxidative damage, inflammation, and intermediate filament protein expression in male rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 30426–30436. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book, 7th ed.; Elsevier Health Sciences: London, UK, 2018. [Google Scholar]
- Yousef, J.M.; Chen, G.; Hill, P.A.; Nation, R.L.; Li, J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J. Antimicrob. Chemother. 2012, 67, 452–459. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Kiupel, M.; Baszler, T.; Bliven, L.; Brodersen, B.; Chelack, B.; Czub, S.; Del Piero, F.; Dial, S.; Ehrhart, E.J.; et al. Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J. Vet. Diagn. Investig. 2008, 20, 393–413. [Google Scholar] [CrossRef]
- Saber, T.M.; Arisha, A.H.; Abo-Elmaaty, A.M.A.; Abdelgawad, F.E.; Metwally, M.M.M.; Saber, T.; Mansour, M.F. Thymol alleviates imidacloprid-induced testicular toxicity by modulating oxidative stress and expression of steroidogenesis and apoptosis-related genes in adult male rats. Ecotoxicol. Environ. Saf. 2021, 221, 112435. [Google Scholar] [CrossRef] [PubMed]
- Saber, T.M.; Farag, M.R.; Cooper, R.G. Ameliorative effect of extra virgin olive oil on hexavalent chromium-induced nephrotoxicity and genotoxicity in rats. Revue Méd. Vét. 2015, 166, 11–19. [Google Scholar]
- Minnema, D.J.; Hammond, P.B. Effect of lead exposure on patterns of food intake in weanling rats. Neurotoxicol. Teratol. 1994, 16, 623–629. [Google Scholar] [CrossRef]
- Al-Attar, A.M. Therapeutic influences of almond oil on male rats exposed to a sublethal concentration of lead. Saudi J. Biol. Sci. 2020, 27, 581–587. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Y.; Shen, Y.; Cheng, Y.; Qiao, M.; Song, L.; Huang, X. Protective effects of folic acid on oxidative damage of rat spleen induced by lead acetate. Ecotoxicol. Environ. Saf. 2021, 211, 111917. [Google Scholar] [CrossRef]
- Salehi, I.; Karamian, R.; Komaki, A.; Tahmasebi, L.; Taheri, M.; Nazari, M.; Shahidi, S.; Sarihi, A. Effects of vitamin E on lead-induced impairments in hippocampal synaptic plasticity. Brain Res. 2015, 1629, 270–281. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Nasuti, C.; Gabbianelli, R.; Falcioni, M.L.; Di Stefano, A.; Sozio, P.; Cantalamessa, F. Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology 2007, 229, 194–205. [Google Scholar] [CrossRef]
- Jones, D.C.; Miller, G.W. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem. Pharmacol. 2008, 76, 569–581. [Google Scholar] [CrossRef]
- Reckziegel, P.; Dias, V.T.; Benvegnú, D.; Boufleur, N.; Silva Barcelos, R.C.; Segat, H.J.; Pase, C.S.; Dos Santos, C.M.; Flores, E.M.; Bürger, M.E. Locomotor damage and brain oxidative stress induced by lead exposure are attenuated by gallic acid treatment. Toxicol. Lett. 2011, 203, 74–81. [Google Scholar] [CrossRef]
