Maintenance over Time of the Effect Produced by Esmolol on the Structure and Function of Coronary Arteries in Hypertensive Heart Diseases
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Model and Treatment
2.2. Blood Pressure and Heart Rate
2.3. Wire Myography for the Study of Coronary Artery Vascular Reactivity
2.4. Confocal Microscopy for the Study of Coronary Artery Structure and Composition
2.5. Biomarkers of Oxidative Status in Plasma
2.5.1. Reduced Glutathione (GSH) Content
2.5.2. Nitrates
2.5.3. Total Protein Carbonyls
2.5.4. Lipid Peroxidation
2.6. Statistical Analysis
3. Results
3.1. Blood Pressure and Heart Rate
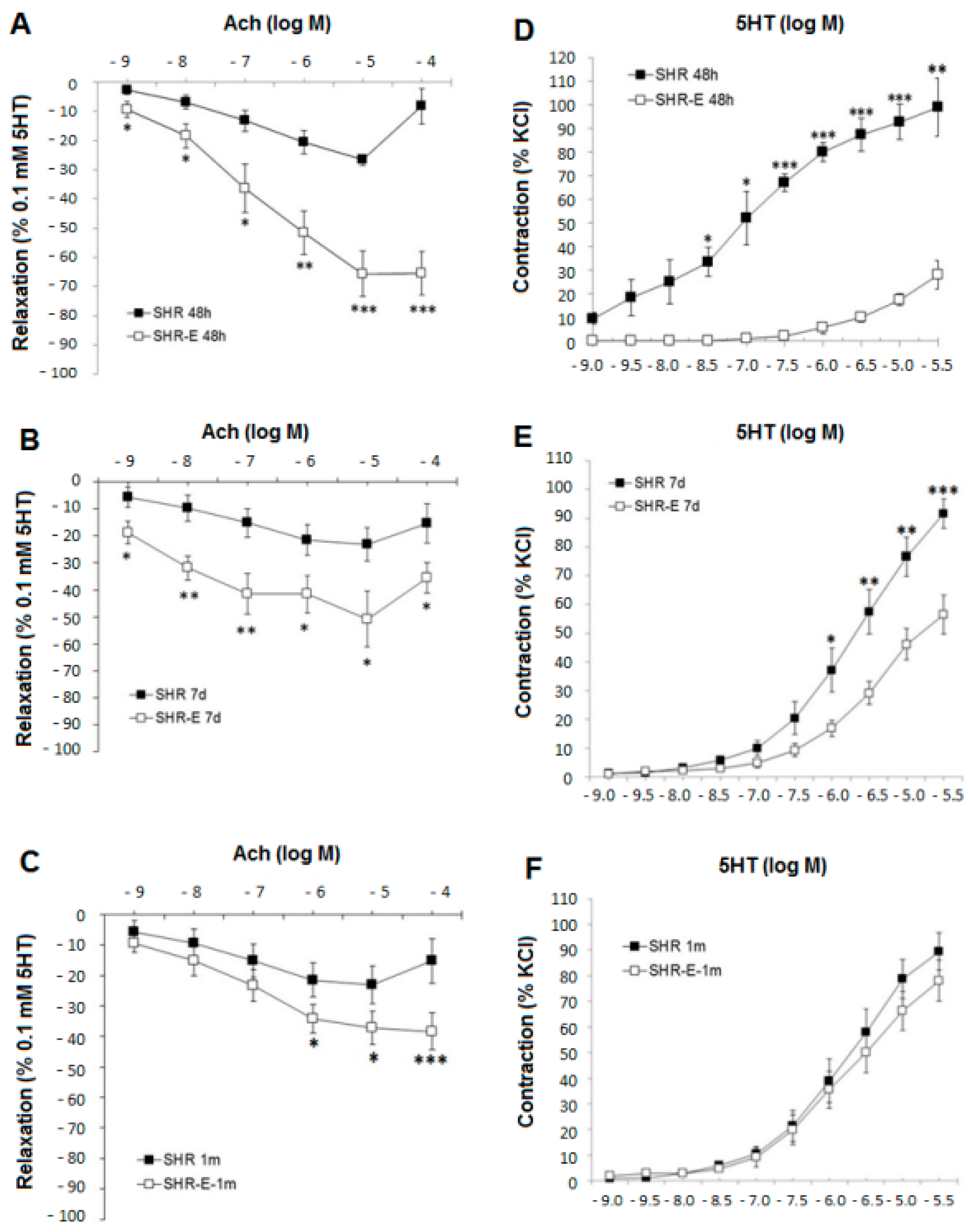
3.2. Esmolol Improves Vascular Function, and This Effect Remains after Withdrawal
3.3. Esmolol Improves Vascular Structure, and This Effect Remains after Withdrawal
3.4. Esmolol Improves Plasma Redox Status, and It Persists after Treatment Withdrawal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fanelli, E.; Enri, L.R.; Pappaccogli, M.; Fasano, C.; Di Monaco, S.; Pignata, I.; Baratta, F.; Eula, E.; Masera, G.; Mana, M.; et al. Knowledge on arterial hypertension in general population: Results from a community pharmacy screening program. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1081–1086. [Google Scholar] [CrossRef]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Rehman, A.; Schiffrin, E.L. Vascular Effects of Antihypertensive Drug Therapy. Curr. Hypertens. Rep. 2010, 12, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Vascular remodeling and endothelial function in hypertensive patients: Effects of antihypertensive therapy. Scand. Cardiovasc. J. Suppl. 1998, 47, 15–21. [Google Scholar] [CrossRef]
- Koprdova, R.; Cebova, M.; Kristek, F. Long-term effect of losartan administration on blood pressure, heart and structure of coronary artery of young spontaneously hypertensive rats. Physiol. Res. 2009, 58, 327–335. [Google Scholar] [CrossRef]
- Sharifi, A.M.; Li, J.S.; Endemann, D.; Schiffrin, E.L. Effects of enalapril and amlodipine on small-artery structure and composition, and on endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 1998, 16, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Fommei, E.; Varela-Carver, A.; Mancini, M.; Ghione, S.; Lombardi, M.; Pisani, P.; Parker, H.; D’amati, G.; Donato, L.; et al. Perindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertension. J. Hypertens. 2011, 29, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Brilla, C.G.; Janicki, J.S.; Weber, K.T. Impaired diastolic function and coronary reservein genetic hypertension: Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ. Res. 1991, 69, 107–115. [Google Scholar] [CrossRef]
- Barone, F.C.; Campbell, W.G., Jr.; Nelson, A.H.; Feuerstein, G.Z. Carvedilol prevents severe hypertensive cardiomyopathy and remodeling. J. Hypertens. 1998, 16, 871–884. [Google Scholar] [CrossRef]
