Abstract
Ascorbic acid, as a one of the basic exogenous vitamins, occurs in the body in the form of ascorbate, known for its strong antioxidant and anti-inflammatory properties. The presented review shows not only the importance of ascorbate as a free radical scavenger but also summarizes its antioxidant action based on other mechanisms, including the activation of intracellular antioxidant systems and its effect on the NFκB/TNFα pathway and apoptosis. Ascorbate interacts with small-molecule antioxidants, including tocopherol, glutathione, and thioredoxin; it can also stimulate biosynthesis and the activation of antioxidant enzymes, such as superoxide dismutase, catalase, or glutathione peroxidase. Moreover, ascorbate promotes the activity of transcription factors (Nrf2, Ref-1, AP-1), which enables the expression of genes encoding antioxidant proteins. Additionally, it supports the action of other exogenous antioxidants, mainly polyphenols. In this regard, both DNA, proteins, and lipids are protected against oxidation, leading to an inflammatory reaction and even cell death. Although ascorbate has strong antioxidant properties, it can also have pro-oxidant effects in the presence of free transition metals. However, its role in the prevention of DNA mutation, inflammation, and cell apoptosis, especially in relation to cancer cells, is controversial.
1. Introduction
Ascorbic acid, commonly known as vitamin C, is one of the basic and best-known compounds necessary for the proper functioning of the human body. It was described and isolated for the first time in 1928 by the Hungarian biochemist Albert Szent-Györgyi, who was awarded the Nobel Prize in 1937. The name ‘ascorbic acid’ refers to scurvy (scorbutus), as the deficiency of this compound was initially associated solely with the development of this disease [1].
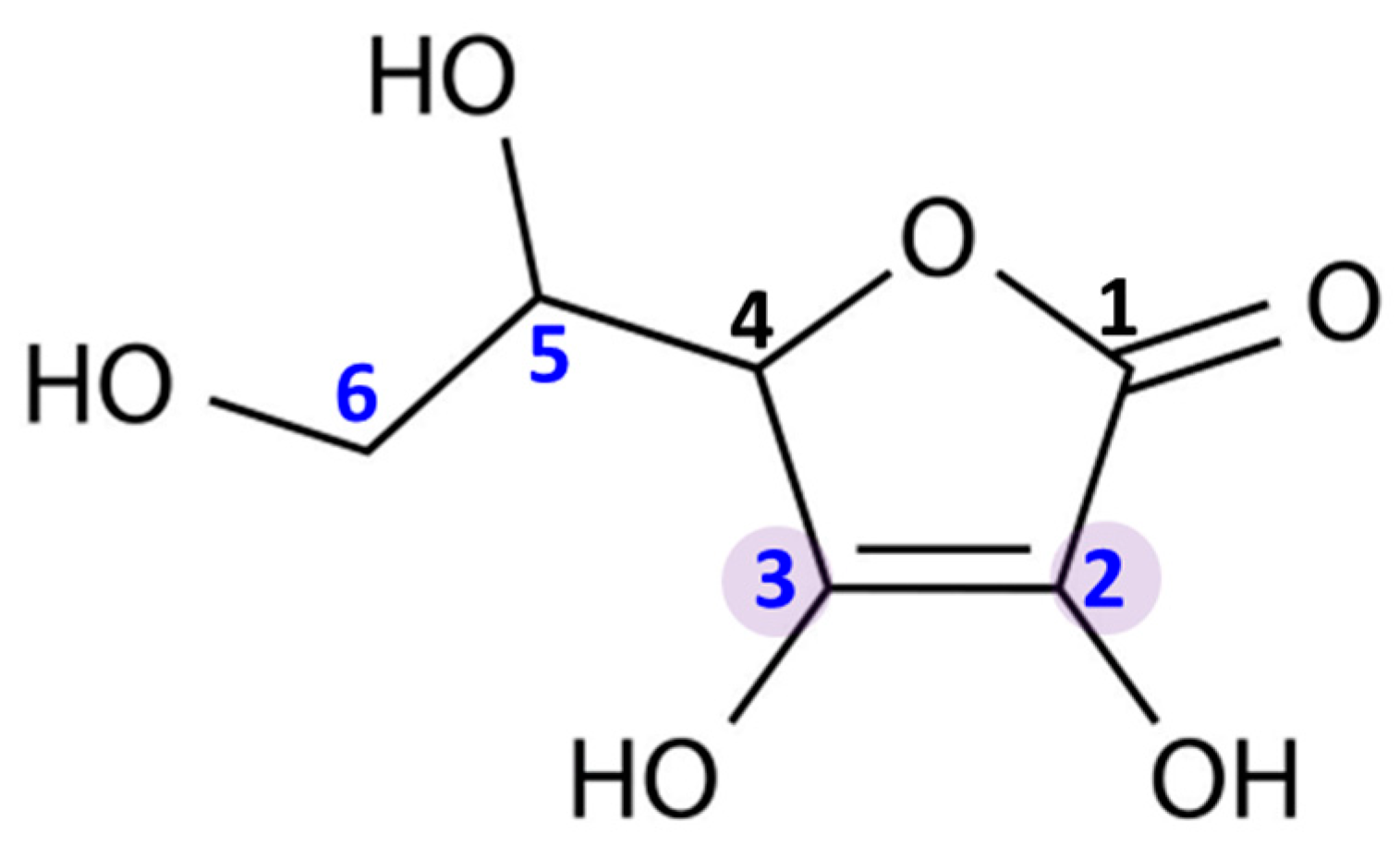
Ascorbic acid is an organic compound belonging to the group of unsaturated polyhydroxy alcohols. It is a water-soluble ketolactone, whose center is formed by a five-membered carbon ring (Figure 1). Ascorbic acid has strong reducing properties, resulting from the presence of double bonds at the C2 and C3 carbons, as well as four hydroxyl groups in positions C2, C3, C5, and C6. Moreover, due to the proximity of the hydroxyl and carbonyl groups, ascorbic acid is an ideal hydrogen or electron donor, which makes it the cofactor of many enzymatic reactions in living organism [2].
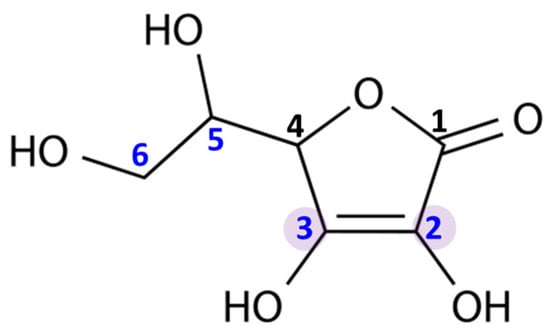
Figure 1.
Chemical structure of ascorbic acid. The marked carbon atoms are important for the antioxidant properties: the carbon atoms with hydroxyl group in positions 2, 3, 5, and 6; and double bonds between carbon atoms in positions 2 and 3.
2. Bioavailability and Main Functions
In most mammals, ascorbic acid can be produced from glucose in a multi-step pathway; however, in humans, its synthesis is not possible due to the lack of L-gulonolactone oxidase. Therefore, ascorbic acid must be supplied in the diet [3]. It is assumed that the daily requirement for ascorbic acid intake is different for women and men, and amounts to 75 mg and 90 mg, respectively [4]. Common opinion regards citrus as the main and most abundant source of ascorbic acid; however, analytical studies show a number of fruit and vegetables, as well as meat, as the foods from which the human body can derive this compound (Table 1) [5,6,7].

Table 1.
The amount of ascorbic acid in basic food products with the greatest levels [8,9,10,11].
Under physiological conditions, ascorbic acid is ionized to ascorbate anion, which, after entering the digestive tract as a nutrient, is absorbed from the lumen of the intestine—mainly by enterocytes—and circulates with the blood throughout the body, so it can be taken up by all cells [12]. Another way of introducing ascorbate into the body is through its transdermal application [13]. Moreover, it can be reabsorbed by the cells of the renal tubular epithelium into the blood filtered by the kidneys [14]. The physiological concentration of ascorbate in the blood is 10–100 µM [4]. As ascorbate is soluble in water, its transfer through lipid plasma membrane is hampered. The simple diffusion of ascorbate plays only a small role in its transport across membranes and is thus assisted by the specific transport systems. The best known mechanisms of ascorbate transport are: (I) diffusion through transmembrane channels; (II) facilitated diffusion through exocytosis in secretory vesicles; (III) transmission by glucose-sensitive transporters; and (IV) secondary active transport through the sodium-dependent transporters SVCT1/2 [15,16]. Therefore, the bioavailability of ascorbate to all the cellular processes where it is needed is high, provided that a varied diet is consumed.
The presented structure of ascorbic acid (Figure 1) is the reason for its wide range of biological activities. It is currently known that ascorbate is necessary for the proper functioning of the human body, because it is responsible for numerous processes, including the strengthening and sealing of blood vessels, the regulation of microbial absorption by leukocytes, and lowering the level of cholesterol, as well as acceleration of wound-healing [17,18]. Other important functions of the compound include the regulation of collagen production, slowing down the aging process of the skin, and lowering blood pressure [19,20]. The participation of ascorbate in the aforementioned processes is partially based on its anti-antioxidant and anti-inflammatory properties. A deficiency of ascorbate is associated with numerous disorders, such as general weakness, fatigue, muscle and joint pain, a lack of appetite lowering the immunity, the tendency to bruise, and swollen and bleeding gums. Moreover, chronic ascorbate deficiency may also contribute to the development of neoplastic changes and atherosclerosis [21,22]. On the other hand, excessive supplementation of this compound can also be unfavorable. It has been reported that ascorbic acid overdosage causes gastrointestinal disturbances, including abdominal pain, nausea, vomiting and diarrhea, and skin rash [23]. Moreover, very high doses of ascorbic acid (>800 mg) may also contribute to the acidification of the urine and, consequently, to the formation of kidney stones [24]. However, ascorbic acid is water-soluble, which means that it is easily excreted with sweat and urine; hence, it is not easy to overdose on this broad-spectrum compound.