- Sabbar, M.; Delaville, C.; De Deurwaerdère, P.; Lakhdar-Ghazal, N.; Benazzouz, A. Lead-induced atypical parkinsonism in rats: Behavioral, electrophysiological, and neurochemical evidence for a role of noradrenaline depletion. Front. Neurosci. 2018, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Mohammed, H.H.; Mohamed, W.A.M. Imidacloprid impacts on neurobehavioral performance, oxidative stress, and apoptotic events in the brain of adolescent and adult rats. J. Agric. Food Chem. 2018, 66, 13513–13524. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; El-Sharkawy, N.I.; Mohammed, H.H.; Ebraheim, L.L.M.; Shalaby, M.A. Camel milk rescues neurotoxic impairments induced by fenpropathrin via regulating oxidative stress, apoptotic, and inflammatory events in the brain of rats. Food Chem. Toxicol. 2020, 135, 111055. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef]
- Virgolini, M.B.; Aschner, M. Molecular mechanisms of lead neurotoxicity. Adv. Neurotoxicol. 2021, 5, 159–213. [Google Scholar]
- Benammi, H.; Erazi, H.; El Hiba, O.; Vinay, L.; Bras, H.; Viemari, J.C.; Gamrani, H. Disturbed sensorimotor and electrophysiological patterns in lead intoxicated rats during development are restored by curcumin I. PLoS ONE 2017, 12, e0172715. [Google Scholar] [CrossRef] [PubMed]
- Sansar, W.; Ahboucha, S.; Gamrani, H. Chronic lead intoxication affects glial and neural systems and induces hypoactivity in adult rat. Acta Histochem. 2011, 113, 601–607. [Google Scholar] [CrossRef]
- Thangarajan, S.; Vedagiri, A.; Somasundaram, S.; Sakthimanogaran, R.; Murugesan, M. Neuroprotective effect of morin on lead acetate- induced apoptosis by preventing cytochrome c translocation via regulation of Bax/Bcl-2 ratio. Neurotoxicol. Teratol. 2018, 66, 35–45. [Google Scholar] [CrossRef]
- Naik, S.R.; Panda, V.S. Hepatoprotective effect of Ginkgoselect Phytosome in rifampicin induced liver injury in rats: Evidence of antioxidant activity. Fitoterapia 2008, 79, 439–445. [Google Scholar] [CrossRef]
- Kumar, V.; Cotran, R.S.; Robbins, S.L. The Liver and the Billary tract. In Robbins Basic Pathology, 7th ed.; Saunders Penns: Philadelphia, PA, USA, 2003. [Google Scholar]
- Lopes, A.C.; Peixe, T.S.; Mesas, A.E.; Paoliello, M.M. Lead exposure and oxidative stress: A systematic review. Rev. Environ. Contam. Toxicol. 2016, 236, 193–238. [Google Scholar]
- Barnes, P.; Yeboah, J.K.; Gbedema, W.; Saahene, R.O.; Amoani, B. Ameliorative effect of Vernonia amygdalina plant extract on heavy metal-induced liver and kidney dysfunction in rats. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 2976905. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Elhady, W.M.; Elewa, Y.H.A.; Abd El-Hameed, N.E.; Ali, S.A. Possible role of Arthrospira platensis in reversing oxidative stress-mediated liver damage in rats exposed to lead. Biomed. Pharmacother. 2018, 97, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating Akt/GSK-3β sgnaling pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, M.; Gurung, A.B.; Bhattacharjee, A.; Joshi, S.R. Analysis of the bioactive metabolites of the endangered Mexican lost fungi Campanophyllum—A Report from India. Mycobiology 2020, 48, 58–69. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Shen, H.-B.; Wang, Z.-X.; Wei, Q.-Y.; Chen, F. Association between δ-aminolevulinic acid dehydratase (ALAD) polymorphism and blood lead levels: A meta-regression analysis. J. Toxicol. Environ. Health Part A 2007, 70, 1986–1994. [Google Scholar] [CrossRef]
