Abstract
Comparative chemical analyses among peel and pulp essential oils (EOs) and methanolic extracts of four Citrus australasica varieties (Red, Collette, Pink Ice, and Yellow Sunshine), and the hybrid Faustrime, were performed using GC-MS and UHPLC-DAD-HR-Orbitrap/ESI-MS. Peel and pulp extracts were also analysed for their in vitro antioxidant activity on a Balb/3T3 clone A31 mouse embryo fibroblast cell line. The results of peel and pulp EOs were mainly characterised by monoterpenes and sesquiterpenes, respectively. All peels displayed a higher total phenol content (TPC) than pulps, and consequently a greater antioxidant activity. Collette peels and Pink Ice pulps showed the highest amount of identified flavonoids (e.g., luteolin, isosakuranetin, and poncirin derivatives). Collette and Red peels were rich in anthocyanins (delphinidin and petunidin glycosides), exhibiting the maximum protective activity against induced oxidative damage. In conclusion, finger lime fruits are good sources of health-promoting phytocomplexes, with the Red, Collette, and Pink Ice varieties being the most promising.
Keywords:
Citrus australasica; finger lime; phenols; volatiles; antioxidant; UHPLC-MS/Orbitrap; chemometrics 1. Introduction
The Citrus genus, belonging to the Rutaceae family, includes widely distributed, consumed, and studied species, such as Citrus limon (L.) Osbeck (lemon), C. medica L. (cedar), C. × aurantium L. (bitter orange), C. paradisi Macfad. (grapefruit), C. reticulata Blanco (tangerine), and C. sinensis (L.) Osbeck (orange), but also the lesser known C. australasica F. Muell [1,2]. Citrus australasica is a small tree native to Australia which is recently acquiring growing commercial interest in Italy and in Europe, in general, due to the uniqueness of its fruits that are used in gourmet culinary preparations. Citrus australasica is commonly called finger lime or lemon caviar, because its spindle-shaped fruits are finger-like, while the vesicles of its pulp are similar to pearls of caviar. There are several varieties and hybrids of C. australasica that differ macroscopically in peel and pulp color, and have more or less acidulous or floral odorous notes [3]. These differences reflect changes in qualitative and quantitative chemical composition, and may mean a greater or lesser content of bioactive compounds and/or secondary metabolites of therapeutic interest. Previous evidence reports that Citrus fruits are a source of both macronutrients (e.g., simple sugars, fibers, and water) and micronutrients (e.g., folic acid, thiamine, niacin, vitamin C, and vitamin B6). Their pulps, peels, and seeds contain minerals (potassium, calcium, phosphorus, and magnesium), and are free of sodium and cholesterol; moreover, they are low in proteins and fats [4]. However, their secondary metabolites are the constituents of major interest, especially flavonoids (flavones, flavonols, flavanones, and flavanonols), phenolic acids (hydroxybenzoic acids and hydroxycinnamic acids), anthocyanins, coumarins, and limonoids, which have demonstrated antioxidant, anti-inflammatory, anti-cancer, and neuro-cardioprotective activities [5,6]. Among the flavonoids, the most common aglycones in the Citrus genus are naringenin, hesperetin, apigenin, nobiletin, tangeretin, and quercetin, often carrying saccharide chains that are composed of glucose, rhamnose, rutinose, and neohesperidose. Sinapic, p-coumaric, ferulic, caffeic, and gallic acids are the most common phenolic acids that have been identified in the fruits of this genus. Furthermore, among the bitter limonoid constituents, limonin, nomilin, obacunone, and limonexic acid are also present [7].
More than 170 molecules with antioxidant activity have been identified in the most common fruits of the Citrus genus. The antioxidant activity could be attributed to the whole phytocomplex, which is capable of ensuring the maintenance of healthy cell structure and function through the inactivation of free radicals, the inhibition of lipid peroxidation, and the prevention of harmful oxidative mechanisms [8]. Free radicals, or reactive oxygen species (ROS), by-products of the metabolism of aerobic cells, can generate other peroxidic and hydroperoxidic radicals that are capable of interacting with lipid molecules, or, in a cytotoxic manner, with nucleic acids and proteins essential for life, damaging them or altering their functionality. Therefore, the search for molecules that are capable of counteracting these free radicals is of great interest [7,9].
In this context, C. australasica fruits attract great attention as a potential source of bioactive molecules with antioxidant properties. To the best of our knowledge, few current studies have been reported in the literature, with most focused on volatile components of Alstonville, Judy’s Everbearing, and Durham’s Emerald varieties, along with Faustrime hybrid [10,11]; total phenolic content of four Florida-grown selections [3]; the phenolic composition of XiangBin and LiSiKe varieties [2]; and a Spanish cultivated plant [12].
Based on the previous promising studies and the growing economic, gastronomic, and health-related demands on this peculiar Citrus fruit, the aim of the present study was to carry out a full comparative chemical analysis on both volatile and non-volatile components of the peel and pulp of C. australasica varieties, with particular attention to phenolic compounds and anthocyanins, still lacking in the literature. To this purpose, four C. australasica varieties (Collette, Yellow Sunshine, Pink Ice, and Red), and the hybrid species Faustrime (Monocitrus australasica × Fortunella sp. × Citrus aurantifolia) (Figure 1A), were selected. The metabolomic profile of all varieties was investigated by means of ultra-high performance liquid chromatography (UHPLC), coupled with a diode array detector (DAD) and a high-resolution Orbitrap-based electrospray ionization source mass spectrometer (HR-Orbitrap/ESI-MS); meanwhile, the EO composition was established through gas chromatography coupled with mass spectrometry (GC/MS). All of the extracts were also investigated for their in vitro antioxidant activity on a Balb/3T3 clone A31 mouse embryo fibroblast cell line. This study is part of a larger project conducted by our research group, which is aimed at re-evaluating the beneficial properties of fruits of the genus Citrus, due to their high content of bioactive compounds belonging to the class of polyphenols and triterpenoids [13,14,15,16,17].
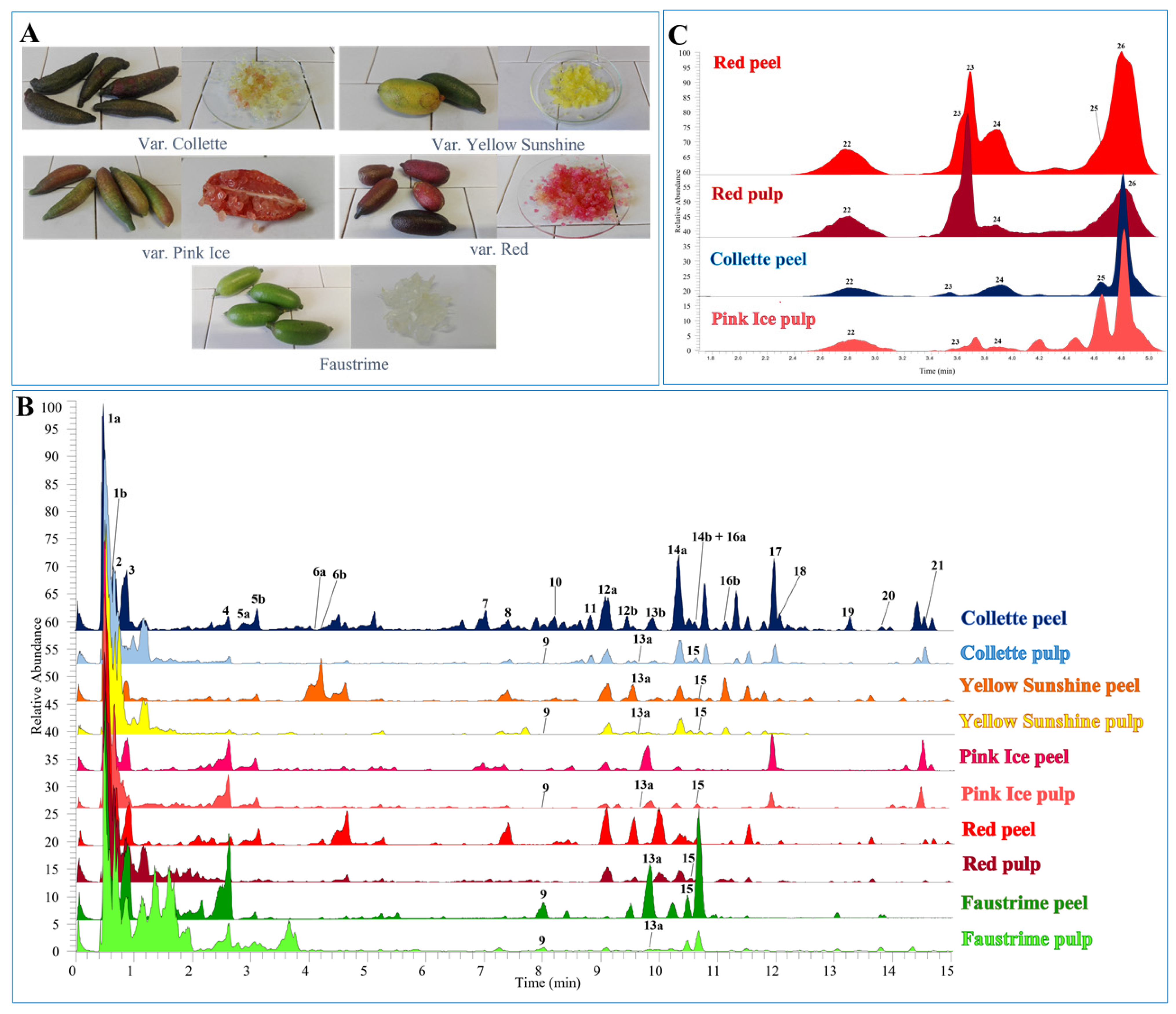
Figure 1.