3. Antioxidant Properties
3.1. Suppression of Generation of Free Radicals
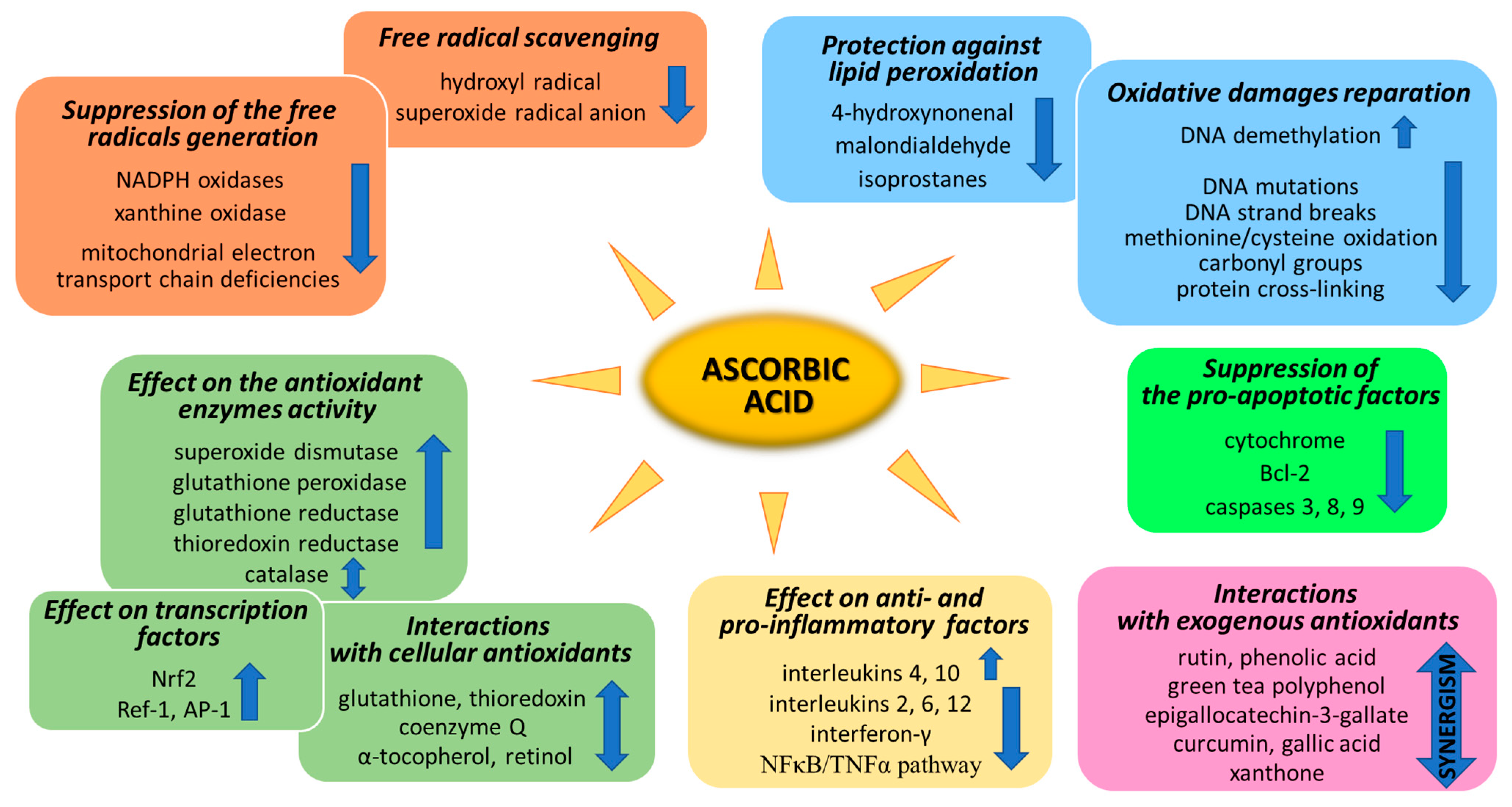
Ascorbic acid is one of the basic low-molecular antioxidants functioning in the human body. It takes part in the regulation of the levels of reactive oxygen species (ROS) and the effectiveness of other antioxidants. Ascorbic acid regulates the level of ROS as early as at the stage of their formation. The main sources of ROS are the mitochondrial respiratory chain and specific enzymes, such as NADPH oxidases (NOXs) or xanthine oxidase (XO) [25]. Ascorbic acid (100 µM) has been shown to modify both of the above systems (Figure 2). XO is an enzyme that generates ROS through the oxidation of hypoxanthine to xanthine and then to uric acid. Both of these reactions are necessary for the functioning of the organism, but the result of their occurrence are hydrogen peroxide and the generation of the superoxide radical anion [26]. It is known that XO can also directly oxidize ascorbate [27]; however, ascorbate supplementation significantly protects the organism against XO hyperactivity [28]. Moreover, the latest studies show that due to the possibility of continuous supplementation with ascorbate, the resulting inhibition of XO activity in plasma significantly contributes to the improvement in gout treatment [29,30,31,32]. On the other hand, ascorbate has no effect on the activity of XO under physiological conditions, as found with skin cells (fibroblasts and keratinocytes). However, in cells under stress caused by, e.g., UV radiation or hydrogen peroxide, treatment with ascorbate (100 µM) inhibited XO hyperactivation [33,34]. Moreover, such decrease in XO activity induced by ascorbate may be useful in preventing or reducing reperfusion injuries also in stimulated neutrophils (6 µM of ascorbate) [35] and delay the progression of hyperuricemic nephropathy (10 mg/kg/day of ascorbate) [32].
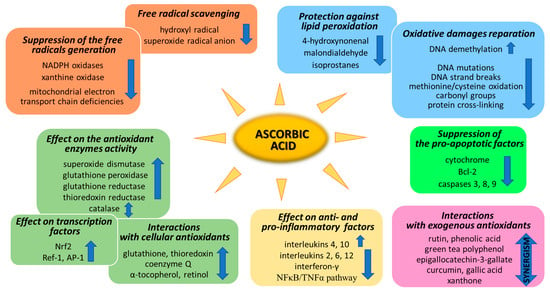
Figure 2.
Pathways of the antioxidant action of ascorbic acid.
NOXs is a group of enzymes widespread in cells transmembrane that, similarly to XO, generate the superoxide radical anion or hydrogen peroxide as the signaling molecules, whose controlled levels enable the proper functioning of cells [36]. However, NOX hyperactivity inevitably leads to oxidative stress, which can be prevented by ascorbate [37]. As described in the case of XO in skin cells, NOX activity is also not affected by ascorbate (100 µM) in healthy cells. Only strong stress inducers, such as hydrogen peroxide or UVB irradiation, activate NOX strongly enough for ascorbate to start inhibiting the enzyme [33,34]. Other data show that, due to the fact that ascorbic acid can inhibit NOX in microvascular endothelial cells, the vitamin may reduce the development of sepsis [38,39]. However, ascorbate (100 µM) also shows the activating properties of NOX in embryonic stem cells, where cardiomyogenesis increases as a result of NOX-induced enhanced levels of ROS [40].
Mitochondria also play an important function in the action of ascorbate antioxidant. On the one hand, due to the activity of Complexes II and III, mitochondria regenerate ascorbate from its oxidized form, thus maintaining redox status both in the mitochondrial matrix and in the cytoplasm [41]. On the other hand, ascorbate (5 mM) favors sealing the mitochondrial electron transport chain [42] and reduces the superoxide radical anion generation, especially in cells with electron transport chain deficiencies [43]. However, the described effect raises concerns as to whether the influence of ascorbate on mitochondrial processes does not reduce the elimination of damaged cells by apoptosis, thus promoting carcinogenesis [44].
3.2. ROS Scavenging by Ascorbic Acid
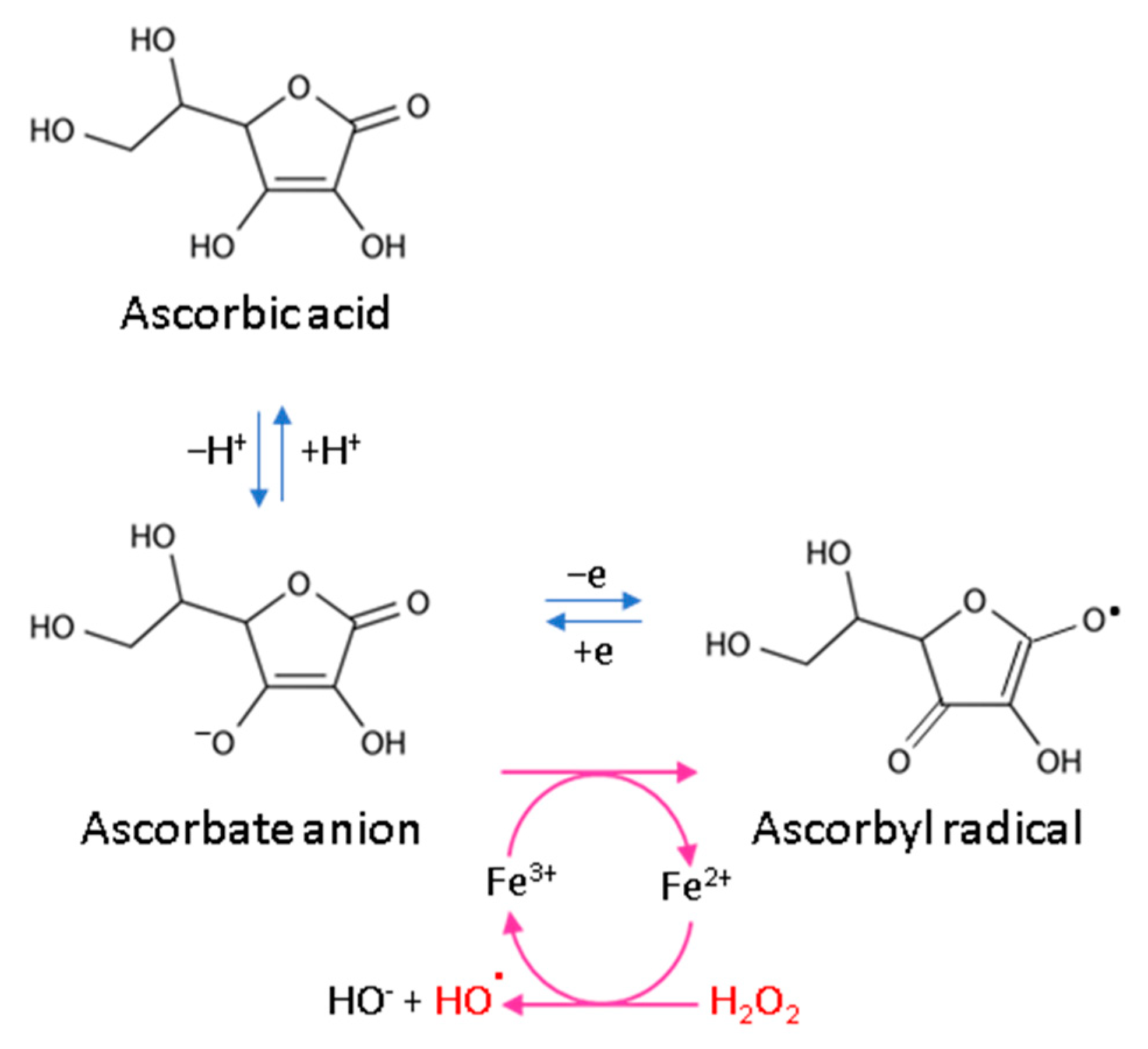
Every living organism constantly generates reactive oxygen and nitrogen species (ROS/RNS) that participate in its physiological activities. However, their level increases significantly in pathological conditions as a result of dysfunction of pro-oxidative systems of cells/biological fluids. In both cases, ROS should be counterbalanced by an effective natural antioxidant system. A disruption of this balance leads to oxidative stress and, as a consequence, the possibility of ROS interactions with the endogenous components of the organism, such as nucleic acids, proteins, lipids, and small molecules, thus causing irreversible oxidative damage to cells and their components. A living cell’s antioxidant system has three lines of defense: (I) free radical scavenging; (II) biosynthesis and activation of antioxidant enzymes; and (III) the repair of oxidative damage. Ascorbate has been shown to be a molecule involved in all these stages (Figure 2). Its antioxidant properties as a scavenger of free radicals are related to its ability to form a stabilized radical (Figure 3). This allows ascorbate to react with more reactive molecules, including the hydroxyl radical or the superoxide radical anion, which prevents their interaction with biomolecules important for proper cell functioning [45]. On the other hand, the strong antioxidant properties of ascorbate can induce the transformation of Fe3 + into Fe2 +. However, the ascorbate–Fe2 + chelate may catalyze ROS generation via Fenton’s reaction [45]. Hence, ascorbate is an antioxidant; however, the products of its transformation show pro-oxidant properties in the presence of oxygen [46,47]. Additionally, the oxidation of ascorbate results in the formation of the ascorbate radical (Asc•−) and a high flux of H2O2 [48]. Therefore, one should be very careful in formulating hypotheses and pay attention to whether the results obtained in the experiments come directly from ascorbate or possibly from reactive oxygen species generated during cell supplementation.