- Gleichmann, M.; Mattson, M.P. Neuronal calcium homeostasis and dysregulation. Antioxid. Redox Signal. 2011, 14, 1261–1273. [Google Scholar] [CrossRef]
- Verma, S.K.; Dua, R.; Gill, K.D. Impaired energy metabolism after co-exposure to lead and ethanol. Basic Clin. Pharmacol. Toxicol. 2005, 96, 475–479. [Google Scholar] [CrossRef]
- Chander, K.; Vaibhav, K.; Ahmed, M.E.; Javed, H.; Tabassum, R.; Khan, A.; Kumar, M.; Katyal, A.; Islam, F.; Siddiqui, M.S. Quercetin mitigates lead acetate-induced behavioral and histological alterations via suppression of oxidative stress, Hsp-70, Bak and upregulation of Bcl-2. Food Chem. Toxicol. 2014, 68, 297–306. [Google Scholar] [CrossRef]
- Manoj Kumar, V.; Henley, A.K.; Nelson, C.J.; Indumati, O.; Prabhakara Rao, Y.; Rajanna, S.; Rajanna, B. Protective effect of Allium sativum (garlic) aqueous extract against lead-induced oxidative stress in the rat brain, liver, and kidney. Environ. Sci. Pollut. Res. 2017, 24, 1544–1552. [Google Scholar] [CrossRef]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int. J. Mol. Sci. 2018, 19, 1813. [Google Scholar] [CrossRef]
- Li, C.; Pan, Z.; Xu, T.; Zhang, C.; Wu, Q.; Niu, Y. Puerarin induces the upregulation of glutathione levels and nuclear translocation of Nrf2 through PI3K/Akt/GSK-3β signaling events in PC12 cells exposed to lead. Neurotoxicol. Teratol. 2014, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alagar Yadav, S.; Ramalingam, S.; Jebamalairaj, A.; Subban, R.; Sundaram, K.M. Biochemical fingerprint and pharmacological applications of Barleria noctiflora L.f. leaves. J. Complement. Integr. Med. 2016, 13, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, M.; Liu, F.; Zeng, S.; Hu, J. Identification of 2, 3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products. Food Res. Int. 2013, 51, 397–403. [Google Scholar] [CrossRef]
- Shukla, R.; Banerjee, S.; Tripathi, Y.B. Antioxidant and antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats. BMC Complement. Altern. Med. 2018, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Boulebd, H. Are thymol, rosefuran, terpinolene and umbelliferone good scavengers of peroxyl radicals? Phytochemistry 2021, 184, 112670. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.; Kim, K. Combined exposure to metals in drinking water alters the dopamine system in mouse striatum. Int. J. Environ. Res. Public Health 2021, 18, 6558. [Google Scholar] [CrossRef]
- Saritha, S.; Davuljigari, C.B.; Kumar, K.P.; Reddy, G.R. Effects of combined arsenic and lead exposure on the brain monoaminergic system and behavioral functions in rats: Reversal effect of MiADMSA. Toxicol. Ind. Health 2019, 35, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Tian, Z.K.; Zhang, Y.J.; Ming, Q.L.; Ma, J.Q.; Ji, L.P. Effects of gastrodin against lead-induced brain injury in mice associated with the Wnt/Nrf2 Pathway. Nutrients 2020, 12, 1805. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 2016, 11, e0158965. [Google Scholar] [CrossRef] [PubMed]