(A) Citrus australasica studied varieties; (B) comparison of chromatograms of C. australasica peels and pulps extracts, recorded by UHPLC-HR-Orbitrap/ESI-MS analyses in negative ion mode; (C) anthocyanin HR-Orbitrap/ESI-MS profiles of C. australasica peels and pulps, recorded in positive ion mode.
2. Materials and Methods
2.1. Chemicals and Reagents
UHPLC-grade n-hexane, acetonitrile, methanol, water, and formic acid Supelco® were purchased from Merck KGaA (Darmstadt, Germany). All analytical-grade solvents were purchased from VWR (Milano, Italy). Standards of rutin, hesperidin, and gallic acid were purchased from Merck KGaA (Darmstadt, Germany), cyanidin 3-O-glucoside chloride was purchased from Extrasynthese (Extrasynthese, France), and luteolin-4′-O-neohesperidoside was previously obtained in our laboratory by isolation from plant materials and characterised by 1D- and 2D-NMR techniques. Folin–Ciocâlteu reagent was purchased from Merck KGaA (Darmstadt, Germany). The Balb/3T3 clone A31 mouse embryo fibroblast cell line was purchased from the American Type Culture Collection LGC standards (ATCC CCL163, Milan, Italy) and propagated as indicated by the supplier. Dulbecco’s Modified Eagle’s medium (DMEM), 0.01 M pH 7.4 phosphate buffer saline without Ca2+ and Mg2+ (PBS), bovine calf serum (BCS), glutamine, and antibiotics (penicillin/streptomycin) were obtained from Merck KGaA (Darmstadt, Germany). Cell proliferation reagent WST-1 was provided by Roche Diagnostic (Milan, Italy).
2.2. Plant Materials and Non-Volatile Extract Preparation
The fruits of C. australasica varieties (Red, Collette, Pink Ice, and Yellow Sunshine), and the hybrid species Faustrime (Figure 1A), were provided by the Agrumi Lenzi Company (Pescia, Pistoia, Italy) in October 2019. Fruits (6 for each variety) were collected at the ripening stage from plants that were growing in pots. For each fruit, peels were separated from pulps. For the extraction of non-volatile compounds, peels were dried in an oven at 40 °C, while pulps were freeze-dried (Modulyo, Pirani 501, Edwards, UK). The dried material was stored at room temperature, and protected from light until extraction. A portion of fresh material was stored at −20 °C for essential oil preparation (see Section 2.5) and anthocyanin extraction.
For each variety, powdered peels and pulps were first defatted with n-hexane, then subjected to extraction with methanol (solid:liquid mg/mL ratio 1:20 w/v) via dynamic maceration (120 rpm) with a digital orbital shaker (IKA™ KS 501, Minerva S.r.l., Pisa, Italy) for three consecutive days at room temperature, renewing the solvent every 24 h. Finally, the solvent was removed under vacuum to obtain dry extracts, as reported in Table S1.
Anthocyanins were extracted from the peels of Red and Collette varieties, and from the pulps of Red and Pink Ice varieties, and characterised by visible pigmentation. For extraction, 500 mg of defrosted plant material were placed in 3 mL of a 2% methanol/hydrochloric acid mixture for 15 min under stirring, then centrifuged for 5 min at 4000 rpm. The supernatants were withdrawn using a syringe, and directly analysed in triplicate using UHPLC-HR-ESI-MS.
2.3. Determination of the Total Polyphenol Content
Total polyphenol content (TPC) was evaluated in the methanolic extracts of C. australasica peels and pulps following the colorimetric method of Folin–Ciocâlteu [18]. Methanol solutions (10 mg/mL) of peel and pulp extracts were diluted with water until a final concentration of 0.48 mg/mL was reached for peels and 0.70 mg/mL for pulps. Samples were prepared by adding 1 mL of distilled water, 100 μL of Folin–Ciocâlteu reagent, and 300 μL of Na2CO3 (20%) to 500 μL of the aqueous solutions; then, the samples were mixed and incubated in the dark at room temperature for 2 h. The absorbance was measured at 765 nm against a blank solution using a UV-VIS spectrophotometer (Lambda 25, Perkin-Elmer, Waltham, MA, USA). Gallic acid was employed as the standard in the concentration range 0.005–0.030 mg/mL (R2 = 0.999). All of the samples were tested in quadruplicate, and the results were expressed as mg of gallic acid equivalents (GAE)/g of dry weight (DW) [19].
2.4. UHPLC-DAD-HR-Orbitrap/ESI-MS Analyses
2.4.1. Quali-Quantitative Analyses of Phenols
Quali-quantitative chemical analyses were performed via UHPLC using a Vanquish Flex Binary pump that was coupled with a DAD and an HR Q Exactive Plus MS, based on Orbitrap technology, equipped with an ESI source, a hybrid-quadrupole analyser and Xcalibur 3.1 software (Thermo Fischer Scientific Inc., Bremem, Germany). Elutions were conducted at a flow rate of 0.5 mL/min, using a splitting system of 1:1 to an MS detector (250 μL/min) and a DAD/UV detector (250 μL/min), respectively.
For each variety of C. australasica, methanol extracts were solubilized in methanol at concentrations of 2 and 10 mg/mL for the peels and the pulps, respectively. These solutions were prepared in triplicate, and centrifuged for 5 min at 4000 rpm in order to remove suspended particles. Volumes of 5 μL of the supernatants were injected into the LC-MS system. Chromatographic analyses were performed using a 2.1 × 100 mm, 2.6 µm, Kinetex® Biphenyl C-18 column provided from a Security GuardTM Ultra Cartridge (Phenomenex, Bologna, Italy), and a mixture of HCOOH in H2O 0.1% v/v (solvent A) and acetonitrile in H2O 0.1% v/v (solvent B) as the mobile phase. A linear gradient was used, increasing from 5 to 35% B in 15 min for both the methanol extracts of peels and pulps. DAD data were recorded in a 200–600 nm range, with the three preferential channels set at 254, 280, and 325 nm, which are typical absorbances for phenolic compounds. The HR-MS results were acquired in a m/z scan range of 250–1200 in negative ion mode, operating in full (resolution 70,000 and maximum injection time of 220 ms) and data dependent-MS/MS (resolution 17,500 and maximum injection time of 60 ms). The used ionization parameters were the following: nebulization voltage of 3500 V, capillary temperature of 300 °C, sheath gas (N2) 20 arbitrary units, auxiliary gas (N2) 3 arbitrary units, and an HCD (higher-energy C- trap dissociation) of 18 eV.