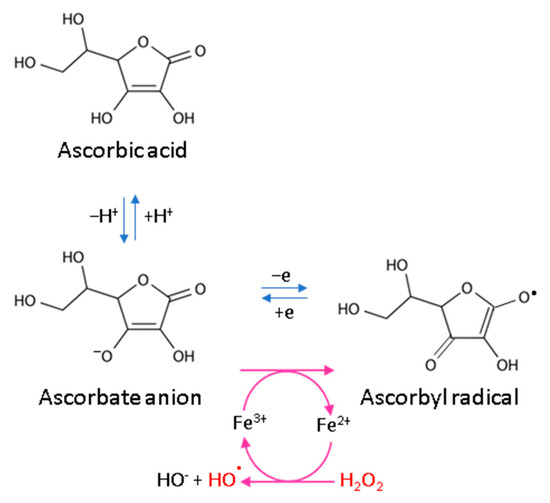
Figure 3.
Scheme of ascorbic acid reaction under oxidative conditions.
3.3. Ascorbic Acid Interaction with the Cellular Antioxidant System
To prevent oxidative stress or reduce its destructive effect on cellular compartments, ascorbate not only suppresses the generation of free radicals or directly reduces their amounts, but it also significantly stimulates the cellular antioxidant system at the level of low molecular weight antioxidants, as well as by acting on antioxidant enzymes (Figure 2).
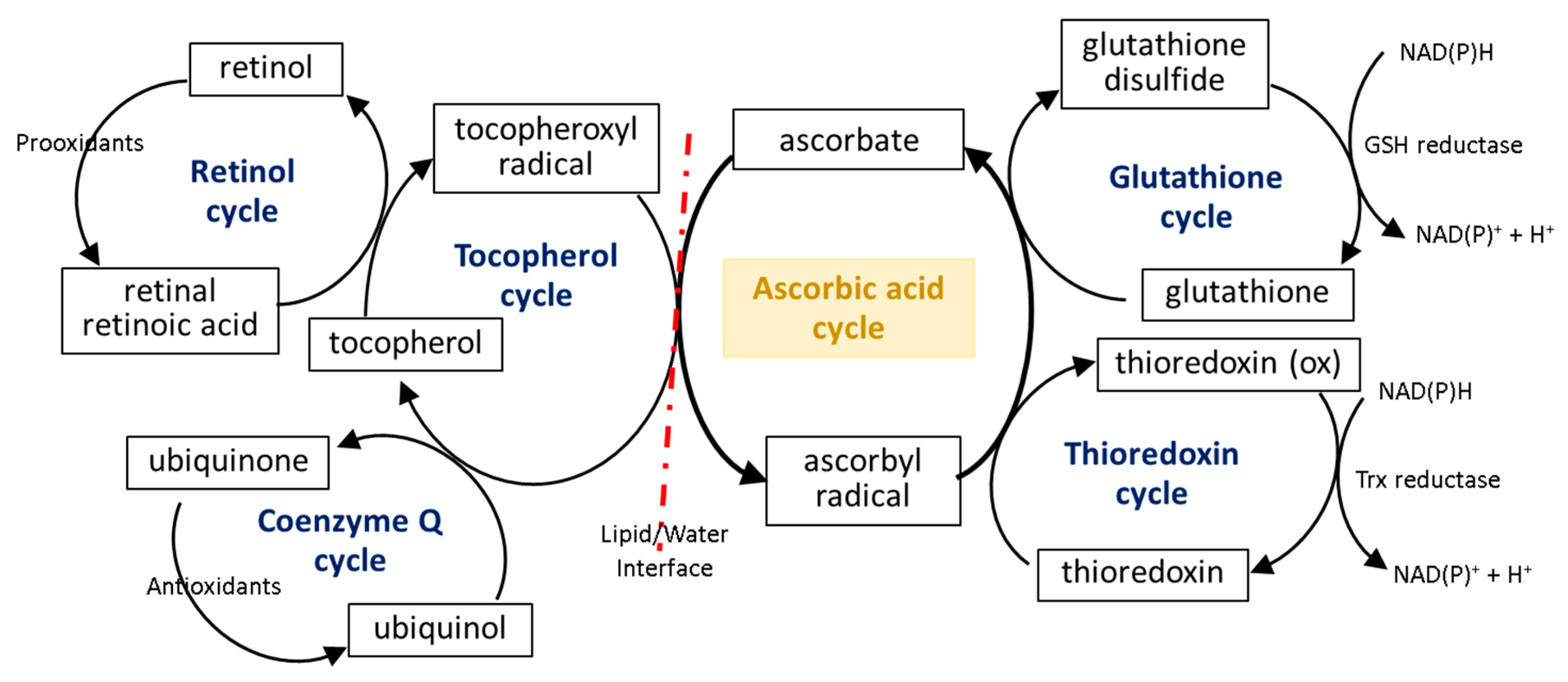
The first line of the natural cell protection against the uncontrolled overproduction of ROS is created by low molecular weight antioxidants, such as glutathione (GSH), thioredoxin (Trx), coenzyme Q, α-tocopherol, and retinol. Ascorbic acid also belongs to this group, but its antioxidant effect is limited to ROS elimination as well as the interaction with the other molecules mentioned earlier. The small molecules in the center of the oxidation–reduction reaction cycle also include tocopherol and GSH/Trx (Figure 4). As some of these compounds (GSH/Trx) are not soluble in lipids, they can act only in cytoplasm and are not capable of protecting the cell membrane against ROS. Furthermore, ascorbate is a hydrophilic molecule; however, it can react with tocopherol and its derivatives over the lipid/water interface. Under oxidative conditions, tocopherol neutralizes free radicals, which attack cell membrane components, and itself becomes an oxidized form (tocopheroxyl radical). Ascorbate from cytoplasm restores the reduced form of tocopherol in the lipid fraction, owing to which it can further protect cell membranes. This reaction leads to the oxidation of ascorbate to the ascorbyl radical, which is reduced in the cytoplasm by the thiol group of GSH or Trx [49,50,51]. In addition, tocopherol reduced by ascorbic acid can interact with lipophilic retinol and control its reduced pool [52,53], which allows the maintenance of the continuity of the membranes. Another lipophilic compound, i.e., coenzyme Q, which interacts with the tocopheroxyl radical, regenerating its antioxidant form, can also participate in these cycles [54]. Moreover, significant data suggest that the antioxidant properties of coenzyme Q not only accompany but also complement the attributes of ascorbic acid [55,56,57,58]. However, so far it has not been possible to accurately describe their relationship.
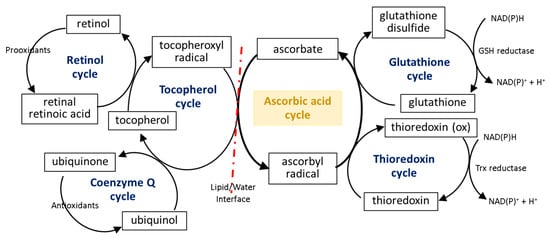
Figure 4.
Scheme of the interaction of ascorbic acid with glutathione (GSH), thioredoxin (Trx), coenzyme Q, α-tocopherol, and retinol [49,50,51,53].
The described reduction/oxidation cycles of low molecular weight antioxidants can also be modified by the effect of ascorbate on the activity of enzymes involved in these reactions. So far, numerous experiments have shown that ascorbic acid can influence the GSH-based action of enzymes, which prevents disturbances in the GSH-based system induced by chemical factors or the development of diseases [59,60]. However, there are two patterns of action of this compound: (I) ascorbic acid significantly increases the level of GSH without affecting or even reducing the activity of enzymes associated with this molecule (glutathione peroxidase, GSH-Px; glutathione reductase, GSSG-R) and (II) the action of ascorbic acid is primarily based on inducing GSH-Px and GSSG-R activity. It has been found that hepatoprotective and gastroprotective effects of ascorbate are mainly based on the activation of GSH-Px and GSSG-R [61,62], while in the case of UV-irradiated skin cells, ascorbic acid leads to GSH-Px and GSSG-R down-regulation with a simultaneous increase in the GSH level [33,34]. On the other hand, the antioxidant effect of ascorbate (14–47 mM) is strong enough to replace the GSH-based system, observed as a decrease in GSH-Px activity and the GSH level in bovine semen [63].
Another important element of the maintenance of redox balance in cells is Trx reductase (TrxR) activity. So far it has been widely shown that the oxidized form of ascorbate activates TrxR, resulting in the restoration of the reduced form of ascorbate [18]. Additionally, it has been noted that the induction of TrxR activity by ascorbate is accompanied by the stimulation of the GSH-based antioxidant system [64]. In skin cells, ascorbic acid (100 µM) is capable of enhancing both the Trx level and TrxR activity under physiologic conditions; it can also prevent UV-induced lowering of these parameters [33]. Moreover, ascorbic acid also affects inflammasome functioning through the stimulation of the thioredoxin-interacting protein (TXNIP), thus reducing ROS production and the expression of pro-inflammatory proteins (interleukins 1β, 18 and caspase-1) in human macrophages [65]. On the other hand, the effect of ascorbic acid on TrxR activity may be a promising tool in cancer therapies focused on TrxR hyperactivity [66].
Ascorbic acid also stimulates the antioxidant system by affecting the activity of other antioxidant enzymes. An example of such an enzyme is superoxide dismutase (Cu, Zn-SOD; SOD), responsible for the conversion of the superoxide radical anion to the less cytotoxic hydrogen peroxide. There are no conclusive data concerning the effect of ascorbic acid on SOD activity in cells under physiologic conditions. However, a series of data points to an essential function of this vitamin on the SOD activity under oxidative stress. In all cases, regardless of whether the oxidative stress was caused by heavy metals, UV radiation, brain damage, or depression, supplementation with ascorbate (0.1–1 mM, 60–200 mg/kg/day) significantly increased the activity of SOD in in vitro cultured cells, as well as in animal plasma [33,34,59,67,68,69]. As a result, not only does this lead to a lowering of the level of the superoxide radical anion but it also protects lipids against oxidation observed as a decreased level of products of lipid peroxidation, including malondialdehyde (MDA) [33,34,68]. On the other hand, ascorbic acid (10–50µM) in SOD-depleted cells also reduces the superoxide radical anion level, thus preventing oxidative stress [70,71]. However, other studies indicate that oral ascorbic acid supplementation (0.2–1 g/day) does not significantly affect the superoxide dismutase activity in human plasma [72].