- Al-Megrin, W.A.; Soliman, D.; Kassab, R.B.; Metwally, D.M.; Ahmed, E.A.M.; El-Khadragy, M.F. Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front. Physiol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Baty, R.; Hassan, K.; Alsharif, K.; El-Hennamy, R.; Elmahallawy, E.; Hafez, M.; Moneim, A.A.; Kassab, R. Neuroprotective role of luteolin against lead acetate-induced cortical damage in rats. Hum. Exp. Toxicol. 2020, 39, 1200–1212. [Google Scholar] [CrossRef]
- Aminzadeh, A.; Salarinejad, A. Citicoline protects against lead-induced oxidative injury in neuronal PC12 cells. Biochem. Cell Biol. 2019, 97, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Al-Khalifa, M.S.; Al-Quraishy, S.; Zrieq, R.; Abdel Moneim, A.E. Indigofera oblongifolia mitigates lead-acetate-induced kidney damage and apoptosis in a rat model. Drug Des. Devel. Ther. 2016, 10, 1847–1856. [Google Scholar] [PubMed]
- Xu, L.H.; Mu, F.F.; Zhao, J.H.; He, Q.; Cao, C.L.; Yang, H.; Liu, Q.; Liu, X.H.; Sun, S.J. Lead induces apoptosis and histone hyperacetylation in rat cardiovascular tissues. PLoS ONE 2015, 10, e0129091. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, L.; Liang, Y.; Peng, D.; Aschner, M.; Jiang, Y. Signal transduction associated with lead-induced neurological disorders: A review. Food Chem. Toxicol. 2021, 150, 112063. [Google Scholar] [CrossRef] [PubMed]
- El-Shetry, E.S.; Mohamed, A.A.; Khater, S.I.; Metwally, M.M.M.; Nassan, M.A.; Shalaby, S.; El-Mandrawy, S.A.M.; Bin Emran, T.; Abdel-Ghany, H.M. Synergistically enhanced apoptotic and oxidative DNA damaging pathways in the rat brain with lead and/or aluminum metals toxicity: Expression pattern of genes OGG1 and P53. J. Trace Elem. Med. Biol. 2021, 68, 126860. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, X.; Kang, D.; Yan, B.; Liu, X.; Gao, Y.; Zhang, K. Apoptosis and expression of the Bcl-2 family of proteins and P53 in human pancreatic ductal adenocarcinoma. Med. Princ. Pract. 2012, 21, 68–73. [Google Scholar] [CrossRef] [PubMed]

| Genes | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) | Product Size/bp | GenBank Accession Numbers |
|---|---|---|---|---|
| Bax | CGAATTGGCGATGAACTGGA | CAAACATGTCAGCTGCCACAC | 109 | NM_017059.2 |
| Bcl-2 | GACTGAGTACCTGAACCGGCATC | CTGAGCAGCGTCTTCAGAGACA | 135 | NM_016993.1 |
| Caspase-3 | GAGACAGACAGTGGAACTGACGATG | GGCGCAAAGTGACTGGATGA | 147 | NM_012922.2 |
| CAT | GTCCGATTCTCCACAGTCGC | CGCTGAACAAGAAAGTAACCTG | 272 | AH004967.1 |
| SOD | ATGGGGACAATACACAAGGC | TCATCTTGTTTCTCGTGGAC | 225 | Z21917.1 |
| GPx | CACAGTCCACCGTGTATGCC | AAGTTGGGCTCGAACCCACC | 292 | S50336.1 |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA | 143 | NM_017008.4 |
| Compound | Molecular Formula | RT (min) | Peak Area Sum % |
|---|---|---|---|
| Beta-D-glucopyranose, 1,6-anhydro- | C6H10O5 | 24.97 | 28.91 |
| 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 15.38 | 23.24 |
| 3-methyl-2-(2-oxopropyl)furan | C8H10O2 | 25.45 | 13.56 |
| 3-methyl-2-(2-methyl-2-butenyl)-furan | C10H14O | 23.19 | 12.84 |
| (3Z,6Z)-3,6-octadien-1-ol | C8H14O | 28.99 | 7.83 |