In order to quantify the phenolic compounds that were identified in the five methanol extracts of C. australasica varieties, four calibration curves were constructed using rutin as the external standard for flavonol glycosides, hesperidin for flavanones glycosides, luteolin 4′-O-neohesperidoside for flavone glycosides, and chlorogenic acid for hydroxycinnamic acids and their esters. Stock solutions of 1 mg/mL of each standard were prepared, and then different concentrations were obtained using serial dilutions. Rutin, hesperidin, and luteolin 4′-O-neoesperidoside were prepared in triplicate acetonitrile solutions in a concentration range of 15.63–1.95 μg/mL, while concentrations of 200–20 μg/mL were used for the chlorogenic acid standard. Integration of the peak areas obtained for each standard in UHPLC-HR-MS was related to the respective concentration, and the equation of the resulting curve was used to quantify the phenolic compounds. The obtained curves showed a good linearity in the range of prepared concentrations and correlation coefficients (R2) equal to 0.993 for rutin, 0.990 for hesperidin, 0.995 for luteolin 4′-O-neoesperidoside, and 0.999 for chlorogenic acid, were obtained. The amount of each compound was calculated using Microsoft® Office Excel, and expressed as μg/g of dried peel or freeze-dried pulp (DW) ± standard deviation.
2.4.2. Quali-Quantitative Analyses of Anthocyanins
The anthocyanin extracts were analysed in triplicate using UHPLC-DAD-HR-ESI-MS. The solutions (5 μL injection volume) were injected in a 2.1 × 100 mm, 2.6 µm, Kinetex® Biphenyl C-18 column provided by a Security GuardTM Ultra Cartridge (Phenomenex, Bologna, Italy), at a flow rate of 0.5 mL/min. A mixture of HCOOH in H2O 0.1% v/v (solvent A) and acetonitrile in H2O 0.1% v/v (solvent B) was used for the elution, according to a linear gradient from 5 to 20% B in 5 min. UV data were recorded using 515 nm as a detection wavelength, the typical absorbance of anthocyanins. The HR-MS data were acquired in a m/z scan range of 120–1200 in positive ion mode, operating in full and data dependent-MS/MS using the same ionization parameters as for phenols.
In order to quantify the anthocyanins in the peels of the Collette and Red varieties, as well as in the pulps of Red and Pink Ice varieties, a calibration curve was constructed with cyanidin 3-O-glucoside as an external standard. Triplicate acetonitrile solutions at the concentrations of 0.05, 0.025, and 0.0025 μg/mL were prepared, beginning from a stock 1 mg/mL solution. By correlating the integrations of the peak areas with the respective standard concentrations, a curve was obtained that showed good linearity in the selected concentration range, and an R2 equal to 0.996. The amount of the anthocyanins identified in the plant material was obtained by Microsoft® Office Excel, and finally expressed as μg/g of dried peel or freeze-dried pulp (DW) ± standard deviation.
2.5. Essential Oils (EOs) Hydrodistillation and Analysis
For all of the samples, 15 g of defrosted peels and 30 g of fresh pulps (after removal of the seeds) were subjected to hydrodistillation in a standard Clevenger apparatus for 2 h. The hydrodistillation duration was experimentally determined as the time necessary for the complete EO volatilisation from the samples. For each sample, triplicates were performed. The hydrodistillation yields could not be evaluated, given the small material amount; thus, the volatile fraction was captured in HPLC-grade n-hexane in the Clevenger apparatus. The EOs in HPLC-grade n-hexane were stored in amber-glass vials and maintained at –20 °C until analysis.
The hydrodistilled samples were injected into a GC-MS apparatus. Gas chromatography–electron impact mass spectrometry (GC-EIMS) analyses were performed with an Agilent 7890B gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) that was equipped with an Agilent HP-5MS (Agilent Technologies Inc., Santa Clara, CA, USA) capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and an Agilent 5977B single quadrupole mass detector (Agilent Technologies Inc., Santa Clara, CA, USA). The analytical conditions used were as follows: injector and transfer line temperatures 220 and 240 °C, respectively; oven temperature programmed from 60 to 240 °C at 3 °C/min; carrier gas helium at 1 mL/min; injection of 1 μL (0.5% HPLC grade n-hexane solution); split ratio 1:25. The acquisition parameters used were as follows: full scan; scan range: 30–300 m/z; scan time: 1.0 sec. The identification of the constituents was based on a comparison of their retention times with those of authentic samples (when available), comparing their linear retention indices relative to the series of n-hydrocarbons. Computer matching was also used against a commercial [20] and a laboratory-developed mass spectra library that was built up from pure substances and components of commercial essential oils of known composition, and from MS literature data [21].
2.6. Cell Viability
Cell viability evaluations of C. australasica extracts were performed using the Balb/3T3 clone A31 cell line. Cells were grown in complete DMEM containing 10% bovine calf serum (BCS), 4 mM glutamine, and 100 U/mL:100 μg/mL penicillin:streptomycin. Balb/3T3 clone A31 fibroblast cells were seeded in 96-well culture plates at a concentration of 104 cells per well, incubated at 37 °C and 5% CO2, and left to proliferate for 24 h prior to the incubation with the samples. The culture medium from each well was removed and replaced with a medium containing pre-dissolved sample in dimethyl sulfoxide (DMSO), and diluted with complete DMEM at different concentrations. Cells incubated with fresh growth medium were used as a control. The DMSO percentage in control and extract samples was kept at 1% v/v. With a view to the assessment of antioxidant effects, cytotoxicity ranges of peel and pulp extracts were set on GAE equivalents, resulting in 15–120 μg/mL for peels, and 30–300 μg/mL for pulps. After 2 h of incubation, cell viability was assessed using WST-1 tetrazolium salt reagent diluted to 1:10, and incubated for 4 h at 37 °C and 5% CO2. Measurements of formazan dye absorbance were carried out at 450 nm, with the reference wavelength of 655 nm, using a microplate reader (BioTek 800/TS, Thermo Scientific).
2.7. Cell Treatment and Oxidative Stress
Adherent Balb/3T3 fibroblast cells, grown on 96-well culture plates, were incubated for 2 h with peel and pulp extracts that were diluted to polyphenol concentrations of 0.25, 0.50, and 1.00 μg/mL GAE in complete DMEM (Table S2). After the treatment, the cells were washed with phosphate buffered saline (PBS), and stressed with 1500 μM of commercial H2O2 for 1 h. Fibroblast cells incubated with H2O2 without sample treatment were considered as reference for the oxidative stress. The cells were evaluated for viability by means of WST-1 reagent. Cell viability percentages were referred to Balb/3T3 control cells, in the absence of treatment and without H2O2 incubation [22].
2.8. Statistical Analyses
All of the analyses were performed with JMP® Pro 14.0.0 (SAS Institute Inc., Cary, NC, USA) software.
For the statistical evaluation of all the EO compositions, an 89 × 10 correlation matrix (89 individual compounds × 10 samples = 890 data) was used, while for composition of phenols and anthocyanins, a 28 × 10 correlation matrix (28 individual compounds × 10 samples = 280 data) was applied. In order to perform the principal component analysis (PCA), linear regressions were operated on mean-centred, unscaled data to select the two highest principal components (PCs). This unsupervised method reduced the dimensionality of the multivariate data of the matrix, whilst preserving most of the variance [23]. For the essential oil analyses, the chosen PC1 and PC2 explained 58.5% and 20.0% of the variance, respectively, for a total explained variance of 78.5%. For the non-volatile components, the chosen PC1 and PC2 explained 52.9% and 25.1% of the studied variance, respectively, for a total studied variance of 78.0%. A hierarchical cluster analysis (HCA) was performed using Ward’s method, with Euclidean distances as a measure of similarity. The observations of the groups of samples performed with HCA and the PCA unsupervised methods can be applied even when there are no available reference samples that can be used as a training set to establish the model.
The significant difference (p value < 0.05) between groups of values was evaluated using a one-way ANOVA.