The hydrogen peroxide formed in the cells as a result of the SOD-catalyzed reaction is decomposed into oxygen and water by another antioxidant enzyme, i.e., catalase (CAT). Ascorbate influences the activity of this enzyme in various ways, depending on the type of cells. In the case of plant cells, high concentrations of ascorbic acid, constituting the signal pointing to a high antioxidant potential of the cell, reduce catalase activity and cause an increase in the level of hydrogen peroxide [73], allowing the molecule to perform the signaling function. A similar effect of ascorbate in the case of CAT activity is also observed in rapidly proliferating mammalian cells, especially cancer cells, e.g., gastric cancer cells, glioblastoma, and carcinoma cells [74,75]. However, in the case of non-neoplastic cells, such as keratinocytes, ascorbic acid (100 µM) significantly enhances CAT activity, thereby increasing the antioxidant potential of these cells, which is important due to the peripheral location of keratinocytes in the skin [33]. In the same experiment, it has been shown that following UV-induced oxidative stress, ascorbate affects catalase activity in different skin cells in various ways. In UV-irradiated keratinocytes, ascorbic acid stimulates CAT activity and protects cells against UV-induced hydrogen peroxide overexpression, while this effect is not observed in UV-irradiated skin fibroblasts [33].
3.4. Effect of Ascorbic Acid on Cytoprotective Gene Transcription
Another aspect of the action of ascorbic acid as an antioxidant is its effect on gene expression, resulting in the biosynthesis of antioxidant proteins. Among the most important transcriptional factors involved in cellular antioxidant response are Nrf2 (nuclear factor, erythroid 2-like 2), Ref-1 (redox effector factor 1), and AP-1 (activator protein 1) (Figure 2).
Nrf2 is a protein common in the cytoplasm regardless of the oxidation–reduction conditions. Under physiological conditions, it is attached to its inhibitor, i.e., Keap1, which under oxidative conditions changes conformation and dissociates from Nrf2. The free form of Nrf2 transfers to the nucleus, where it heterodimerizes with sMaf protein. The created complex is capable of binding to the DNA in a sequence-specific manner, i.e., to the antioxidant response element (ARE), and starts the biosynthesis of antioxidant proteins [76,77,78]. Ascorbate (2.9–224.5 mg/kg/day) is known as an activator of the Nrf2 factor, as well as the whole Keap1/Nrf2/ARE pathway, and its deficiency leads to impaired Nrf2 action resulting in inflammation and apoptosis [79,80]. It is especially pronounced in cells under chemically or physically induced oxidative stress [33,81]. That is the most important in the case of cells that are constantly exposed to the harmful stressors, such as hepatocytes and keratinocytes [33,82,83]. On the one hand, in keratinocytes, ascorbate (100 µM) reduces the level of Nrf2 inhibitor, i.e., Keap1 protein, and increases free Nrf2 expression, as well as its activators, including p62 and KAP1, on the other [33,83]. At the same time, ascorbate (1 mM) favors Keap1 conformational changes induced by other antioxidants, such as polyphenols, which additionally stimulates Nrf2 dissociation [84]. In hepatocytes, Nrf2 activation by ascorbic acid (1–10 µM) results in the expression of antioxidant enzymes observed as a reduced level of lipid hydroperoxides [82]. On the other hand, some data indicate that a high ascorbic acid (1 mM) concentration may lead to a disturbed Keap1/Nrf2/ARE pathway activation [85]. However, in accordance with the dual role of Nrf2 in cancers, its activation by ascorbate becomes an ambiguous and possibly a dangerous outcome [86,87].
The activities of Ref-1 and AP-1 factors are closely related to each other. Ref-1 is a Trx-dependent endonuclease that facilitates AP-1 DNA-binding activity [88], while AP-1 is a heterodimer composed of proteins belonging to the c-Fos, c-Jun, ATF, and Maf families and manifests transcriptional activity through the regulation of gene expression in response to a variety of stimuli, including cytokines, growth factors, and oxidative stress [89]. Only oxidized ascorbate (1 µM) indirectly affects Ref-1 activity, in accordance with the decrease in Trx levels [64]. In the case of AP-1 ascorbic acid, it has been recognized as a molecule that mutes ascorbate action in epidermal keratinocytes, thus preventing these cells from staying alive when their DNA is oxidatively modified, which would pose a threat consisting of the formation of cancer [90]. Moreover, in keratinocytes exposed to UV radiation, where AP-1 should be activated, supplementation with ascorbic acid induces reduced levels of the active positive component, i.e., c-Jun, and increases the levels of fra-1 messenger, which is an AP-1 inhibitor [91]. On the other hand, the similar silencing of AP-1 activity by ascorbate (200 µM) is observed in respiratory epithelial cells, resulting in reduced levels of signaling molecules, such as pro-inflammatory chemokines [92].
4. Ascorbic Acid and Oxidative Modifications
4.1. Oxidative Damage Repair
One of the most dangerous types of damage in cells occurring under oxidative stress are oxidative DNA modifications, including single-strand breaks and oxidative base damage with 8-oxoguanine (8-oxoG) formation [93]. DNA polymerase β is involved in the repair of both of these accumulated damages [94]. There are no clear data on how ascorbate affects the activity of DNA polymerases; however, it has been found that ascorbate can directly reduce DNA mutations induced by H2O2, including 8-oxoG [95], as well as DNA strand break levels [96] (Figure 2). On the other hand, ascorbate (100 µM) enhances the activity of TET (ten eleven translocation) dioxygenases in the oxidation of 5-methylcytosine in a various cell types [97,98]. Hence, as a result of the action of ascorbate, the silencing mechanism of gene expression induced by methylation is canceled out. As a result of this ascorbate (5 mM) effect, the expression of a known tumor suppressor gene, i.e., SMAD1, in lymphoma cells is increased [99], which can also be used in therapy of other cancer types [100,101].
ROS also interact with amino acids and lead to the generation of a number of oxidatively modified proteins with impaired functions. The most common oxidative modifications of proteins are methionine/cysteine oxidation, carbonyl group formation, and cross-linking [102]. Some of them are reversible and, as a donor of hydrogen or electron, ascorbate can reduce these proteins and restore their functions [2]. However, many modifications are irreversible and, moreover, their proteolysis is impossible due to disruptions in the functioning of proteasomes under oxidative stress [103]. Thus, the protective properties of ascorbate (1 g/day) are based on the elimination of the action of various proteasome inhibitors [104,105] and the promotion of the removal of oxidatively damaged proteins from the cytoplasm of cells (Figure 2). Unfortunately, the protective action of ascorbate against protein oxidation with the formation of protein radicals is strictly dependent on the ascorbate concentration, which is often insufficient in tissues in vivo (physiological conditions) [106,107,108].
4.2. Prevention Lipid Peroxidation
The destructive activity of ROS also relates to lipids. Molecules included in the lipid bilayer as well as non-membrane-forming lipids often undergo non-enzymatic or enzymatic oxidative reactions with a simultaneous formation of lipid peroxidation products. These are reactive aldehydes (e.g., 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA)) or cyclization products, including isoprostanes [109]. The compounds are important signaling molecules in cell functioning; however, their overproduction under oxidative stress disturbs the continuity of biological membranes, as well as the basic metabolic processes of cells [110]. So far, it has been described that when reduced by approximately 20%, the levels of ascorbate in cells or plasma inevitably favor the formation of lipid peroxidation products in the whole organism [111,112,113]. Therefore, it is clear that ascorbate is an important molecule in the prevention of lipid peroxidation [61,114,115] (Figure 2). It has been found that ascorbate (100 µM, 60–500 mg/kg/day) significantly protects cells/organisms against an increase in 4-HNE and MDA levels under stress conditions, including UV-induced oxidative stress in skin cells [33,34,84] or cornea [116] and the toxic effects of xenobiotics in liver cells [117,118,119,120] and erythrocytes [121], as well as oxidative stress-related myonecrosis [122]. The described protective action of ascorbate connected with the level of reactive aldehydes is strongly correlated with oxidative stress. In the case of the action of ascorbic acid on the level of isoprostanes, one of the main aspects is to arrest the pro-inflammatory effect of these products of lipid peroxidation. Ascorbate (1 g/day) efficiently reduces the expression of isoprostanes in the plasma of patients with a wide variety of diseases, including diabetes or hypertension [123,124]. Thus, through its antioxidant properties, the molecule prevents inflammation mediated by lipid peroxidation.
5. Anti-Inflammatory Properties
Ascorbic acid is widely recognized as a molecule with versatile anti-inflammatory properties (Table 2). Hence, it is believed that its consumption provides a low level of C-reactive protein (CRP), which constitutes a stable downstream marker of inflammation in plasma. However, an analysis of the obtained results shows that the activity of the vitamin is not unequivocal [125], as according to one set of data, the CRP level in human plasma is significantly reduced by ascorbate supplementation (causing a 4-fold increase in the concentration of ascorbate in plasma) [126], and according to others, the vitamin has no effect on CRP [127]. Additionally, there are no clear data about the effect of ascorbate on plasma anti-/pro-inflammatory cytokines, due to the fact that it is used in a mixture with other protective compounds, such as α-tocopherol, β-carotene, or 25-hydroxyvitamin D, which significantly downregulate pro-inflammatory molecules, such as interleukin 6 (IL-6) or interferon-γ (IFN-γ), and, to a different extent, influences the levels of anti-inflammatory interleukin 4 (IL-4) in human plasma [128,129]. On the other hand, ascorbate (3 g per oral dose) has no regulatory effect on IL-6 and IL-8 level in human plasma under high oxidative stress, such as during the initiation of a cardiopulmonary bypass [130].
Regardless of the above, ascorbate has a more singular effect on inflammatory signaling in cells, where it unambiguously lowers the expression of pro-inflammatory mediators, thus reducing the inflammatory reaction. One of the main known pro-inflammatory signaling pathways affected by ascorbic acid is the NFκB/TNFα pathway [131]. Under physiologic conditions, NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) creates a complex with IκK (inhibitor of nuclear factor kappa-B), which causes its inactivation. Only the phosphorylation of IκK by IKK (an inhibitor of nuclear factor kappa-B kinase) causes the dissociation of active NFκB subunits. As a result, pro-inflammatory cytokines, including interleukins and TNF-α (tumor necrosis factor α), are transcribed [132]. So far the effect of ascorbate on the NFκB/TNFα pathway has been strongly connected with its antioxidative properties, including the reduced ROS production [133], leading to lowered NFκB levels in cells exposed to the harmful effects of the external environment [33,134]. Moreover, ascorbate (20 mM) also decreases NFκB-depended genes transcription through the activation of kinases involved in IκK phosphorylation [135,136], as well as the suppression of the DNA-binding activity of NFκB [137]. As a result, the levels of pro-inflammatory factors in cells are lowered. It has been described that ascorbate (500 mg/day) significantly reduces IL-6 and TNF-α levels in brain tissue [138] and inhibits IL-6 protein release from a contracting skeletal muscle [139]. Moreover, ascorbic acid significantly downregulates the expression of pro-inflammatory factors (IL-6, IL-12, and TNF-α) and upregulates anti-inflammatory cytokines (IL-4 and IL-10) [140] in mouse splenocytes. Additionally, ascorbic acid (2.3 mM) reduces IL-2 and IL-6 production through the reduction of the proliferation of mononuclear cells in porcine peripheral blood [141,142]. On the other hand, ascorbate does not decrease TNF-α nor upregulate the IL-10 level under high oxidative stress, as observed in the endometrial tissue of rats [143].