- Arnalich-Montiel, A.; González, M.C.; Delgado-Baeza, E.; Delgado-Martos, M.J.; Condezo-Hoyos, L.; Martos-Rodríguez, A.; Rodriguez-Rodriguez, P.; Quintana-Villamandos, B. Short term esmolol improves coronary artery remodeling in spontaneously hypertensive rats through increased nitric oxide bioavailability and superoxide dismutase activity. Biomed. Res. Int. 2014, 2014, 531087. [Google Scholar] [CrossRef]
- Quintana-Villamandos, B.; Arnalich-Montiel, A.; Arribas, S.; Lüneburg, N.; Böger, R.H.; Delgado-Martos, M.J.; Fernández-Criado, C.; Delgado-Baeza, E.; González, M.A. Early regression of coronary artery remodeling with esmolol and DDAH/ADMA pathway in hypertensive rats. Hypertens. Res. 2016, 39, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Villamandos, B.; Pazó-Sayós, L.; Arribas, S.M.; Rodríguez-Rodríguez, P.; Böger, R.H.; Lüneburg, N.; Delgado-Baeza, E.; González, M.C. Dronedarone induces regression of coronary artery remodeling related to better global antioxidant status. Hypertens. Res. 2019, 42, 1485–1494. [Google Scholar] [CrossRef]
- Quintana-Villamandos, B.; Delgado-Martos, M.J.; Delgado-Baeza, E. Impact of a multichannel blocker in attenuating intramyocardial artery remodeling in hypertensive rats through increased nitric oxide bioavailability. Biomed. Int. Res. 2019, 2019, 6374582. [Google Scholar] [CrossRef]
- Wiest, D.B.; Haney, J.S. Clinical pharmacokinetics and therapeutic efficacy of esmolol. Clin. Pharmacokinet. 2012, 51, 347–356. [Google Scholar] [CrossRef]
- Zangrillo, A.; Bignami, E.; Noè, B.; Nardelli, P.; Licheri, M.; Gerli, C.; Crivellari, M.; Oriani, A.; Di Prima, A.L.; Fominskiy, E.; et al. Esmolol in Cardiac Surgery: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Ollila, A.; Vikatmaa, L.; Sund, R.; Pettilä, V.; Wilkman, E. Efficacy and safety of intravenous esmolol for cardiac protection in non-cardiac surgery. A systematic review and meta-analysis. Ann. Med. 2019, 51, 17–27. [Google Scholar] [CrossRef]
- Conde, M.V.; González, M.C.; Quintana-Villamandos, B.; Abderrahim, F.; Briones, A.M.; Condezo-Hoyos, L.; Regadera, J.; Susin, C.; Gómez de Diego, J.; Delgado-Baeza, E.; et al. Liver growth factor treatment restores cell-extracellular matrix balance in resistence arteries and improves left ventricular hypertrophy in SHR. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1153–H1165. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, P.R.; López de Pablo, A.L.L.; Condezo-Hoyos, L.; Martín-Cabrejas, M.A.; Aguilera, Y.; Ruiz-Hurtado, G.; Gutierrez-Arzapalo, P.Y.; Ramiro-Cortijo, D.R.; Fernandez-Alfonso, M.S.; González, M.C.; et al. Fetal undernutrition is associated with perinatal sex-dependent alterations in oxidative status. J. Nutr. Biochem. 2015, 26, 1650–1659. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; De la Calle, M.; Böger, R.; Hannemann, J.; Lüneburg, N.; López-Giménez, M.R.; Rodríguez-Rodríguez, P.; Martín-Cabrejas, M.A.; Benítez, V.; López de Pablo, A.L.; et al. Male fetal sex is associated with low maternal plasma anti-inflammatory cytokine profile in the first trimester of healthy pregnancies. Cytokine 2020, 136, 155290. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Gila-Díaz, A.; Carrillo, G.H.; Cañas, S.; De Pipaón, M.S.; Martínez-Orgado, J.A.; Rodríguez-Rodríguez, P.; De Pablo, L.L.; Martin-Cabrejas, M.A.; Ramiro-Cortijo, D.; Arribas, S.M. Influence of Maternal Age and Gestational Age on Breast Milk Antioxidants During the First Month of Lactation. Nutrients 2020, 12, 2569. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O. Remodelling of the heart and vessels in experimental hypertension: Advances in protection. J. Hypertens. 2010, 28 (Suppl. S1), S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Esmolol: A review of its use in the short-term treatment of tachyarrhythmias and the short-term control of tachycardia and hypertension. Drugs 2012, 72, 109–132. [Google Scholar] [CrossRef]
- Poveda-Jaramillo, R.; Monaco, F.; Zangrillo, A.; Landoni, G. Ultra-Short–Acting β-Blockers (Esmolol and Landiolol) in the Perioperative Period and in Critically Ill Patients. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1415–1425. [Google Scholar] [CrossRef]
- Deblois, D.; Tea, B.-S.; Dam, T.-V.; Tremblay, J.; Hamet, P. Smooth Muscle Apoptosis During Vascular Regression in Spontaneously Hypertensive Rats. Hypertension 1997, 29, 340–344. [Google Scholar] [CrossRef]
- Giummelly, P.; Lartaud-Idjouadiene, I.; Marque, V.; Niederhoffer, N.; Chillon, J.M.; Capdeville-Atkinson, C.; Atkinson, J. Effects of aging and antihypertensive treatment on aortic internal diameter in spontaneously hypertensive rats. Hypertension 1999, 34, 207–211. [Google Scholar] [CrossRef][Green Version]
- Vaja, V.; Ochodnicky, P.; Krenek, P.; Klimas, J.; Bajuszova, Z.; Kyselovic, J. Rapid large artery remodeling following the administration and withdrawal of calcium channel blockers in spontaneously hypertensive rats. Eur. J. Pharmacol. 2009, 619, 85–91. [Google Scholar] [CrossRef]
- Marchand, E.L.; Sarkissian, S.D.; Hamet, P.; deBlois, D. Caspase-dependent cell death mediates the early phase of aortic hypertrophy regression in losartan-treated spontaneously hypertensive rats. Circ. Res. 2003, 92, 777–784. [Google Scholar] [CrossRef]
- Guerrero, E.I.; Ardanaz, N.; Sevilla, M.A.; Arévalo, M.A.; Montero, M.J. Cardiovascular effects of nebivolol in spontaneously hypertensive rats persist after treatment withdrawal. J. Hypertens. 2006, 24, 151–158. [Google Scholar] [CrossRef]
- Martinez-Quinones, P.; McCarthy, C.G.; Watts, S.W.; Klee, N.S.; Komic, A.; Calmasini, F.B.; Priviero, F.; Warner, A.; Chenghao, Y.; Wenceslau, C.S. Hypertension induced morphological and physiological changes in cells of the arterial wall. Am. J. Hypertens. 2018, 31, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H. Asymmetric dimethylarginine (ADMA) modulates endothelial function--therapeutic implications. Vasc. Med. 2003, 8, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Feihl, F.; Liaudet, L.; Levy, B.Í.; Waeber, B. Hypertension and microvascular remodelling. Cardiovasc. Res. 2008, 78, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.K.; Chaudhary, M.; Sharma, B.; Bhattacharjee, J.; Ghambhir, D.S.; Arora, N.; Sudha, R. Effect of esmolol on oxidant status and antioxidant activity in acute myocardial infarction. J. Assoc. Physicians India 2003, 51, 677–680. [Google Scholar]