3. Results and Discussion
3.1. Polyphenol Content of Finger Lime Fruits
The TPC was determined for both peels and pulps of the four varieties of C. australasica (Red, Collette, Pink Ice, and Yellow Sunshine), and the hybrid species Faustrime (Table 1). In general, the results highlighted significantly different TPC among the varieties, but always a higher content of polyphenols in peels than pulps. Specifically, Red and Pink Ice peels had similar TPC values that were higher than the other varieties (9.1 ± 0.2 and 8.2 ± 0.2 mg of GAE/g of DW, respectively). Meanwhile, the hybrid species Faustrime was the most lacking in TPC, as shown by the quantitative datum 4.9 ± 0.1 mg of GAE/g DW. Regarding the pulps, Pink Ice and Collette were the two varieties that were most abundant in polyphenols (6.4 ± 0.2 and 5.6 ± 0.2 mg of GAE/g DW, respectively); Faustrime was confirmed to be poor in TPC, as was Yellow Sunshine variety (3.1 ± 0.2 and 2.6 ± 0.1 mg of GAE/g DW, respectively). Even though Yellow Sunshine peels had a TPC value that was comparable to the other C. australasica varieties, a significant decrease was observed in the pulps (Table 1). Compared to previous results reported by [12], all of the extracts showed higher TPC levels in both peels and pulps.

Table 1.
Total polyphenol content (TPC) of Citrus australasica peel and pulp extracts, expressed as mg of gallic acid equivalents (mg GAE) for mass of dry weight (g DW) ± standard deviation (SD).
3.2. Metabolomic Fingerprint and Quantitative Analysis of Non-Volatile Components
3.2.1. Phenol Composition
The quali-quantitative analyses of C. australasica fruits were performed using UHPLC-DAD-HR-Orbitrap/ESI-MS technique. The chromatographic profiles of all peels and pulps showed similarities and differences, both within the same variety and among different varieties (Figure 1B).
The tentative identification of constituents was performed by comparing their elution order, UV data, HR full mass spectra, and fragmentation patterns, with data that were reported in the literature [2,24]. The level of the identification led to the proposal of tentative candidates, since it was not possible to establish the position of substituents based only on full MS and MS/MS experiments. In addition, a mass error < 5 ppm on the experimental molecular formula was considered for the annotation. Following this approach, 7 hydroxycinnamic acid derivatives, 18 glycosylated flavonoids, and a limonoid, were tentatively identified from all of the analysed finger lime fruits (Table 2). Compounds 7, 16, and 24 were confirmed on the basis of injection of reference standards.

Table 2.
Chromatographic data (retention time, tR) and HR-ESI-MS/MS data of compounds 1–26, detected in peels and pulps of Citrus australasica F. Muell. C = Collette; F = Faustrime; PI = Pink Ice; R = Red; YS = Yellow Sunshine. pe = only peel; pu = only pulp.
In the first chromatographic region (0–5 min; Figure 1B), hydroxycinnamic acid derivatives were characterised. Compounds 1a and 1b (tR = 0.50 and 0.66 min) are two caffeoylisocitric acid isomers, as indicated by HR mass data, showing the deprotonated ion [M-H]− at m/z 353.0723, the ion product [M-162-H]− at m/z 191.02 corresponding to isocitric acid that was generated by the loss of 162 u due to the cleavage of an ester bond with a caffeic acid residue. Compound 2 was annotated as caffeoylmethylisocitric acid, due to the deprotonated ion [M-H]− at m/z 367.0879, and the presence of a methylisocitric product ion at m/z 205.03 that was generated by the loss of a caffeoyl residue (−162 u). These hydroxycinnamic acid tricarboxylic acid esters are metabolites that are not commonly found in plants, especially isocitric acid derivatives [25]. Compounds 5a and 5b were identified as two p-coumaroylglucoside acid isomers. In the mass spectrum recorded in full scan, a parent ion at m/z 325.0927 was observed for both molecules, which under collision energy lost a hexose residue, and generated a p-coumaroyl fragment at m/z 163.04. Compounds 6a and 6b showed the same deprotonated ion [M-H]− at m/z 355.1034 in the full MS, while in the MS/MS experiment a feruloyl ion product at m/z 193.05 that was generated by the cleavage of the glycosidic bond was observed; thus, the two compounds were tentatively attributed to feruloylglucoside acid isomers. Compounds 3 and 4 were not fully identified, but information about a portion of the molecule was deduced by the analyses of their MS fragmentation patterns. Compound 3 ([M-H]− at m/z 433.0596) showed product ions that were in common with compound 2 at m/z 205.03, 143.03, and 111.00, indicating the occurrence of a methylisocitric acid derivative. Compound 4 ([M-H]− at m/z 365.1451) showed the presence of typical product ions at m/z 303.14, 263.11, and 221.10, due to the loss of 62, 102, and 144 u, respectively, indicating the occurrence of a 3-hydroxy-3-methylglutaric acid derivative.
In the chromatographic region within 7–15 min, nine flavonol glycosides (compounds 7, 8, 11, 12a, 12b, 14a, 14b, 18, and 19), seven flavanone glycosides (compounds 9, 13a, 13b, 16a, 16b, 17, and 21), and two flavone glycosides (compounds 10 and 15) were found. Both compounds 7 and 8 displayed the flavonol quercetin (m/z 301.04) as an aglycon portion. In particular, compound 7 was identified as rutin, as deduced by the parent ion at m/z 609.1458 and the observed loss of a rutinose residue (308 u); meanwhile, for compound 8 (deprotonated ion [M-H]− at m/z 463.0880), a loss of a hexose unit was observed, suggesting the occurrence of a quercetin glucoside. Compounds 11 and 19 were kaempferol derivatives, as indicated by the product ion at m/z 285.04 in the MS/MS. Compound 11 (deprotonated molecular ion [M-H]− at m/z 447.1035) was annotated as a kaempferol glucoside, due to the loss of a hexose residue (−162 u), while for compound 19 ([M-H]− at m/z 771.2354), a kaempferol triglucoside structure was suggested ([M-162-162-162-H] − at m/z 285.04). Compounds 12a, 12b, 14a, 14b, and 18 all exhibited a base ion peak at m/z 315.04 in the MS/MS, which was attributed to isorhamnetin. Compounds 12a and 12b ([M-H]− at m/z 477.1035) were assigned as isorhamnetin glucoside isomers, showing the loss of a hexose unit. Compounds 14a and 14b showed the same deprotonated ion at m/z 621.1457, and diagnostic fragments for a hexose unit (−162), and a 3-hydroxy-3-methylglutaryl residue (−62, −102, −144 u). Compound 18 ([M-H]− at m/z 765.1881) differed from 14a and 14b, only for having one more unit of 3-hydroxy-3-methylglutaric acid; thus, it was annotated as a di-(3-hydroxy-3-methylglutaryl) isorhamnetin glucoside. Compound 9 was revealed only in the fruits of the hybrid species Faustrime, and it could correspond to neoeriocitrin or eriocitrin, two flavanones glycosides that are commonly found in the genus Citrus [9]. In addition to the deprotonated ion [M-H]− at m/z 595.1666, a base ion peak at m/z 287.06 that corresponded to the aglycone portion of eriodictyol was observed, due to the loss of a disaccharide (308 u) which could be attributed to a rutinose or a neohesperidose, since they cannot be distinguished only on the basis of mass spectra. Peaks 13a and 13b displayed the same parent ion at m/z 579.1713 and the same base ion peak at m/z 271.02 attributed to naringenin. The loss of a disaccharide unit [M-H–308]− due to a rutinose or neohesperidose residue suggested the presence of two isomers, tentatively identified as naringin (naringenin neohesperidoside) and naringenin rutinoside. Compounds 16a and 16b were two isomeric forms of the same molecule, as deduced from the same deprotonated molecular ion [M-H]− at m/z 609.1029, and from the overlapping fragmentation mass spectra in which the base ion peak at m/z 301.07 was attributed to hesperetin. The two isomers were annotated as neohesperidin (hesperetin neohesperidoside) and hesperidin (hesperetin rutinoside). Peak 17 ([M-H]− at m/z 755.2415) was tentatively identified as isosakuranetin rhamnosyldiglucoside, since in the ESI-MS/MS, a base ion peak at m/z 285.08 ([M-162-162-146-H] −) generated by the loss of two hexose residues (probably glucose) and a deoxyhexose (probably rhamnose) was observed. Poncirin (21, [M-H]− at m/z 593.1878) displayed a fragment ion at m/z 285.08 that was assigned to isosakuranetin, previously reported in C. australasica by Wang et al. (2019). The full MS ([M-H]− at m/z 593.1514) and MS/MS (base ion peak at m/z 285.04) suggested compound 10 as luteolin 7-O-neohesperidoside or luteolin 7-O-rutinoside, according to the aforementioned previous study (Wang et al., 2019). Compound 15 exhibited a parent ion at m/z 607.1666, and a diagnostic product ion at m/z 299.06, which was assigned to diosmetin. For its glycosidic portion, the option between two disaccharides (308 u), neohesperidose and rutinose, was considered; therefore, 15 could be annotated as diosmin or neodiosmin. Compound 20 ([M-H]− at m/z 501.1763) was identified as limonexic acid, which belongs to the class of limonoids, typical terpenoids of the genus Citrus and responsible for their bitter taste [26]. The fragmentation peaks observed in the ESI-MS/MS at m/z 457.18 and 413.20 are in agreement with the data reported in the literature [27].