The effect of ascorbate on the NFκB activity may also be connected with its influence on the interaction between the NFκB/TNFα and Nrf2/Keap1 pathways. The Nrf2-induced expression of heme oxygenase 1 inhibits the pro-inflammatory signaling operated by NFκB [144], while free Keap1 following Nrf2 dissociation can additionally amplify this effect, through the production of adducts with IKK, which is a positive regulator of NFκB [145]. Thus, the activation of Nrf2 by ascorbate contributes significantly to the reduction of the activity of NFκB. Therefore, as a result of all the above, ascorbate has a preventive effect at different levels of pro-inflammatory pathways’ activation and action [146].

Table 2.
Summary of the effects of ascorbate on individual inflammatory responses.
Table 2.
Summary of the effects of ascorbate on individual inflammatory responses.
| Factor | Biological Material | Conditions | Effect of Ascorbic Acid | Refs. |
|---|---|---|---|---|
| CRP | Plasma | Physiological conditions | Downregulation | [126] |
| CRP | Plasma | Inflammation (cardiopulmonary bypass graft surgery) | No effect | [127] |
| IFN-γ | Plasma | Physiological conditions | Downregulation | [128] |
| IL-4 | Plasma | Oxidative stress (Alzheimer’s disease) | Upregulation | [129] |
| IL-6 | Plasma | Oxidative stress (Alzheimer’s disease) | Downregulation | [129] |
| IL-6 IL-8 | Plasma | Oxidative stress (cardiopulmonary bypass initiation) | No effect | [130] |
| NFκB | Skin cells | Oxidative stress (UV irradiation) | Downregulation | [33,134] |
| NFκB | Cell lines ECV304, HUVEC, HeLa, U937, HL-60, MCF7 | Inflammation (induced experimentally/tumor proliferation) | Activation of kinases involved in IκK phosphorylation | [135,136] |
| NFκB | Acute myeloid leukemia | Inflammation (tumor proliferation) | Suppression of NFκB binding to DNA | [137] |
| TNF-α | Brain tissue | Physiological conditions neurotoxicity (induced experimentally) | Downregulation | [138] |
| TNF-α | Splenocytes | Inflammation (induced experimentally) | Downregulation | [140] |
| TNF-α | Endometrial tissue | Oxidative stress (endometritis) | No effect | [143] |
| IL-6 | Brain tissue | Physiological conditions neurotoxicity (induced experimentally) | Downregulation | [138] |
| IL-6 | Skeletal muscle | Contracting skeletal muscle | Downregulation | [139] |
| IL-6 IL-12 | Splenocytes | Inflammation (induced experimentally) | Downregulation | [140] |
| IL-4 | Splenocytes | Inflammation (induced experimentally) | Upregulation | [140] |
| IL-2 IL-6 | Peripheral blood mononuclear cells | Animals with hereditary deficiency in ascorbate synthesis | Downregulation | [141,142] |
| IL-10 | Splenocytes | Inflammation (induced experimentally) | Upregulation | [140] |
| IL-10 | Endometrial tissue | Oxidative stress (endometritis) | Upregulation | [143] |
6. Ascorbic Acid and Apoptosis
Uncontrolled ROS generation leading to oxidative stress has been described as an inherent factor causing apoptosis [147]. Thus, a number of antioxidants have the capacity to prevent apoptosis in its various stages [148]. Ascorbate is also not insignificant in this regard. Its primary importance is the suppression of drug-induced apoptosis through the direct scavenging of mitochondrial superoxide anions [149]. At the same time, ascorbate (100 µM) significantly reduces the level of DNA damage, thereby preventing pro-apoptotic signaling [150]. This highly active vitamin also influences the levels of pro-apoptotic factors, including cytochrome c, Bcl-2, and caspases 3, 8 and 9, which have been established in the case of UV-irradiated skin cells particularly [33], as well as in rat brains with potassium dichromate-induced damage [151].
In the case of cells with disturbed metabolism, the action of ascorbate is completely different, as observed in neoplastic cells. It has been found that in melanoma or acute myeloid leukemia cells, ascorbic acid (0.25–1 mM) induces apoptosis through Bcl-2 overexpression and caspase 3 and 9 activation [152,153]. There is also a number of data ascorbate-induced (1–15 mM) apoptosis in cancer cells, such as human colon cancer cells [154], breast cancer cells [155,156], cervical cancer cells [157], or colorectal cancer cells [158]. However, the mechanism and the selective nature of its action is still unknown and may be related to the pro-oxidative activity of ascorbate [159]. On the other hand, a number of studies suggest that in pharmacologic doses (10 mM), ascorbate exhibits anti-cancer effects and may have a potential for use in the treatment of cancer through the induction of both oxidative stress and DNA demethylation [160,161].
7. Ascorbic Acid Cooperation with Other Antioxidants
Despite the fact that ascorbate itself has strong antioxidant properties and anti-inflammatory capacity, it can interact with other exogenous molecules and induce a synergistic protective effect (Figure 2). The compounds with which ascorbate interacts include substances used in pharmacotherapies of different diseases, e.g., antibiotics, such as ceftriaxone, gallic acid, or xanthone [162,163,164]. However, the group of the best-studied compounds that cooperate effectively with ascorbate includes natural polyphenols, especially curcumin, epigallocatechin-3-gallate, phenolic acid, and green tea polyphenol (GTP) [164,165,166,167,168,169]. Moreover, in the case of the best-known combination of ascorbate and a polyphenol, i.e., rutin, which is often used in oral fever and cold pharmaceuticals, the synergistic action consists of the enhanced antioxidant effect of the particles by mutually restoring their reduced forms. As has been wildly described following in vitro experiments on UV-irradiated skin cells, ascorbate (100 µM) and rutin (25 µM) support each other’s cytoprotective properties at every possible stage of activity [33,84,170,171,172]. As mentioned before, ascorbate uses many pathways to penetrate cell membranes, while the bioavailability of rutin is limited. Therefore, ascorbic acid promotes the penetration of cells by rutin, e.g., through the activation of bilitranslocase [33]. As a result, ascorbate-induced enhanced levels of rutin lead to an effective action of these antioxidants in both the scavenging of free radicals and the activation of the cellular antioxidant system [33,173]. To enhance this antioxidant effect, ascorbate and rutin also cooperate in the effective activation of Nrf2, including direct creation of adducts with Keap1 [33,84]. Moreover, at the same time, ascorbate supports the protective properties of rutin against the formation of protein complexes with highly reactive lipid peroxidation products [84], thus significantly inhabiting pro-inflammatory signaling, described owing to the efforts of complex proteomic research studies [170]. As a result, ascorbate in cooperation with rutin lowers the level of pro-inflammatory factors NFκB and TNFα [172,173]. This results in a more successful protection against UV-induced changes in the functioning of skin cells as well as in their viability and whole skin condition [84,170,172].
8. Conclusions
As can be seen in the presented review, ascorbic acid exhibits its antioxidant and anti-inflammatory properties at various levels of the functioning of living cells—starting from the direct scavenging of free radicals and the silencing of pro-inflammatory pathways, through the activation of intracellular antioxidant systems, to supporting the action of other exogenous antioxidants. In this regard, DNA, proteins, and lipids are protected against oxidation, leading to an inflammatory reaction and even apoptosis. Although ascorbate has strong antioxidant properties, it can also have pro-oxidant effects in the presence of free transition metals. Moreover, its role in the prevention of DNA mutation and cell apoptosis is controversial, especially in relation to cancer cells.
Author Contributions
Conceptualization, A.G. and E.S.; data curation, A.G.; writing—original draft preparation, A.G.; writing—review and editing, E.S.; visualization, A.G.; supervision, E.S.; project administration, A.G.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moss, R.W. Free Radical: Albert Szent-Gyorgyi and the Battle over Vitamin C. J. Hist. Biol. 1989, 22, 180–181. [Google Scholar]
- Davey, M.W.; Montagu, M.V.; Inz, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.; Strain, J.J.; Favell, D.; Fletcher, J. PlantL-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Nishikimi, M.; Yagi, K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1203S–1208S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Mazurek, A.; Pankiewicz, U. Changes of dehydroascorbic acid content in relation to total content of vitamin C in selected fruits and vegetables. Acta Sci. Pol. Hortorum Cultus 2012, 6, 169–177. [Google Scholar]
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- Vanderslice, J.T.; Higgs, D.J.; Hayes, J.M.; Block, G. Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J. Food Compos. Anal. 1990, 3, 105–118. [Google Scholar] [CrossRef]
- Hill, C.H. 20 Foods That Are High in Vitamin C. Available online: https://www.healthline.com/nutrition/vitamin-c-foods#TOC_TITLE_HDR_2 (accessed on 12 March 2022).
- García-Closas, R.; Berenguer, A.; Tormo, M.J.; Sánchez, M.J.; Quirós, J.R.; Navarro, C.; Arnaud, R.; Dorronsoro, M.; Chirlaque, M.D.; Barricarte, A.; et al. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br. J. Nutr. 2004, 91, 1005–1011. [Google Scholar] [CrossRef]
- Douglas, R.; Hemilä, H.; Chalker, E.; D’Souza, R.; Treacy, B. Vitamin C for preventing and treating the common cold. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central; 2019. Available online: fdc.nal.usda.gov (accessed on 1 October 2022).