- Tsuchiya, H.; Mizogami, M. Characteristic interactivity of landiolol, an ultra-short-acting highly selective β1-blocker, with biomimetic membranes: Comparisons with β1-selective esmolol and non-selective propranolol and alprenolol. Front. Pharmacol. 2013, 4, 150. [Google Scholar] [CrossRef]
- Bernatova, I. Endothelial dysfunction in experimental models of arterial hypertension: Cause or consequence? Biomed. Res. Int. 2014, 2014, 598271. [Google Scholar] [CrossRef]
- Frohlich, E.D. Is the spontaneously hypertensive rat a model for human hypertension? J. Hypertens. Suppl. 1986, 4, S15–S19. [Google Scholar]




| SHR (n = 9/Group) | SHR-E (n = 9/Group) | |
|---|---|---|
| Blood pressure (mmHg) | ||
| Treatment group | 187 ± 20 | 139 ± 23 * |
| Withdrawal week group | 183 ± 19 | 179 ± 16 # |
| Withdrawal month group | 179 ± 15 | 189 ± 10 # |
| Heart rate (beats/min) | ||
| Treatment group | 380 ± 6 | 305 ± 10 * |
| Withdrawal week group | 372 ± 9 | 369 ± 15 # |
| Withdrawal month group | 389 ± 11 | 373 ± 7 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Oropesa, R.; Rodríguez-Rodríguez, P.; Pazó-Sayós, L.; Arnalich-Montiel, A.; Arribas, S.M.; González, M.C.; Quintana-Villamandos, B. Maintenance over Time of the Effect Produced by Esmolol on the Structure and Function of Coronary Arteries in Hypertensive Heart Diseases. Antioxidants 2022, 11, 2042. https://doi.org/10.3390/antiox11102042
Martín-Oropesa R, Rodríguez-Rodríguez P, Pazó-Sayós L, Arnalich-Montiel A, Arribas SM, González MC, Quintana-Villamandos B. Maintenance over Time of the Effect Produced by Esmolol on the Structure and Function of Coronary Arteries in Hypertensive Heart Diseases. Antioxidants. 2022; 11(10):2042. https://doi.org/10.3390/antiox11102042
Chicago/Turabian StyleMartín-Oropesa, Raquel, Pilar Rodríguez-Rodríguez, Laia Pazó-Sayós, Ana Arnalich-Montiel, Silvia Magdalena Arribas, Maria Carmen González, and Begoña Quintana-Villamandos. 2022. "Maintenance over Time of the Effect Produced by Esmolol on the Structure and Function of Coronary Arteries in Hypertensive Heart Diseases" Antioxidants 11, no. 10: 2042. https://doi.org/10.3390/antiox11102042
APA StyleMartín-Oropesa, R., Rodríguez-Rodríguez, P., Pazó-Sayós, L., Arnalich-Montiel, A., Arribas, S. M., González, M. C., & Quintana-Villamandos, B. (2022). Maintenance over Time of the Effect Produced by Esmolol on the Structure and Function of Coronary Arteries in Hypertensive Heart Diseases. Antioxidants, 11(10), 2042. https://doi.org/10.3390/antiox11102042