From a qualitative point of view, our results confirmed the presence of some components that were identified in C. australasica peel and pulp via UHPLC-MS/MS from a previous study [12]. To the best of our knowledge, the chemical composition of the hybrid species Faustrime has herein been reported for the first time. There are differences among the five varieties, especially in the case of the hybrid species Faustrime (Table 2), which showed several typical constituents of the Citrus genus, such as (neo)eriocitrin, (neo)diosmin, and naringenin rutinoside/naringin, that are rarely found in the other C. australasica varieties.
The quantitative estimation of all of the constituents (Table 3) that were obtained through UHPLC-MS highlighted greater differences among all studied fruits. Generally, it was confirmed that all phenol constituents are more abundant in peels than in pulps. The hydroxycinnamic acid derivatives were present in peels and the pulps of all varieties in very similar amounts, with the exception of Pink Ice, which was particularly rich in caffeoylisocitric acid. The largest amount of total flavonoids found among peels was in Collette (3432 ± 239 μg/g dry weight, DW), and in Pink Ice among pulps (897 ± 43 μg/g DW). Considering the whole fruit, among the identified compounds, the most abundant ones were rutin, luteolin 7-O-neohesperidoside/rutinoside, isosakuranetin rhamnosyldiglucoside, and poncirin, in Collette and Pink Ice; quercetin glucoside, isorhamnetin glucoside, and neohesperidin in Yellow Sunshine; quercetin glucoside, naringin, and poncirin in Red. The hybrid species Faustrime was distinguished by the significant presence, especially in the peels, of (neo)eriocitrin (316 ± 27 μg/g DW), (neo)diosmin (606 ± 41 μg/g DW), and naringenin rutinoside/naringin (145 ± 12 μg/g DW).

Table 3.
Content of phenolic compounds (μg/g of dried peel or pulp ± standard deviation) and anthocyanins (μg/g of fresh peel or pulp ± standard deviation) in C. australasica peel and pulp extracts. nd = not determined.
3.2.2. Anthocyanins Characterisation
Anthocyanins were identified in the extracts that were obtained from Red and Collette peels, and from Red and Pink Ice pulps, by comparing the data obtained through UHPLC-UV-ESI-MS/MS (Figure 1C) with those from a previous study on the Citrus fruits [28]. Five anthocyanins derived from cyanidin, delphinidin, petunidin, and peonidin were found (compounds 22–26, Table 2).
Compound 22 (tR = 2.78 min) was characterised as cyanidin 3-O-glucoside, as deduced by ESI-MS/MS data showing a molecular ion [M]+ at m/z 449.1068, and a base ion peak at m/z 287.05 that corresponded to the aglycone portion of cyanidin, and generated by the loss of a hexose residue (−162 u). Compound 23 (tR = 3.68 min) was assigned a molecular weight equal to 787.2272 u, on the basis of molecular ion [M]+ recorded in full scan MS. In the fragmentation spectrum, a base ion peak at m/z 317.06 ([M-162-146-162]+) was observed, corresponding to the aglycone portion, petunidin, generated by the loss of two hexose and one deoxyhexose residues; thus, 23 was tentatively identified as petunidin rhamnosyldiglucoside. Cyanidin 3-(6″-malonylglucoside) (24, tR = 3.87 min) was characterised by a molecular ion [M]+ at m/z 535.1071, and a base ion peak at m/z 287.05 that was attributed to the aglycone, cyanidin, generated by the loss of a malonyl residue and a hexose [M-162-86]+. Peonidin 3-(6″-malonylglucoside) (25, tR = 4.85 min) displayed a molecular ion [M]+ at m/z 549.1227, a product ion at m/z 301 corresponding to peonidin, and similarly to compound 24, the loss of 86 and 162 u residues. Compound 26 (tR = 4.76 min, [M]+ = 611.1593) was annotated as delphinidin rhamnosylglucoside. The analysis of the fragmentation pattern highlighted the aglycone portion at m/z 303.05, which was identified as delphinidin, and the loss of a disaccharide ([M-162-146]+) was attributable to hexose and deoxyhexose residues.
Based on the quantitative analysis (Table 3), cyanidin 3-O-glucoside was found to be the most representative anthocyanin, both in peels and pulps, of all of the investigated varieties. In agreement with our results, cyanidin 3-O-glucoside was found to be the most abundant anthocyanin from a previous study of red finger lime [29]. The peels of the Collette variety were the richest in anthocyanins (143.2 ± 3.2 μg/g DW), followed by the peels (96.2 ± 2.3 μg/g DW) and the pulps (8.26 ± 0.56 μg/g DW) of the Red variety.
3.2.3. Multivariate Statistical Analyses of the Non-Volatile Components
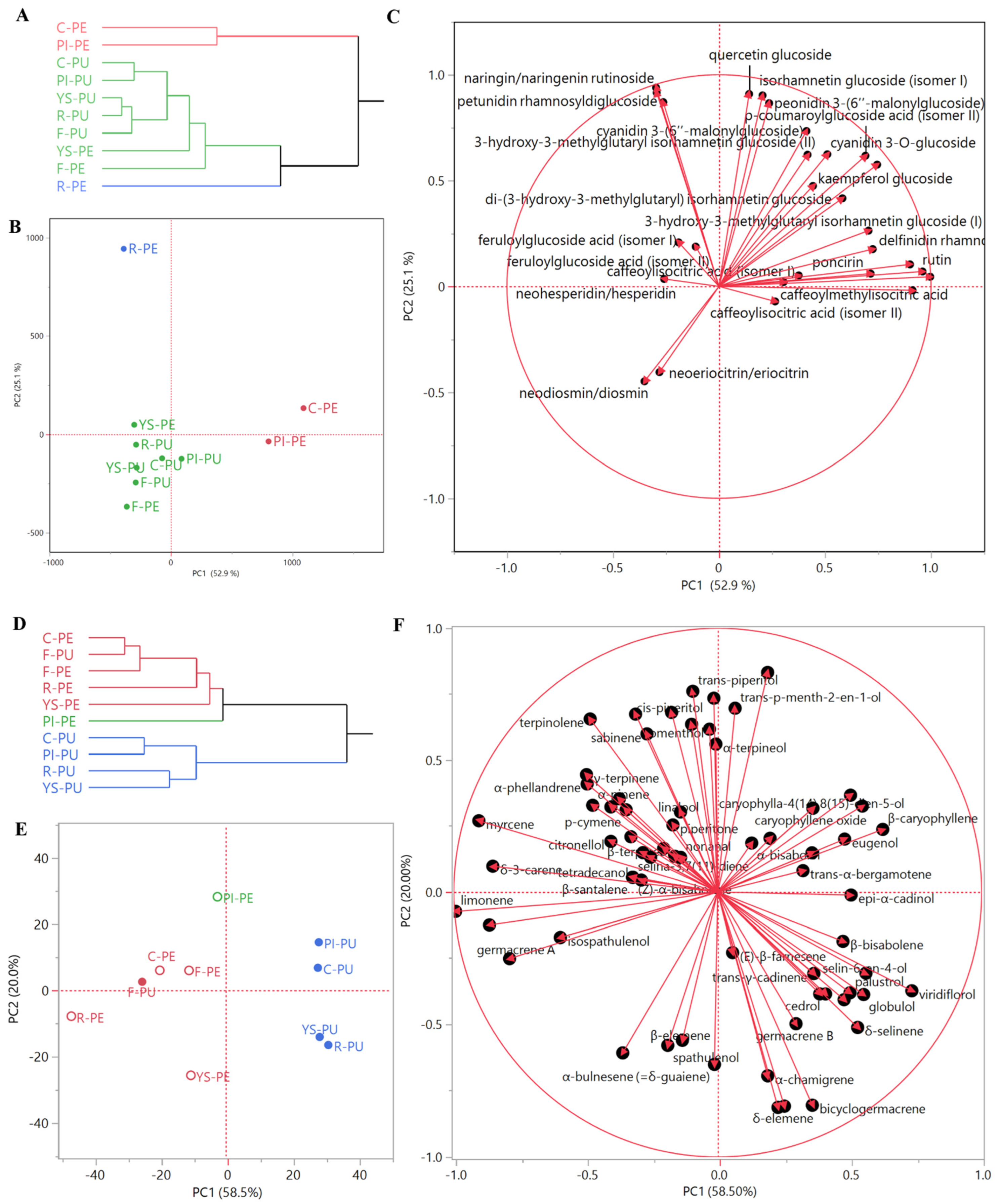
The dendrogram of the HCA (Figure 2A) shows a sample distribution into two macro-clusters: the first one (red samples) comprised Collette and Pink Ice peels, while the second one comprised two clusters (blue and green). The blue cluster is composed only of Red peel, while the green cluster includes the pulps of all of the varieties, as well as the Faustrime and Yellow Sunshine peels.