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Yang, H.; Omar, M.; Riviere, N.M.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-Ascorbic Acid: Percutaneous Absorption Studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Rose, R.C. Transport of ascorbic acid and other water-soluble vitamins. BBA–Rev. Biomembr. 1988, 947, 335–366. [Google Scholar] [CrossRef]
- Wilson, J.X. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005, 25, 105–125. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, L.; Thumser, A.E.; Sharp, P. Decreased expression of the vitamin C transporter SVCT1 by ascorbic acid in a human intestinal epithelial cell line. Br. J. Nutr. 2002, 87, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Ströhle, A.; Hahn, A. Vitamin C und immunfunktion. Med. Monatsschr. Pharm. 2009, 32, 49–54. [Google Scholar]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [PubMed]
- Crisan, D.; Roman, I.; Crisan, M.; Scharffetter-Kochanek, K.; Badea, R. The role of vitamin C in pushing back the boundaries of skin aging: An ultrasonographic approach. Clin. Cosmet. Investig. Dermatol. 2015, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef]
- Ang, A.; Pullar, J.M.; Currie, M.J.; Vissers, M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018, 46, 1147–1159. [Google Scholar] [CrossRef]
- Langlois, M.; Duprez, D.; Delanghe, J.; De Buyzere, M.; Clement, D.L. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 2001, 103, 1863–1868. [Google Scholar] [CrossRef]
- Kodentsova, V.M. Gradation in the level of vitamin consumption: Possible risk of excessive consumption. Vopr. Pitan. 2014, 83, 41–51. [Google Scholar]
- Lamarche, J.; Nair, R.; Peguero, A.; Courville, C. Vitamin C-Induced Oxalate Nephropathy. Int. J. Nephrol. 2011, 2011, 146927. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Hille, R.; Nishino, T. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem. Biophys. Res. Commun. 1975, 63, 463–468. [Google Scholar] [CrossRef]
- Francis, A.J.; Anderson, D.; Jenkinson, P.C.; Parke, D.V.W. The protective effects of L-ascorbic acid and DL-α-tocopherol on cultured rat embryos treated with xanthine/xanthine oxidase. Mutat. Res.–Fundam. Mol. Mech. Mutagen. 1989, 214, 137–145. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Althumali, J.S.; Almalki, M.M.M.; Almasoudi, A.S.; Almuntashiri, A.H.; Almuntashiri, A.H.; Mohammed, A.I.; Alkinani, A.A.; Almahdawi, M.S.; Mahzari, M.A.H. An Overview on the Role of Xanthine Oxidase Inhibitors in Gout Management. Arch. Pharm. Pract. 2021, 12, 94–99. [Google Scholar] [CrossRef]
- Linani, A.; Benarous, K.; Bou-Salah, L.; Yousfi, M. The inhibitory kinetics of vitamins B9, C, E, and D3 on bovine xanthine oxidase: Gout treatment. Chem. Biol. Interact. 2022, 359, 109922. [Google Scholar] [CrossRef] [PubMed]
- Moonrungsee, N.; Jakmunee, J.; Peamaroon, N.; Boonmee, A.; Kasemsuk, T.; Seeda, S.; Suwancharoen, S. Phytochemical and Xanthine Oxidase Inhibitory Activity in Nypa fruticans Wurmb. Fruit Extracts. Trends Sci. 2022, 19, 2583. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Lee, M.; Li, H. Vitamin C alleviates hyperuricemia nephropathy by reducing inflammation and fibrosis. J. Food Sci. 2021, 86, 3265–3276. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef]
- Dwenger, A.; Funck, M.; Lueken, B.; Schweizer, G.; Lehmann, U. Effect of Ascorbic Acid on Neutrophil Functions and Hypoxanthine/Xanthine Oxidase-Generated, Oxygen-Derived Radicals. Clin. Chem. Lab. Med. 1992, 30, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schröder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin e on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Schuster, D.P.; Tyml, K.; Wilson, J.X. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic. Biol. Med. 2007, 42, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. Mechanism of action of vitamin C in sepsis: Ascorbate modulates redox signaling in endothelium. BioFactors 2009, 35, 5–13. [Google Scholar] [CrossRef]
- Bartsch, C.; Bekhite, M.M.; Wolheim, A.; Richter, M.; Ruhe, C.; Wissuwa, B.; Marciniak, A.; Müller, J.; Heller, R.; Figulla, H.R.; et al. NADPH oxidase and eNOS control cardiomyogenesis in mouse embryonic stem cells on ascorbic acid treatment. Free Radic. Biol. Med. 2011, 51, 432–443. [Google Scholar] [CrossRef]
- Li, X.; Cobb, C.E.; May, J.M. Mitochondrial recycling of ascorbic acid from dehydroascorbic acid: Dependence on the electron transport chain. Arch. Biochem. Biophys. 2002, 403, 103–110. [Google Scholar] [CrossRef]
- Martinovich, G.G.; Golubeva, E.N.; Martinovich, I.V.; Cherenkevich, S.N. Redox Regulation of Calcium Signaling in Cancer Cells by Ascorbic Acid Involving the Mitochondrial Electron Transport Chain. J. Biophys. 2012, 2012, 921653. [Google Scholar] [CrossRef]
- Sharma, P.; Morgan, P.D. Ascorbate reduces superoxide production and improves mitochondrial respiratory chain function in human fibroblasts with electron transport chain deficiencies. Mitochondrion 2001, 1, 191–198. [Google Scholar] [CrossRef]
- Bakalova, R.; Zhelev, Z.; Miller, T.; Aoki, I.; Higashi, T. Vitamin C versus Cancer: Ascorbic Acid Radical and Impairment of Mitochondrial Respiration? Oxid. Med. Cell. Longev. 2020, 2020, 1504048. [Google Scholar] [CrossRef]
- Tariq, S.A. Role of ascorbic acid in scavenging free radicals and lead toxicity from biosystems. Mol. Biotechnol. 2007, 37, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.P.; Trofimova, S.V.; Piskarev, I.M. Evaluation of prooxidant properties of ascorbic acid. Biophysics 2013, 58, 453–456. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Du, J.; Wagner, B.A.; Buettner, G.R.; Cullen, J.J. Role of labile iron in the toxicity of pharmacological ascorbate. Free Radic. Biol. Med. 2015, 84, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of α-tocotrienol antioxidant action and cell signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Peake, J.M. Vitamin C: Effects of exercise and requirements with training. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 125–151. [Google Scholar] [CrossRef]
- Li, X.; Qu, Z.C.; May, J.M. GSH is required to recycle ascorbic acid in cultured liver cell Lines. Antioxid. Redox Signal. 2001, 3, 1089–1097. [Google Scholar] [CrossRef]
- Sünder, A.; Flachowsky, G. Influence of high vitmin E dosages on retinol and carotinoid concentration in body tissues and eggs of laying hens. Arch. Anim. Nutr. 2001, 55, 43–52. [Google Scholar]
- Young, A.M.; Gregoriadis, G. Photolysis of Retinol in Liposomes and Its Protection with Tocopherol and Oxybenzone. Photochem. Photobiol. 1996, 63, 344–352. [Google Scholar] [CrossRef]
- Beyer, R.E. The role of ascorbate in antioxidant protection of biomembranes: Interaction with vitamin E and coenzyme Q. J. Bioenerg. Biomembr. 1994, 26, 349–358. [Google Scholar] [CrossRef]
- Nemati, M.; Shahir, M.; Harakinezhad, M.; Lotfalhian, H. Cold-Induced Ascites in Broilers: Effects of Vitamin C and Coenzyme Q10. Rev. Bras. Ciência Avícola 2017, 19, 537–544. [Google Scholar] [CrossRef]
- Shargorodsky, M.; Debby, O.; Matas, Z.; Zimlichman, R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr. Metab. 2010, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Kobori, Y.; Ota, S.; Sato, R.; Yagi, H.; Soh, S.; Arai, G.; Okada, H. Antioxidant cosupplementation therapy with vitamin C, vitamin E, and coenzyme Q10 in patients with oligoasthenozoospermia. Arch. Ital. Urol. Androl. 2014, 86, 1–4. [Google Scholar] [CrossRef]
- Pavlović, S.Z.; Ognjanović, B.I.; Štajn, A.Š.; Źikić, R.V.; Saičić, Z.S.; Petrović, V.M. The effect of coenzyme Q10 on blood ascorbic acid, vitamin E, and lipid peroxide in chronic cadmium intoxication. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Budni, J.; Dos Santos, D.B.; Antunes, A.; Daufenbach, J.F.; Manosso, L.M.; Farina, M.; Rodrigues, A.L.S. Protective effects of ascorbic acid on behavior and oxidative status of restraint-stressed mice. J. Mol. Neurosci. 2013, 49, 68–79. [Google Scholar] [CrossRef]
- Muthuvel, R.; Venkataraman, P.; Krishnamoorthy, G.; Gunadharini, D.N.; Kanagaraj, P.; Jone Stanley, A.; Srinivasan, N.; Balasubramanian, K.; Aruldhas, M.M.; Arunakaran, J. Antioxidant effect of ascorbic acid on PCB (Aroclor 1254) induced oxidative stress in hypothalamus of albino rats. Clin. Chim. Acta 2006, 365, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Adikwu, E.; Deo, O. Hepatoprotective Effect of Vitamin C (Ascorbic Acid). Pharmacol. Pharm. 2013, 4, 84–92. [Google Scholar] [CrossRef]
- Koc, M.; Imik, H.; Odabasoglu, F. Gastroprotective and anti-oxidative properties of ascorbic acid on indomethacin-induced gastric injuries in rats. Biol. Trace Elem. Res. 2008, 126, 222–236. [Google Scholar] [CrossRef]
- Hu, J.H.; Tian, W.Q.; Zhao, X.L.; Zan, L.S.; Wang, H.; Li, Q.W.; Xin, Y.P. The cryoprotective effects of ascorbic acid supplementation on bovine semen quality. Anim. Reprod. Sci. 2010, 121, 72–77. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D.; Kłudkiewicz, B.; Sehnal, F. Glutathione-ascorbic acid redox cycle and thioredoxin reductase activity in the digestive tract of Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2009, 39, 180–188. [Google Scholar] [CrossRef]
- Choe, J.Y.; Kim, S.K. Quercetin and Ascorbic Acid Suppress Fructose-Induced NLRP3 Inflammasome Activation by Blocking Intracellular Shuttling of TXNIP in Human Macrophage Cell Lines. Inflammation 2017, 40, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yang, J.; Ji, S.; Li, T.; Gao, S.; Sun, C.; Xu, H. Cancer therapy by targeting thioredoxin reductase based on selenium-containing dynamic covalent bond. CCS Chem. 2020, 2, 225–235. [Google Scholar] [CrossRef]
- Ishaq, G.M.; Saidu, Y.; Bilbis, L.S.; Muhammad, S.A.; Jinjir, N.; Shehu, B.B. Effects of α-tocopherol and ascorbic acid in the severity and management of traumatic brain injury in albino rats. J. Neurosci. Rural Pract. 2013, 4, 292–297. [Google Scholar] [CrossRef]
- Erdogan, Z.; Erdogan, S.; Celik, S.; Unlu, A. Effects of ascorbic acid on cadmium-induced oxidative stress and performance of broilers. Biol. Trace Elem. Res. 2005, 104, 19–31. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Meredith, M.E. Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem. Biophys. Res. Commun. 2012, 426, 148–152. [Google Scholar] [CrossRef]
- Saffi, J.; Sonego, L.; Varela, Q.D.; Salvador, M. Antioxidant activity of L-ascorbic acid in wild-type and superoxide dismutase deficient strains of Saccharomyces cerevisiae. Redox Rep. 2006, 11, 179–184. [Google Scholar] [CrossRef]
- Tamari, Y.; Nawata, H.; Inoue, E.; Yoshimura, A.; Yoshii, H.; Kashino, G.; Seki, M.; Enomoto, T.; Watanabe, M.; Tano, K. Protective roles of ascorbic acid in oxidative stress induced by depletion of superoxide dismutase in vertebrate cells. Free Radic. Res. 2013, 47, 1–7. [Google Scholar] [CrossRef]