| Beta-l-arabinopyranoside, methyl | C6H12O5 | 25.28 | 7.51 |
| Phenyllactic acid benzyl ester | C12H16O3 | 28.36 | 2.99 |
| Anti-3,3-dimethyl-6-hydroxybicyclo [2.2.2] octan-2-one | C10H16O2 | 27.42 | 1.72 |
| (4R,6S)-4-(t-butyldimethylsilyl)-6-fluoro-5-nonanone | C15H31FOSi | 27.23 | 1.40 |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Control | Alhagi | Lead | Lead + Alhagi | p-Value | |
| Initial body weight (g) | 216.00 ± 3.06 | 216.67 ± 4.41 | 208.33 ± 4.26 | 222.67 ± 3.71 | 0.157 |
| Final body weight (g) | 307.67 ± 1.45 | 311.33 ± 5.21 | 275.67 ± 3.18 *** | 308.00 ± 1.53 ### | 0.000 |
| Body weight change (g) | 91.67 ± 1.67 | 94.67 ± 1.45 | 67.33 ± 1.45 *** | 85.33 ± 2.73 ### | 0.000 |
| Relative liver weight (%) | 2.69 ± 1.21 | 2.82 ± 4.41 | 2.50 ± 0.04 | 2.58 ± 0.01 | 0.064 |
| Relative brain weight (%) | 0.57 ± 0.01 | 0.56 ± 0.03 | 0.60 ± 0.02 | 0.56 ± 0.01 | 0.518 |
| Groups | |||||
|---|---|---|---|---|---|
| Parameters | Control | Alhagi | Lead | Lead + Alhagi | p-Value |
| ALT (U/L) | 24.33 ± 2.96 | 30.33 ± 1.45 | 98.67 ± 4.49 *** | 44.67 ± 2.91 ### | 0 |
| AST(U/L) | 25.00 ± 3.51 | 28.67 ± 1.86 | 82.00 ± 3.61 *** | 34.00 ± 2.08 ### | 0 |
| GPx (U/g tissue) | |||||
| Liver | 24.51 ± 0.29 | 24.86 ± 0.14 | 16.63 ± 0.62 *** | 23.89 ± 0.49 ### | 0 |
| Brain | 21.07 ± 0.38 | 21.05 ± 0.53 | 14.00 ± 0.58 *** | 19.52 ± 0.29 ### | 0 |
| SOD (U/g tissue) | |||||
| Liver | 61.45 ± 0.53 | 62.51 ± 0.42 | 36.61 ± 0.49 *** | 60.28 ± 1.23 ### | 0 |
| Brain | 40.69 ± 0.43 | 40.71 ± 0.23 | 24.86 ± 0.81 *** | 39.19 ± 0.41 ### | 0 |
| GSH (µmol/g tissue) | |||||
| Liver | 1.33 ± 0.02 | 1.33 ± 0.01 | 0.60 ± 0.04 *** | 1.28 ± 0.03 ### | 0 |
| Brain | 3.21 ± 0.12 | 3.31 ± 0.12 | 1.63 ± 0.09 *** | 2.89 ± 0.06 ### | 0 |
| MDA (nmol/g tissue) | |||||
| Liver | 90. 33 ± 0.33 | 89.67 ± 1.20 | 183.33 ± 1.76 *** | 123. 67 ± 2.03 ### | 0 |
| Brain | 60.33 ± 0.33 | 59.00 ± 0.58 | 92.33 ± 1.45 *** | 73.00 ± 1.16 ### | 0 |
| Dopamine (ng/mL) | 26.77 ± 1.91 | 24.20 ± 2.98 | 10.25 ± 0.50 ** | 20.54 ± 0.87 ## | 0.001 |
| Groups | ||||||
|---|---|---|---|---|---|---|
| Organ | Lesion | Control | Alhagi | Lead | Lead + Alhagi | p-Value |
| Liver | Congestion of central vein | 0 ± 0 | 0 ± 0 | 3.33 ± 0.33 *** | 1.67 ± 0.33 ### | 0 |
| Congestion of portal vein | 0 ± 0 | 0 ± 0 | 4.00 ± 0.58 ** | 1.33 ± 0.88 ## | 0.002 | |
| Congestion of hepatic sinusoids | 0 ± 0 | 0 ± 0 | 1.33 ± 0.33 * | 0.33 ± 0.33 # | 0.012 | |
| Dilatation of hepatic sinusoids | 0.67 ± 0.33 | 0.33 ± 0.33 | 3.00 ± 0 *** | 1.00 ± 0 ### | 0 | |
| Lymphocytic infiltration | 0 ± 0 | 0 ± 0 | 2.00 ± 0.58 ** | 0.67 ± 0.33 ## | 0.009 | |
| Disruption of hepatocytes | 0 ± 0 | 0 ± 0 | 1.33 ± 0.33 * | 0.33 ± 0.33 # | 0.012 | |
| Cerebral cortex | Congestion of meninges | 0 ± 0 | 0 ± 0 | 4.00 ± 0.58 *** | 3.00 ± 0.58 ### | 0 |
| Congestion of cerebral cortex | 0 ± 0 | 0 ± 0 | 2.33 ± 0.33 *** | 0.33 ± 0.33 ### | 0 | |
| Congestion of choroid plexus | 0.33 ± 0.33 | 0 ± 0 | 4.00 ± 0.58 *** | 3.00 ± 0.58 ### | 0 | |
| Demyelination of nerve fibers | 0 ± 0 | 0 ± 0 | 4.00 ± 0 *** | 2.00 ± 0.58 ### | 0 | |