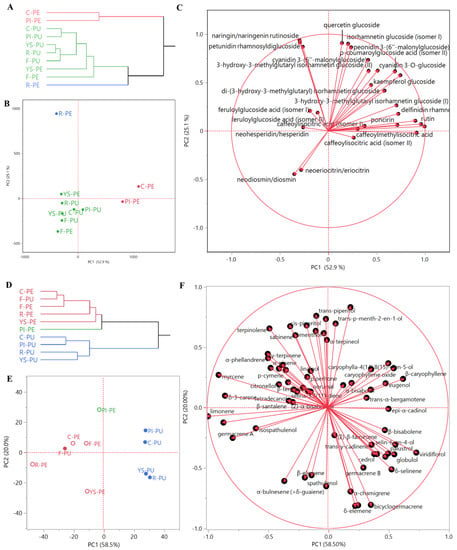
Figure 2.
Non-volatile (A) and EO (D) dendrograms of the hierarchical cluster analysis (HCA), and respective score (B,E) and loadings (C,F) plots of the principal component analysis (PCA) performed on peels (PE) and pulps (PU) for essential oil and non-volatile compositions of all of the samples (C = Collette; F = Faustrime; PI = Pink Ice; R = Red; YS = Yellow Sunshine).
This organ-driven statistical distribution was confirmed through the PCA. Collette and Pink Ice peels were plotted on the right quadrants (PC1 > 0) of the score plot (Figure 2B). Red peels were plotted in the right area of the upper left quadrant (PC1 < 0, PC2 > 0) (score plot, Figure 2B), due to their high anthocyanin content (loadings plot, Figure 2C). The pulps of all of the varieties and of Yellow Sunshine peels were plotted on the left quadrants (PC1 < 0) of the score plot (Figure 2B), due to their content of neodiosmin/diosmin and neoeriocitrin/eriocitrin. Pink Ice and Yellow Sunshine varieties were plotted along the PC1 axis, and in the upper region of the bottom quadrants (PC2 < 0) (score plot, Figure 2B), due to their content of caffeoylisocitric acid (isomers I and II) and hydroxycinnamic acid derivatives (loadings plot, Figure 2C).
3.3. Essential Oil (EO) Composition of All of the Samples
The complete composition of all of the EOs that were hydrodistilled from both the peels and pulps of all the C. australasica varieties (Collette, Pink Ice, Red, and Yellow Sunshine), and the Faustrime hybrid, are reported in Table 4. Overall, 89 compounds were identified from the EO compositions.

Table 4.
Complete composition of the peel and pulp essential oils hydrodistilled from all of the analysed Citrus australasica varieties (Collette, Pink Ice, Red, and Yellow Sunshine), and for the Faustrime hybrid.
Monoterpenes were the most abundant compounds found in all of the peel EOs, with the exception of the Yellow Sunshine variety, where sesquiterpenes prevailed. The Collette and Red varieties, as well as the Faustrime peel EOs, can be considered a limonene chemotype, whereas the Pink Ice and the Yellow Sunshine EOs exhibited a 4-terpineol/limonene and a limonene/bicyclogermacrene chemotype, respectively. This difference in peel EO chemotypes was found to be consistent with previous literature studies for other finger lime varieties [10,30,31], although the latter analysed a dichloromethane extract of the volatile peel constituents. Among the monoterpenes, their hydrocarbon form prevailed in all of the peel EOs, with the exception of the Pink Ice variety, where the oxygenated monoterpenes were more abundant. Among the monoterpene hydrocarbons, limonene was found to be the most quantitatively relevant in all of the samples, accounting for up to 73.6% in the Red variety. With the exception of the Red and Yellow Sunshine varieties, γ-terpinene and α-phellandrene followed, as relative concentrations. Among the peel EOs, oxygenated monoterpenes were more abundant in the Pink Ice variety and the Faustrime hybrid, exhibiting a relative presence of 48.0 and 35.5%, respectively. Among this class, 4-terpineol was observed to be the most abundant in the former, while citronellal and piperitone prevailed in the latter. This quantitatively relevant presence of citronellal and piperitone in the Faustrime hybrid EO is in accordance with [32], although their analysis was performed with an essential oil that was obtained by peel cold-pressing, and [11]. Bicyclogermacrene was the sesquiterpene hydrocarbon that exhibited the highest relative abundance in all of the peel EOs. Sesquiterpenes dominated all of the pulp EO compositions, with the only exception being the Faustrime hybrid, whose composition was mainly represented by monoterpenes. The Yellow Sunshine pulp EO was mainly composed of oxygenated sesquiterpenes, among which viridiflorol and globulol were the most represented; however, like the Red variety, the most characterising compound in its composition was bicyclogermacrene, a sesquiterpene hydrocarbon. Among the latter chemical classes, β-caryophyllene was detected as the main relevant compound in the Collette variety, while it exhibited a comparable presence to its oxidized counterpart (caryophyllene oxide) in the leaf EO of the Pink Ice variety. α-Humulene and β-bisabolene followed within this class, reaching up to 12.7% in the Pink Ice pulp EO and 24.6% in the Red variety pulp EO, respectively. Viridiflorol, globulol, and guaiol were detected as the most abundant oxygenated sesquiterpenes in the pulp EOs of all of the analysed samples, with the exception of the Faustrime hybrid. The latter was, indeed, chiefly composed of monoterpene hydrocarbons, which represented over 70% of its complete composition, with limonene being the most abundant compound (48.3%), followed by γ-terpinene (10.8%), and α-phellandrene (7.2%).
Multivariate Statistical Analyses of the EO Compositions
The dendrogram of the HCA (Figure 2D) evidenced a distribution of the samples into two macro-clusters: the first comprised two clusters (red and green) and included all of the peel Eos, and the Faustrime pulp sample; the second macro-cluster was homogeneous (blue samples), and comprised all of the C. australasica pulp EOs, except the Faustrime pulp.
This organ-driven statistical distribution was confirmed with principal component analysis. All of the peel EOs were plotted on the left quadrants (PC1 < 0) of the score plot (Figure 2E), together with the Faustrime pulp sample. The Pink Ice peel EO was plotted in the right area of the upper left quadrant (PC1 < 0, PC2 > 0) (score plot, Figure 2E), due to its 4-terpineol content (loadings plot, Figure 2F). Collette and Faustrime peel EOs were closely grouped towards the center of the same quadrant, very close to the Faustrime pulp EO (score plot, Figure 2E): all of these samples were grouped in this area due to the contribution of γ-terpinene and α-phellandrene (loadings plot, Figure 2F). Both the Red and Yellow Sunshine peel EOs were plotted in the bottom right quadrant (PC1 and PC2 > 0) of the PCA score plot (Figure 2E): as evidenced in the loadings plot (Figure 2F), their position was due to their high relative content of limonene. With the exception of the Faustrime hybrid sample, all of the pulp EOs were plotted in the right quadrants (PC1 > 0) of the PCA score plot (Figure 2E). Collette and Pink Ice pulp EOs were grouped in the upper quadrant (PC2 > 0): the positioning of the former was mainly due to its high relative content of caryophyllene oxide, while β-caryophyllene and α-humulene showed a high relative presence in both samples. Finally, Yellow Sunshine and Red pulp EOs were plotted in the bottom right quadrant (PC1 > 0, PC2 < 0) of the PCA score plot (Figure 2E), due to the contributions of bicyclogermacrene, globulol, viridiflorol, and guaiol vectors (loadings plot, Figure 2F).