- Washio, K.; Inagaki, M.; Tsuji, M.; Morio, Y.; Akiyama, S.; Gotoh, H.; Gotoh, T.; Gotoh, Y.; Oguchi, K. Oral Vitamin C Supplementation in Hemodialysis Patients and Its Effect on the Plasma Level of Oxidized Ascorbic Acid and Cu/Zn Superoxide Dismutase, an Oxidative Stress Marker. Nephron Clin. Pract. 2008, 109, c49–c54. [Google Scholar] [CrossRef]
- Botanicae Horti Agrobotanici Cluj-Napoca, N.; Dolatabadian, A.; Ali Mohammad Modarres Sanavy, S. Effect of the Ascorbic Acid, Pyridoxine and Hydrogen Peroxide Treatments on Germination, Catalase Activity, Protein and Malondialdehyde Content of Three Oil Seeds. Hort. Agrobot. Cluj 2008, 36, 61–66. [Google Scholar]
- Sun, Y.; Zheng, Q.; LI, G.; Guo, D.; Wang, Z. Mechanism of ascorbic acid-induced reversion against malignant phenotype in human gastric cancer cells. Biomed. Environ. Sci. 2006, 19, 385–391. [Google Scholar]
- Klingelhoeffer, C.; Kämmerer, U.; Koospal, M.; Mühling, B.; Schneider, M.; Kapp, M.; Kübler, A.; Germer, C.T.; Otto, C. Natural resistance to ascorbic acid induced oxidative stress is mainly mediated by catalase activity in human cancer cells and catalase-silencing sensitizes to oxidative stress. BMC Complement. Altern. Med. 2012, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-κB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 52, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depressed the gill physical barriers and immune barriers referring to Nrf2, apoptosis, MLCK, NF-κB and TOR signaling in grass carp (Ctenopharyngodon idella) under infection of Flavobacterium columnare. Fish Shellfish Immunol. 2016, 58, 177–192. [Google Scholar] [CrossRef]
- Vineetha, R.C.; Binu, P.; Arathi, P.; Nair, R.H. L-ascorbic acid and α-tocopherol attenuate arsenic trioxide-induced toxicity in H9c2 cardiomyocytes by the activation of Nrf2 and Bcl2 transcription factors. Toxicol. Mech. Methods 2018, 28, 353–360. [Google Scholar] [CrossRef]
- Li, Y.; Darwish, W.S.; Chen, Z.; Hui, T.; Wu, Y.; Hirotaka, S.; Chiba, H.; Hui, S.P. Identification of lead-produced lipid hydroperoxides in human HepG2 cells and protection using rosmarinic and ascorbic acids with a reference to their regulatory roles on Nrf2-Keap1 antioxidant pathway. Chem. Biol. Interact. 2019, 314, 108847. [Google Scholar] [CrossRef]
- Wagner, A.E.; Ernst, I.; Iori, R.; Desel, C.; Rimbach, G. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp. Dermatol. 2010, 19, 137–144. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Cytoprotective Effect of Ascorbic Acid and Rutin against Oxidative Changes in the Proteome of Skin Fibroblasts Cultured in a Three-Dimensional System. Nutrients 2020, 12, 1074. [Google Scholar] [CrossRef]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Breckwoldt, D.; Schrader, C.; Schmelzer, C.; Döring, F.; Hashida, K.; Hori, O.; Matsugo, S.; Rimbach, G. Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes—Role of the redox-regulated transcription factor Nrf2. BMC Complement. Altern. Med. 2011, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi-Pour, Z.; Ramezani, F.; Keshavarzi, F.; Samadi, N. The role of quercetin and vitamin c in NRF2-dependent oxidative stress production in breast cancer cells. Oncol. Lett. 2017, 13, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Mostafavi-Pour, Z.; Amiri, A.; Keshavarzi, F.; Nejabat, N.; Ramezani, F.; Sardarian, A.; Zal, F. Chemoprevention of Prostate Cancer Cells by Vitamin C plus Quercetin: Role of Nrf2 in Inducing Oxidative Stress. Nutr. Cancer 2021, 73, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Xanthoudakis, S.; Curran, T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992, 11, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.; Dong, Z. Inhibitory effects of ascorbic acid on AP-1 activity and transformation of JB6 cells. Int. J. Oncol. 1996, 8, 389–393. [Google Scholar] [CrossRef]
- Catani, M.V.; Savini, I.; Rossi, A.; Melino, G.; Avigliano, L. Biological Role of Vitamin C in Keratinocytes. Nutr. Rev. 2005, 63, 81–90. [Google Scholar] [CrossRef]
- Lee, A.J.; Lim, J.W.; Kim, H. Ascorbic Acid Suppresses House Dust Mite-Induced Expression of Interleukin-8 in Human Respiratory Epithelial Cells. J. Cancer Prev. 2021, 26, 64–70. [Google Scholar] [CrossRef]
- Lu, A.L.; Li, X.; Gu, Y.; Wright, P.M.; Chang, D.Y. Repair of oxidative DNA damage: Mechanisms and functions. Cell Biochem. Biophys. 2001, 35, 141–170. [Google Scholar] [CrossRef]
- Lan, L.; Nakajima, S.; Oohata, Y.; Takao, M.; Okano, S.; Masutani, M.; Wilson, S.H.; Yasui, A. In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 13738–13743. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, E.A.; Cárcamo, J.M.; Golde, D.W. Vitamin C prevents DNA mutation induced by oxidative stress. J. Biol. Chem. 2002, 277, 16895–16899. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Häder, D.P. UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: Protective effects of ascorbic acid and N-acetyl-L-cysteine. J. Photochem. Photobiol. B Biol. 2002, 66, 115–124. [Google Scholar] [CrossRef]
- Yin, R.; Mao, S.Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic acid enhances tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martínez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A.; et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef]
- Shenoy, N.; Bhagat, T.; Nieves, E.; Stenson, M.; Lawson, J.; Choudhary, G.S.; Habermann, T.; Nowakowski, G.; Singh, R.; Wu, X.; et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. 2017, 7, e587. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, N.; Bhagat, T.D.; Cheville, J.; Lohse, C.; Bhattacharyya, S.; Tischer, A.; Machha, V.; Gordon-Mitchell, S.; Choudhary, G.; Wong, L.F.; et al. Ascorbic acid–induced TET activation mitigates adverse hydroxymethylcytosine loss in renal cell carcinoma. J. Clin. Investig. 2019, 129, 1612–1625. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.P.; Miles, S.L. Silencing HIF-1α induces TET2 expression and augments ascorbic acid induced 5-hydroxymethylation of DNA in human metastatic melanoma cells. Biochem. Biophys. Res. Commun. 2017, 490, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein Oxidation. Ann. N. Y. Acad. Sci. 2006, 899, 191–208. [Google Scholar] [CrossRef]
- Friguet, B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006, 580, 2910–2916. [Google Scholar] [CrossRef]
- Perrone, G.; Hideshima, T.; Ikeda, H.; Okawa, Y.; Calabrese, E.; Gorgun, G.; Santo, L.; Cirstea, D.; Raje, N.; Chauhan, D.; et al. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia 2009, 23, 1679–1686. [Google Scholar] [CrossRef]
- Zou, W.; Yue, P.; Lin, N.; He, M.; Zhou, Z.; Lonial, S.; Khuri, F.R.; Wang, B.; Sun, S.-Y.Y. Vitamin C inactivates the proteasome inhibitor PS-341 in human cancer cells. AACR 2006, 12, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Nauser, T.; Gebicki, J.M. Physiological concentrations of ascorbate cannot prevent the potentially damaging reactions of protein radicals in humans. Chem. Res. Toxicol. 2017, 30, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Domazou, A.S.; Koppenol, W.H.; Gebicki, J.M. Efficient repair of protein radicals by ascorbate. Free Radic. Biol. Med. 2009, 46, 1049–1057. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T. Initiation and prevention of biological damage by radiation-generated protein radicals. Int. J. Mol. Sci. 2022, 23, 396. [Google Scholar] [CrossRef] [PubMed]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Ramana, K.V.; Srivastava, S.; Singhal, S.S. Lipid peroxidation products in human health and disease. Oxid. Med. Cell. Longev. 2013, 2013, 583438. [Google Scholar] [CrossRef]
- Ranjan, R.; Swarup, D.; Naresh, R.; Patra, R.C. Enhanced erythrocytic lipid peroxides and reduced plasma ascorbic acid, and alteration in blood trace elements level in dairy cows with mastitis. Vet. Res. Commun. 2005, 29, 27–34. [Google Scholar] [CrossRef]
- Marchlewicz, M.; Wiszniewska, B.; Gonet, B.; Baranowska-Bosiacka, I.; Safranow, K.; Kolasa, A.; Głąbowski, W.; Kurzawa, R.; Jakubowska, K.; Rać, M.E. Increased lipid peroxidation and ascorbic acid utilization in testis and epididymis of rats chronically exposed to lead. BioMetals 2007, 20, 13–19. [Google Scholar] [CrossRef]
- Surapaneni, K.; Venkataramana, G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J. Med. Sci. 2007, 61, 9–14. [Google Scholar] [CrossRef]
- Sönmez, M.; Türk, G.; Yüce, A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 2005, 63, 2063–2072. [Google Scholar] [CrossRef]
- Krishna Mohan, S.; Venkataramana, G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with pregnancy—Induced hypertension. Indian J. Physiol. Pharmacol. 2007, 51, 284–288. [Google Scholar] [PubMed]
- Chen, W.; Guo, J.; Guo, H.; Kong, X.; Bai, J.; Long, P. Protective Effect of Vitamin C against Infancy Rat Corneal Injury Caused by Acute UVB Irradiation. Biomed Res. Int. 2020, 2020, 8089273. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Elbe, H.; Eris, C.; Dogan, Z.; Toprak, G.; Otan, E.; Erdemli, E.; Turkoz, Y. Cytoprotective effects of amifostine, ascorbic acid and N-acetylcysteine against methotrexate-induced hepatotoxicity in rats. World J. Gastroenterol. 2014, 20, 10158–10165. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Baba, N.A.; Verma, P.K.; Sultana, M.; Singh, M. Hepatotoxicity induced by subchronic exposure of fluoride and chlorpyrifos in Wistar rats: Mitigating effect of ascorbic acid. Biol. Trace Elem. Res. 2015, 166, 157–162. [Google Scholar] [CrossRef]
- Ray, S.; Roy, K.; Sengupta, C. Cisplatin-induced lipid peroxidation and its inhibition with ascorbic acid. Indian J. Pharm. Sci. 2006, 68, 199–204. [Google Scholar]
- Hernández-Guerra, M.; García-Pagán, J.C.; Turnes, J.; Bellot, P.; Deulofeu, R.; Abraldes, J.G.; Bosch, J. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology 2006, 43, 485–491. [Google Scholar] [CrossRef]
- Das, K.K.; Gupta, A.D.; Dhundasi, S.A.; Patil, A.M.; Das, S.N.; Ambekar, J.G. Protective role of L-ascorbic acid on antioxidant defense system in erythrocytes of albino rats exposed to nickel sulfate. BioMetals 2007, 20, 177–184. [Google Scholar] [CrossRef]
- Tonon, E.; Ferretti, R.; Shiratori, J.H.; Santo Neto, H.; Marques, M.J.; Minatel, E. Ascorbic acid protects the diaphragm muscle against myonecrosis in mdx mice. Nutrition 2012, 28, 686–690. [Google Scholar] [CrossRef]