| Vacuolation of perineural spaces | 0.67 ± 0.33 | 0.67 ± 0.33 | 2.33 ± 0.33 | 1 ± 0.58 | 0.059 | |
| Neuropil vacuolation | 0 ± 0 | 0 ± 0 | 2.00 ± 0 *** | 0.33 ± 0.33 ### | 0 | |
| Cerebellar cortex | Congestion of cerebellar cortex | 0 ± 0 | 0 ± 0 | 1.33 ± 0.33 * | 0.33 ± 0.33 # | 0.012 |
| Disappearance of Purkinje cells | 0 ± 0 | 0 ± 0 | 2.67 ± 0.33 *** | 1.67 ± 0.33 ### | 0 | |
| Change of normal shape of Purkinje cells | 0 ± 0 | 0 ± 0 | 2.00 ± 0 *** | 2.67 ± 0.33 ### | 0 | |
| Coalescing of Purkinje cells together | 0 ± 0 | 0 ± 0 | 1.67 ± 0.33 ** | 0.33 ± 0.33 ## | 0.003 | |
| Hippocampus | Degenerated neurons | 0 ± 0 | 0 ± 0 | 4.00 ± 0.58 *** | 1.67 ± 0.33 ### | 0 |
| Widening of blood capillaries | 0 ± 0 | 0 ± 0 | 3.00 ± 0.58 *** | 2.00 ± 0 ### | 0 | |
| Vacuolation of neuropil | 0 ± 0 | 0 ± 0 | 3.00 ± 0 *** | 1.33 ± 0.33 ### | 0 |
| Groups | ||||||
|---|---|---|---|---|---|---|
| Organ | Negative Control | Control | Alhagi | Lead | Lead + Alhagi | p-Value |
| Liver | 0 ± 0 | 26.67 ± 1.67 | 42.33 ± 5.04 | 3.67 ± 0.67 *** | 13.00 ± 1.00 ### | 0 |
| Cerebral cortex | 0 ± 0 | 30.00 ± 2.89 | 60.00 ± 5.77 | 12.33 ± 1.45 *** | 28.33 ± 1.67 ### | 0 |
| Cerebellar cortex | 0 ± 0 | 13.00 ± 1.00 | 38.33 ± 6.01 | 1.67 ± 0.33 *** | 11.67 ± 1.67 ### | 0 |
| Hippocampus | 0 ± 0 | 30.00 ± 2.89 | 60.00 ± 5.77 | 3.67 ± 0.67 *** | 13.33 ± 0.88 ### | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, T.M.; Abo-Elmaaty, A.M.A.; Said, E.N.; Beheiry, R.R.; Moselhy, A.A.A.; Abdelgawad, F.E.; Arisha, M.H.; Saber, T.; Arisha, A.H.; Fahmy, E.M. Alhagi maurorum Ethanolic Extract Rescues Hepato-Neurotoxicity and Neurobehavioral Alterations Induced by Lead in Rats via Abrogating Oxidative Stress and the Caspase-3-Dependent Apoptotic Pathway. Antioxidants 2022, 11, 1992. https://doi.org/10.3390/antiox11101992
Saber TM, Abo-Elmaaty AMA, Said EN, Beheiry RR, Moselhy AAA, Abdelgawad FE, Arisha MH, Saber T, Arisha AH, Fahmy EM. Alhagi maurorum Ethanolic Extract Rescues Hepato-Neurotoxicity and Neurobehavioral Alterations Induced by Lead in Rats via Abrogating Oxidative Stress and the Caspase-3-Dependent Apoptotic Pathway. Antioxidants. 2022; 11(10):1992. https://doi.org/10.3390/antiox11101992
Chicago/Turabian StyleSaber, Taghred M., Azza M. A. Abo-Elmaaty, Enas N. Said, Rasha R. Beheiry, Attia A. A. Moselhy, Fathy Elsayed Abdelgawad, Mariam H. Arisha, Taisir Saber, Ahmed Hamed Arisha, and Esraa M. Fahmy. 2022. "Alhagi maurorum Ethanolic Extract Rescues Hepato-Neurotoxicity and Neurobehavioral Alterations Induced by Lead in Rats via Abrogating Oxidative Stress and the Caspase-3-Dependent Apoptotic Pathway" Antioxidants 11, no. 10: 1992. https://doi.org/10.3390/antiox11101992
APA StyleSaber, T. M., Abo-Elmaaty, A. M. A., Said, E. N., Beheiry, R. R., Moselhy, A. A. A., Abdelgawad, F. E., Arisha, M. H., Saber, T., Arisha, A. H., & Fahmy, E. M. (2022). Alhagi maurorum Ethanolic Extract Rescues Hepato-Neurotoxicity and Neurobehavioral Alterations Induced by Lead in Rats via Abrogating Oxidative Stress and the Caspase-3-Dependent Apoptotic Pathway. Antioxidants, 11(10), 1992. https://doi.org/10.3390/antiox11101992