3.4. Cell Viability Assay and In Vitro Cellular Assessment of Antioxidant Properties
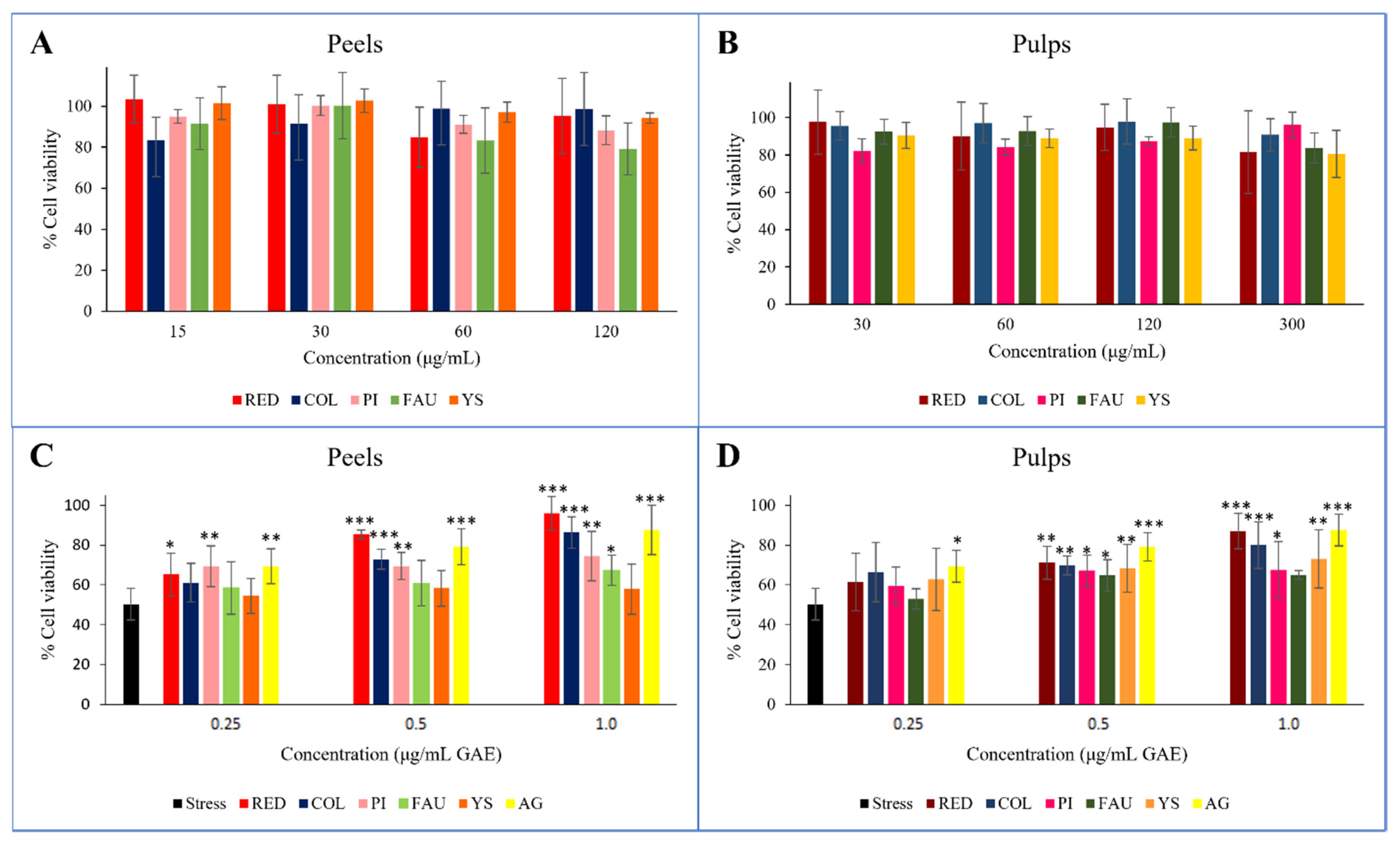
Before evaluating the extent of the protection they provided from oxidative stress, the cytotoxicity on Balb/3T3 of the peel and pulp extracts was assayed. The concentration ranges were set on the basis of relevant GAE equivalents, resulting in 15–120 μg/mL for peels, and 30–300 μg/mL for pulps. Neither the peel nor pulp extracts showed cytotoxic effects at any of the tested concentrations (Figure 3A,B).
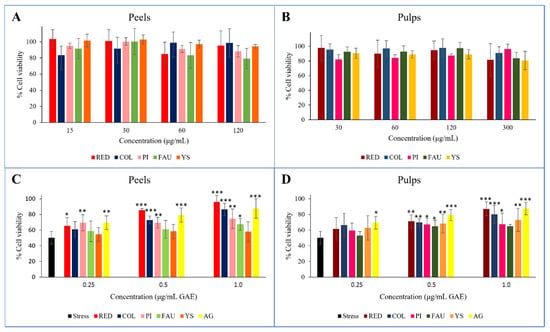
Figure 3.
In vitro cell evaluation of the Balb/3T3 cell line. Cytotoxicity screening after 2-h treatments with peel (A) and pulp (B) extracts. Protective effects of peel (C) and pulp (D) extracts from H2O2-induced oxidative stress, reported as cell viability % after 2-h treatments with 1500 μM H2O2 of pre-treated Balb/3T3 cells. H2O2 = stressed control; C = Collette; F = Faustrime; GA = gallic acid; PI = Pink Ice; R = Red; YS = Yellow Sunshine; *** = p value < 0.0001; ** = p value < 0.005; * = p value < 0.05 vs. stress.
The antioxidant protective effect was assessed in vitro for all peel (Figure 3C) and pulp (Figure 3D) extracts. Three different concentrations were evaluated, namely 0.25, 0.50, and 1.0 μg/mL GAE, and compared to gallic acid that was used as a reference. The oxidative treatment with H2O2 resulted in a drastic decrease in cell viability (45%) with respect to untreated and unstressed control cells. Cell viability was increased with the pretreatment of all extracts, thus protecting cells from the induced oxidative stress. In general, the observed effects were directly proportional to the corresponding concentration of gallic acid, with greater accordance recorded for the Red and Collette extracts. In these cases, the effects on cell viability correlated with increasing GAE concentration treatments, incrementing from 65 to 96%, and from 61 to 87% for Red peel and pulp, respectively; and from 61 to 86%, and from 66 to 80% for Collette peel and pulp, respectively. Concerning the hybrid species Faustrime, a poor protection effect was detected for both its peel and pulp. This evidence could be explained on the basis of the higher polyphenol content in the Red and Collette varieties, and moreover, due to the valuable amounts of anthocyanins in these varieties.
4. Conclusions
The chemical investigation of the four finger lime varieties revealed an interesting profile of potentially health-promoting agents that were represented by hydroxycinnamic acids (ferulic, p-coumaric, and caffeic acid derivatives) and glycosylated flavonols (kaempferol, quercetin, and isorhamnetin derivatives), flavanones (naringenin, eriodictyol, and hesperetin derivatives), and flavones (luteolin and diosmetin derivatives). Furthermore, the glycosides of cyanidin, delfinidin, petunidin, and peonidin were the anthocyanins that were detected in the Red, Pink Ice, and Collette varieties. Among limonoids, triterpenoids typically found in Citrus fruits that are responsible for their bitter taste, only limonexic acid was revealed. For each variety, the peel and pulp showed similar qualitative profiles; among all of the samples, the hybrid species Faustrime differed for the presence of neoeriocitrin, eriocitrin, neodiosmin, and diosmin, components that are usually predominant in other common Citrus fruits. All of the peels differed from their relative pulps in terms of phenol amount; they were richer in bioactive components, as confirmed by their higher antioxidant capacity observed in their provided protection from oxidative damage. Similarly, the volatile compositions of peel EOs of all of the samples were characterised mainly by monoterpenes, while pulp EOs were rich in sesquiterpenes.