- Mason, S.A.; Rasmussen, B.; van Loon, L.J.C.; Salmon, J.; Wadley, G.D. Ascorbic acid supplementation improves postprandial glycaemic control and blood pressure in individuals with type 2 diabetes: Findings of a randomized cross-over trial. Diabetes Obes. Metab. 2019, 21, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Dohi, Y.; Kojima, M.; Miyagawa, K.; Takase, H.; Katada, E.; Suzuki, S. Effects of Ascorbic Acid on Ambulatory Blood Pressure in Elderly Patients with Refractory Hypertension. Arzneimittelforschung 2011, 56, 535–540. [Google Scholar] [CrossRef]
- Jialal, I.; Singh, U. Is vitamin C an antiinflammatory agent? Am. J. Clin. Nutr. 2006, 83, 525–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wannamethee, S.G.; Lowe, G.D.O.; Rumley, A.; Bruckdorfer, K.R.; Whincup, P.H. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am. J. Clin. Nutr. 2006, 83, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Colby, J.A.; Chen, W.T.; Baker, W.L.; Coleman, C.I.; Reinhart, K.; Kluger, J.; White, C.M. Effect of ascorbic acid on inflammatory markers after cardiothoracic surgery. Am. J. Health Pharm. 2011, 68, 1632–1639. [Google Scholar] [CrossRef]
- García-Bailo, B.; Roke, K.; Mutch, D.M.; El-Sohemy, A.; Badawi, A. Association between circulating ascorbic acid, -tocopherol, 25-hydroxyvitamin D, and plasma cytokine concentrations in young adults: A cross-sectional study. Nutr. Metab. 2012, 9, 102. [Google Scholar] [CrossRef]
- de Oliveira, B.F.; Veloso, C.A.; Nogueira-Machado, J.A.; de Moraes, E.N.; dos Santos, R.R.; Cintra, M.T.G.; Chaves, M.M. Ascorbic acid, alpha-tocopherol, and betacarotene reduce oxidative stress and proinflammatory cytokines in mononuclear cells of Alzheimer’s disease patients. Nutr. Neurosci. 2012, 15, 244–251. [Google Scholar] [CrossRef]
- Jouybar, R.; Kabgani, H.; Hamid, K.; Shahbazi, S.; Allahyary, E.; Rasouli, M.; Akhlagh, S.H.; Shafa, M.; Ghazinoor, M.; Moeinvaziri, M.T.; et al. The Perioperative Effect of Ascorbic Acid on Inflammatory Response in Coronary Artery Bypass Graft Surgery. Cardiovasc. Res. J. 2012, 6, 13–17. [Google Scholar]
- Tan, P.H.; Sagoo, P.; Chan, C.; Yates, J.B.; Campbell, J.; Beutelspacher, S.C.; Foxwell, B.M.J.; Lombardi, G.; George, A.J.T. Inhibition of NF-κB and Oxidative Pathways in Human Dendritic Cells by Antioxidative Vitamins Generates Regulatory T Cells. J. Immunol. 2005, 174, 7633–7644. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. WIREs Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Peng, Y.; Kwok, K.H.H.; Yang, P.H.; Ng, S.S.M.; Liu, J.; Wong, O.G.; He, M.L.; Kung, H.F.; Lin, M.C.M. Ascorbic acid inhibits ROS production, NF-κB activation and prevents ethanol-induced growth retardation and microencephaly. Neuropharmacology 2005, 48, 426–434. [Google Scholar] [CrossRef]
- Gegotek, A.; Biernacki, M.; Ambrozewicz, E.; Surazyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Bowie, A.G.; O’Neill, L.A.J. Vitamin C Inhibits NF-κB Activation by TNF Via the Activation of p38 Mitogen-Activated Protein Kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D.W. Vitamin C suppresses TNFα-induced NFκB activation by inhibiting IκBα phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef]
- Han, S.-S.; Kim, K.; Hahm, E.-R.; Lee, S.J.; Surh, Y.-J.; Park, H.K.; Kim, W.S.; Jung, C.W.; Lee, M.H.; Park, K.; et al. L-ascorbic acid represses constitutive activation of NF-?B and COX-2 expression in human acute myeloid leukemia, HL-60. J. Cell. Biochem. 2004, 93, 257–270. [Google Scholar] [CrossRef]
- Alhusaini, A.M.; Fadda, L.M.; Alsharafi, H.; Alshamary, A.F.; Hasan, I.H. L-Ascorbic Acid and Curcumin Prevents Brain Damage Induced via Lead Acetate in Rats: Possible Mechanisms. Dev. Neurosci. 2022, 44, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P.; Hiscock, N.J.; Penkowa, M.; Basu, S.; Vessby, B.; Kallner, A.; Sjöberg, L.-B.; Pedersen, B.K. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J. Physiol. 2004, 558, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.H.; Ma, S.Y.; Jeong, J.Y.; Kim, K.H. Effects of L-ascorbic acid on the production of pro-inflammatory and anti-inflammatory cytokines in C57BL/6 mouse splenocytes. Kosin Med. J. 2015, 30, 41–49. [Google Scholar] [CrossRef]
- Schwager, J.; Schulze, J. Modulation of interleukin production by ascorbic acid. Vet. Immunol. Immunopathol. 1998, 64, 45–57. [Google Scholar] [CrossRef]
- Schwager, J.; Schulze, J. Influence of ascorbic acid on the response to mitogens and interleukin production of porcine lymphocytes. Int. J. Vitam. Nutr. Res. 1997, 67, 10–16. [Google Scholar]
- Prabudi, M.O.; Siregar, M.F.G.; Nasution, I.P.A.; Ilyas, S. The Effect of Ascorbic Acid on Interleukin-10 and Tumor Necrosis Factor-α Cytokines in Rattus norvegicus with Endometritis. Open Access Maced. J. Med. Sci. 2021, 9, 798–801. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, B.F.; Di, J.H.; Jiang, G.; Chen, F.F.; Li, H.Z.; Li, L.T.; Pei, D.S.; Zheng, J.N. Keap1: One stone kills three birds Nrf2, IKKβ and Bcl-2/Bcl-xL. Cancer Lett. 2012, 325, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic Acid: Its Role in Immune System and Chronic Inflammation Diseases. Mini Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Wenzel, U. Ascorbic acid suppresses drug-induced apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Carcinogenesis 2003, 25, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Anusuyadevi, M.; Shila, S.; Panneerselvam, C. Ascorbic acid and α-tocopherol as potent modulators of apoptosis on arsenic induced toxicity in rats. Toxicol. Lett. 2005, 156, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Abu Zeid, E.H.; Hussein, M.M.A.; Ali, H. Ascorbic acid protects male rat brain from oral potassium dichromate-induced oxdative DNA damage and apoptotic changes: The expression patterns of caspase-3, P 53, Bax, and Bcl-2 genes. Environ. Sci. Pollut. Res. 2018, 25, 13056–13066. [Google Scholar] [CrossRef]
- Park, S.; Han, S.S.; Park, C.H.; Hahm, E.R.; Lee, S.J.; Park, H.K.; Lee, S.H.; Kim, W.S.; Jung, C.W.; Park, K.; et al. L-Ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int. J. Biochem. Cell Biol. 2004, 36, 2180–2195. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, Y.; Qu, C.J.; Pan, Z.H.; Qin, Y.; Zhang, X.; Liu, W.J.; Li, D.F.; Zheng, Q. Vitamin C induces human melanoma A375 cell apoptosis via Bax- And Bcl-2-mediated mitochondrial pathways. Oncol. Lett. 2019, 18, 3880–3886. [Google Scholar] [CrossRef]
- Jung, S.A.; Lee, D.H.; Moon, J.H.; Hong, S.W.; Shin, J.S.; Hwang, I.Y.; Shin, Y.J.; Kim, J.H.; Gong, E.Y.; Kim, S.M.; et al. L-Ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free Radic. Biol. Med. 2016, 95, 200–208. [Google Scholar] [CrossRef]
- Frajese, G.V.; Benvenuto, M.; Fantini, M.; Ambrosin, E.; Sacchetti, P.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Bei, R. Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines in vitro. Oncol. Lett. 2016, 11, 4224–4234. [Google Scholar] [CrossRef] [PubMed]
- Bourbour, M.; Khayam, N.; Noorbazargan, H.; Tavakkoli Yaraki, M.; Asghari Lalami, Z.; Akbarzadeh, I.; Eshrati Yeganeh, F.; Dolatabadi, A.; Mirzaei Rad, F.; Tan, Y.N. Evaluation of anti-cancer and anti-metastatic effects of folate-PEGylated niosomes for co-delivery of letrozole and ascorbic acid on breast cancer cells. Mol. Syst. Des. Eng. 2022, 7, 1102–1118. [Google Scholar] [CrossRef]
- Wu, T.-M.; Liu, S.-T.; Chen, S.-Y.; Chen, G.-S.; Wu, C.-C.; Huang, S.-M. Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells. Front. Oncol. 2020, 10, 1483. [Google Scholar] [CrossRef]
- Pires, A.S.; Marques, C.R.; Encarnação, J.C.; Abrantes, A.M.; Marques, I.A.; Laranjo, M.; Oliveira, R.; Casalta-Lopes, J.E.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; et al. Ascorbic Acid Chemosensitizes Colorectal Cancer Cells and Synergistically Inhibits Tumor Growth. Front. Physiol. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Putchala, M.C.; Ramani, P.; Sherlin, H.J.; Premkumar, P.; Natesan, A. Ascorbic acid and its pro-oxidant activity as a therapy for tumours of oral cavity-A systematic review. Arch. Oral Biol. 2013, 58, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010, 16, 509–520. [Google Scholar] [CrossRef]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 2018, 34, 700–706. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; El-Ghoneimy, A. Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Ren. Fail. 2015, 37, 297–304. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M. Synergistic protective role of ceftriaxone and ascorbic acid against subacute diazinon-induced nephrotoxicity in rats. Cytotechnology 2016, 68, 279–289. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discov. Ther. 2015, 9, 136–141. [Google Scholar] [CrossRef]
- Enko, J.; Gliszczyńska-Świgło, A. Influence of the interactions between tea (Camellia sinensis) extracts and ascorbic acid on their antioxidant activity: Analysis with interaction indexes and isobolograms. Food Addit. Contam.—Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, J.X.; Tu, Y.Y. Synergistic effects of tea polyphenols and ascorbic acid on human lung adenocarcinoma SPC-A-1 cells. J. Zhejiang Univ. Sci. B 2010, 11, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Alexa, T.; Dondaş, A.; Andron, G.; Bǎdescu, M.; Alexa, I.D.; Bohotin, C. Pain modulation by curcumin and ascorbic acid in mice. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2014, 118, 346–351. [Google Scholar] [PubMed]
- Andarwulan, N.; Kurniasih, D.; Apriady, R.A.; Rahmat, H.; Roto, A.V.; Bolling, B.W. Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. J. Funct. Foods 2012, 4, 339–347. [Google Scholar] [CrossRef]
- Li, L.; Shao, J.; Zhu, X.; Zhou, G.; Xu, X. Effect of plant polyphenols and ascorbic acid on lipid oxidation, residual nitrite and N-nitrosamines formation in dry-cured sausage. Int. J. Food Sci. Technol. 2013, 48, 1157–1164. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Natural Exogenous Antioxidant Defense against Changes in Human Skin Fibroblast Proteome Disturbed by UVA Radiation. Oxid. Med. Cell. Longev. 2020, 2020, 3216415. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Herrero, J.A.; Frutos, M.J. Influence of rutin and ascorbic acid in colour, plum anthocyanins and antioxidant capacity stability in model juices. Food Chem. 2015, 173, 495–500. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Synergistic cytoprotective effects of rutin and ascorbic acid on the proteomic profile of 3D-cultured keratinocytes exposed to UVA or UVB radiation. Nutrients 2019, 11, 2672. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jastrząb, A.; Dobrzyńska, M.; Biernacki, M.; Skrzydlewska, E. Exogenous Antioxidants Impact on UV-Induced Changes in Membrane Phospholipids and the Effectiveness of the Endocannabinoid System in Human Skin Cells. Antioxidants 2021, 10, 1260. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).