The uniqueness of the organoleptic characteristics of these fruits, jointly with their composition that is rich in antioxidant metabolites, make them promising candidates for their use as fresh fruits, or for the development of nutraceutical products with beneficial properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11102047/s1, Table S1: Results of peel and pulp extraction from Citrus australasica varieties. Table S2: Concentrations of peel and pulp extracts from Citrus australasica varieties applied in the in vitro assay for protection from H2O2 oxidative stress on Balb/3T3.
Author Contributions
Conceptualization, A.M.P., M.D.L., L.P. and Y.Z.; methodology, A.M.P., G.F., M.D.L. and Y.Z.; validation, A.M.P., B.M. and E.C.; formal analysis, B.M., C.M., E.C. and R.A.; investigation, C.M., E.C., R.A. and M.D.L.; resources, M.D.L., L.P. and Y.Z; data curation, B.M., C.M., E.C. and R.A.; writing—original draft preparation, B.M., C.M., E.C. and R.A.; writing—review and editing, A.M.P., M.D.L., G.F., L.P. and Y.Z.; supervision, A.M.P., G.F., L.P. and M.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the data are contained within the article.
Acknowledgments
The authors are grateful to Agrumi Lenzi Company (Pescia, Pistoia, Italy) for kindly supplying the C. australasica fruits used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Favela-Hernández, J.M.J.; González-Santiago, O.; Ramírez-Cabrera, M.A.; Esquivel-Ferriño, P.C.; Camacho-Corona, M. Chemistry and pharmacology of Citrus sinensis. Molecules 2016, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, S.; Zang, W.; Wang, N.; Cao, J.; Li, X.; Sun, C. Identification of phenolic compounds from a unique Citrus species, finger lime (Citrus australasica) and their inhibition of LPS-induced NO-releasing in BV-2 cell line. Food Chem. Toxicol. 2019, 129, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Dutt, M.; Vashisth, T. Comparative phytochemical analysis of the fruits offour Florida-grown finger lime (Citrus australasica) selections. LWT-Food Sci.Technol. 2020, 135, 110003. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of Citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms ofaction. Adv. Nutr. 2014, 5, 404417. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agr. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Marin, F.R.; Ortuño, A.; Del Río, J.A. Uses and properties of Citrus flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Decorzant, E.; Chapuis, C.; Casilli, A.; Frérot, E. Comparative analysis of three australian finger lime (Citrus australasica) cultivars: Identification of unique citrus chemotypes and new volatile molecules. Phytochemistry 2015, 109, 111–124. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Zappia, G.; Bonaccorsi, I.; Cotroneo, A.; Russo, M.T. The composition of the volatile fraction and the enantiomeric distribution of five volatile components of faustrime oil (Monocitrus australatica × Fortunella sp. × Citrus aurantifolia). J. Essential Oil Res. 2004, 16, 4. [Google Scholar] [CrossRef]
- Aznar, R.; Rodríguez-Pérez, C.; Rai, D.K. Comprehensive characterization and quantification of antioxidant compounds in finger lime (Citrus australasica L.) by HPLC-QTof-MS and UPLC-MS/MS. Appl. Sci. 2022, 12, 1712. [Google Scholar] [CrossRef]
- Da Pozzo, E.; De Leo, M.; Faraone, I.; Milella, L.; Cavallini, C.; Piragine, E.; Testai, L.; Calderone, V.; Pistelli, L.; Braca, A.; et al. Antioxidant and antisenescence effects of bergamot juice. Ox. Med. Cell. Longev. 2018, 2018, 9395804. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.; Piragine, E.; Pirone, A.; Braca, A.; Pistelli, L.; Calderone, V.; Miragliotta, V.; Testai, L. Protective effects of bergamot (Citrus bergamia Risso & Poiteau) juice in rats fed with high-fat diet. Planta Med. 2020, 86, 180–189. [Google Scholar] [CrossRef]
- Flamini, G.; Pistelli, L.; Nardoni, S.; Ebani, V.; Zinnai, A.; Mancianti, F.; Ascrizzi, R.; Pistelli, L. Essential oil composition and biological activity of “Pompia”, a sardinian Citrus ecotype. Molecules 2019, 24, 908. [Google Scholar] [CrossRef]
- Giovanelli, S.; Ciccarelli, D.; Giusti, G.; Mancianti, F.; Nardoni, S.; Pistelli, L. Comparative assessment of volatiles in juices and essential oils from minor Citrus fruits (Rutaceae). Flavour Fragr. J. 2020, 35, 639–652. [Google Scholar] [CrossRef]
- Testai, L.; De Leo, M.; Flori, L.; Polini, B.; Braca, A.; Nieri, P.; Pistelli, L.; Calderone, V. Contribution of irisin pathway in protective effects of mandarin juice (Citrus reticulata Blanco) on metabolic syndrome in rats fed with high fat diet. Pharmacol. Res. 2021, 35, 4324–4333. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Felice, F.; Fabiano, A.; De Leo, M.; Piras, A.M.; Beconcini, D.; Cesare, M.M.; Braca, A.; Zambito, Y.; Di Stefano, R. Antioxidant effect of cocoa by-product and cherry polyphenol extracts: A comparative study. Antioxidants 2020, 9, 132. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library. In NIST Standard Reference Database Number 69; (No. 2014); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-based nanoparticles containing cherry extract from Prunus avium L. to improve the resistance of endothelial eells to oxidative stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Giusiani, M.; Stefanelli, F.; Deriu, V.; Chericoni, S. VOCs as fingerprints for the chemical profiling of hashish samples analyzed by HS-SPME/GC–MS and multivariate statistical tools. Forensic Toxicol. 2018, 36, 243–260. [Google Scholar] [CrossRef]
- Parveen, I.; Winters, A.; Threadgill, M.D.; Hauck, B.; Morris, P. Extraction, structural characterisation and evaluation of hydroxycinnamate esters of orchard grass (Dactylis glomerata) as substrates for polyphenol oxidase. Phytochemistry 2008, 69, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Leicht, P.; Bokern, M.; Wray, V.; Grotjahn, L. Hydroxycinnamic acid esters of isocitric acid: Accumulation and enzymatic synthesis in Amaranthus cruentus. Phytochemistry 1987, 26, 2919–2922. [Google Scholar] [CrossRef]
- Russo, M.; Arigò, A.; Calabrò, M.L.; Farnetti, S.; Mondello, L.; Dugo, P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: Limonoids and flavonoids. J. Funct. Foods 2016, 20, 10–19. [Google Scholar] [CrossRef]
- Biavatti, M.W.; Vieira, P.C.; Da Silva, M.F.; Fernandes, J.B.; Albuquerque, S. Limonoids from the endemic brazilian species Raulinoa echinata. Z. Naturforsch. C 2001, 56c, 570–574. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Rapisarda, P. Anthocyanins in different Citrus species: An UHPLC-PDA-ESI/MSn-assisted qualitative and quantitative investigation. J. Sci. Food Agric. 2016, 96, 4797–4808. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Johnson, J.B.; Batley, R.; Manson, D.; White, S.; Naiker, M. Volatile compounds, phenolic acid profiles and phytochemical content of five Australian finger lime (Citrus australasica) cultivars. LWT 2022, 154, 112640. [Google Scholar] [CrossRef]
- Lota, M.-L.; de Rocca Serra, D.; Tomi, F.; Jacquemond, C.; Casanova, J. Volatile components of peel and leaf oils of lemon and lime species. J. Agric. Food Chem. 2002, 50, 796–805. [Google Scholar] [CrossRef]
- Trozzi, A.; Verzera, A.; d’Alcontres, I.S. Constituents of the cold-pressed oil of Faustrime, a trigeneric hybrid of Monocitrus australasica × Fortunella sp. × Citrus aurantifolia. J. Essent. Oil Res. 1995, 5, 97–100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).