Mechanisms of Epithelial-Mesenchymal Transition and Prevention of Dispase-Induced PVR by Delivery of an Antioxidant αB Crystallin Peptide †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. RNA Isolation and Real-Time Quantitative RT-PCR
2.3. Western Blot Analysis
2.4. Detection of Mitochondrial Superoxide with MitoSOX
2.5. Measurement of Cellular Respiration
2.6. Measurement of Cellular Glycolysis
2.7. Cell Migration Assay
2.8. Dispase-Induced PVR in Mouse
2.9. Immunofluorescence
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Results
3.1. αBC-P Suppressed EMT-Associated Genes in hRPE Cells
3.2. αBC-P Suppressed EMT-Associated Proteins in hRPE Cells
3.3. αBC-ELP Treatment Inhibited Mitochondrial ROS Production in hRPE Cells Exposed to TGFβ2
3.4. αBC-ELP Treatment Inhibited SMAD4 Mitochondrial Translocation in hRPE Cells
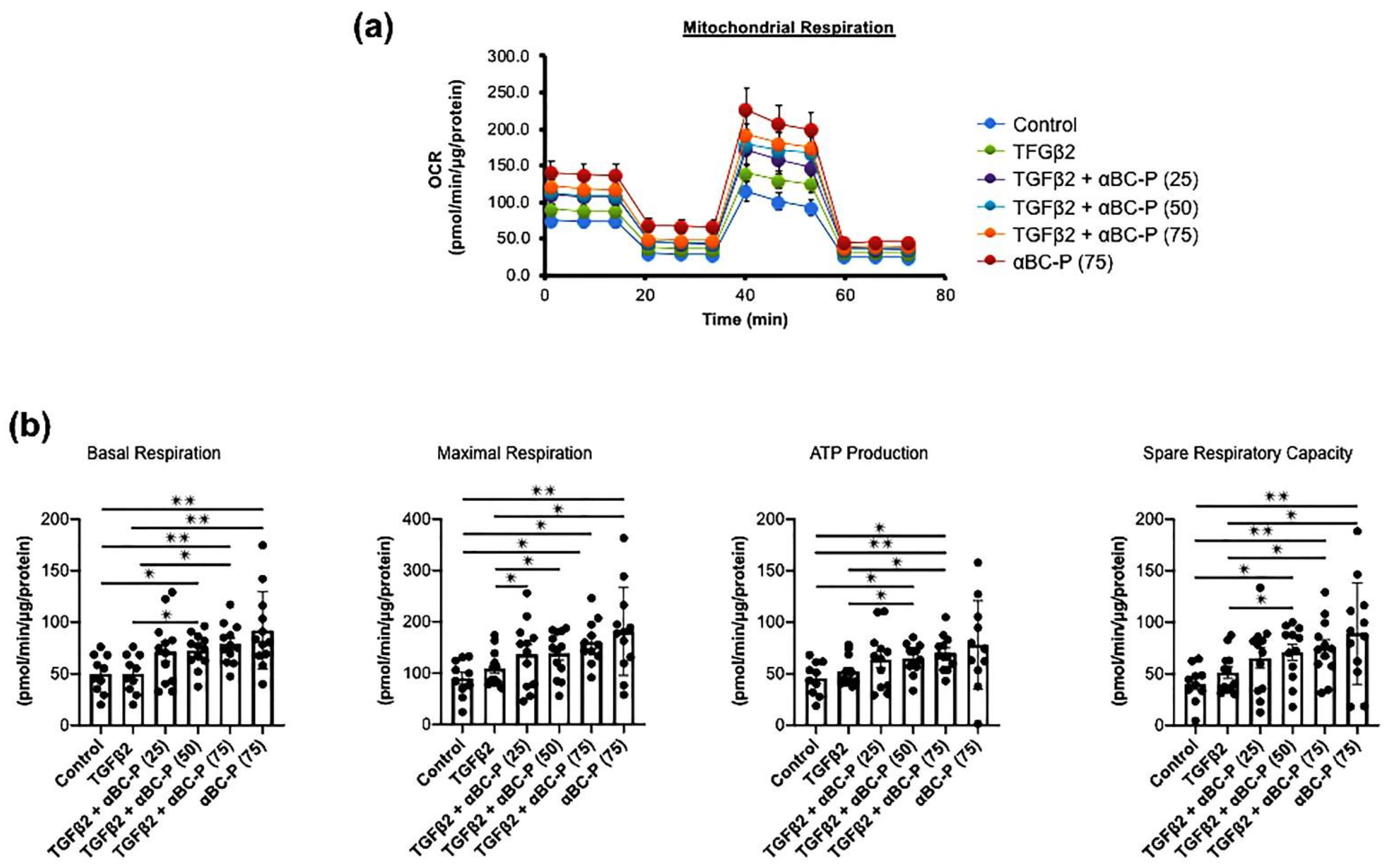
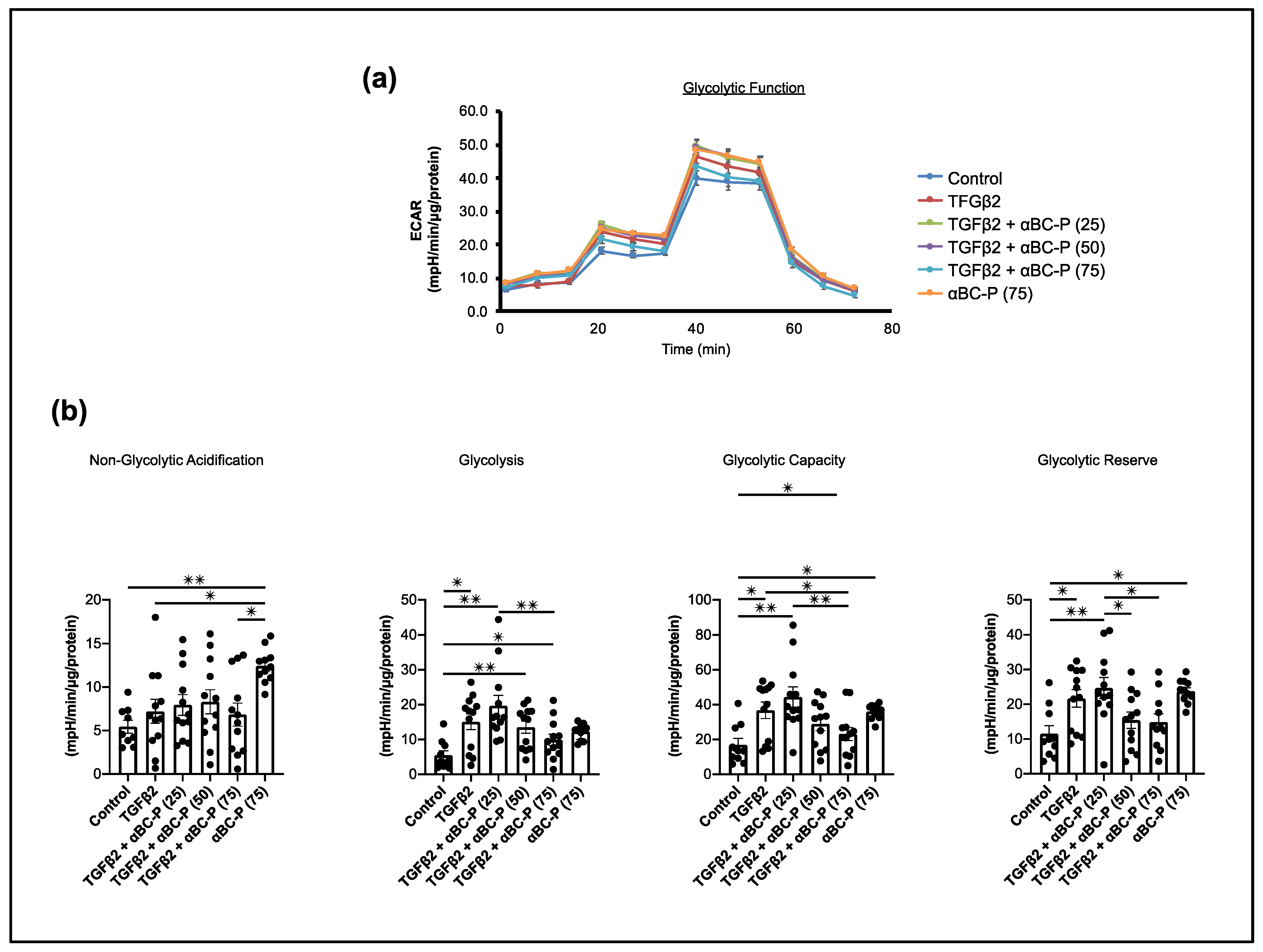
3.5. TGFβ2-Induced Changes in Energy Metabolism and the Role of αBC-P in RPE
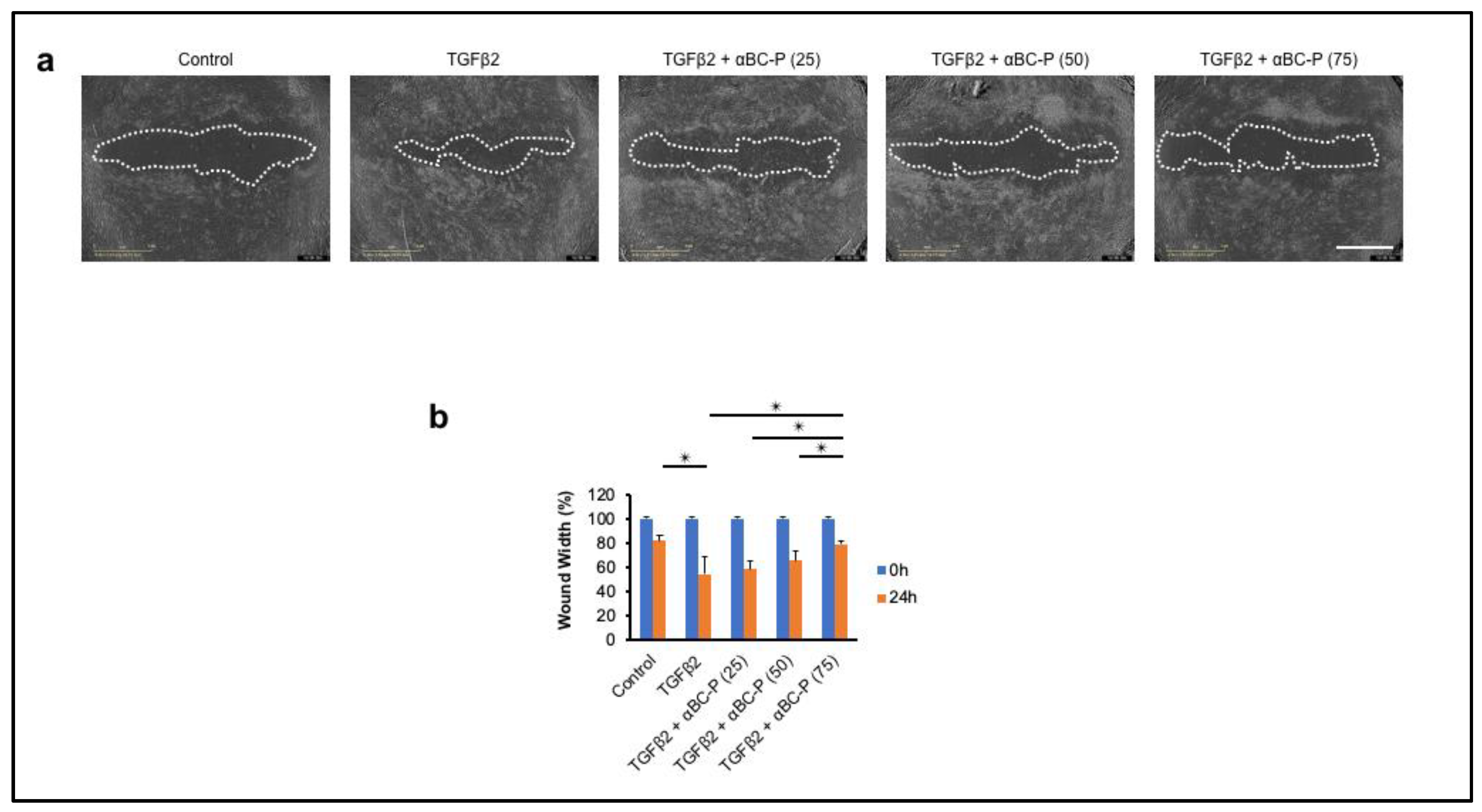
3.6. αBC-P Inhibits TGFβ2-Induced Migration of hRPE Cells
3.7. αBC-P Inhibits Matrix Deposition in Dispase-Induced PVR in Mice
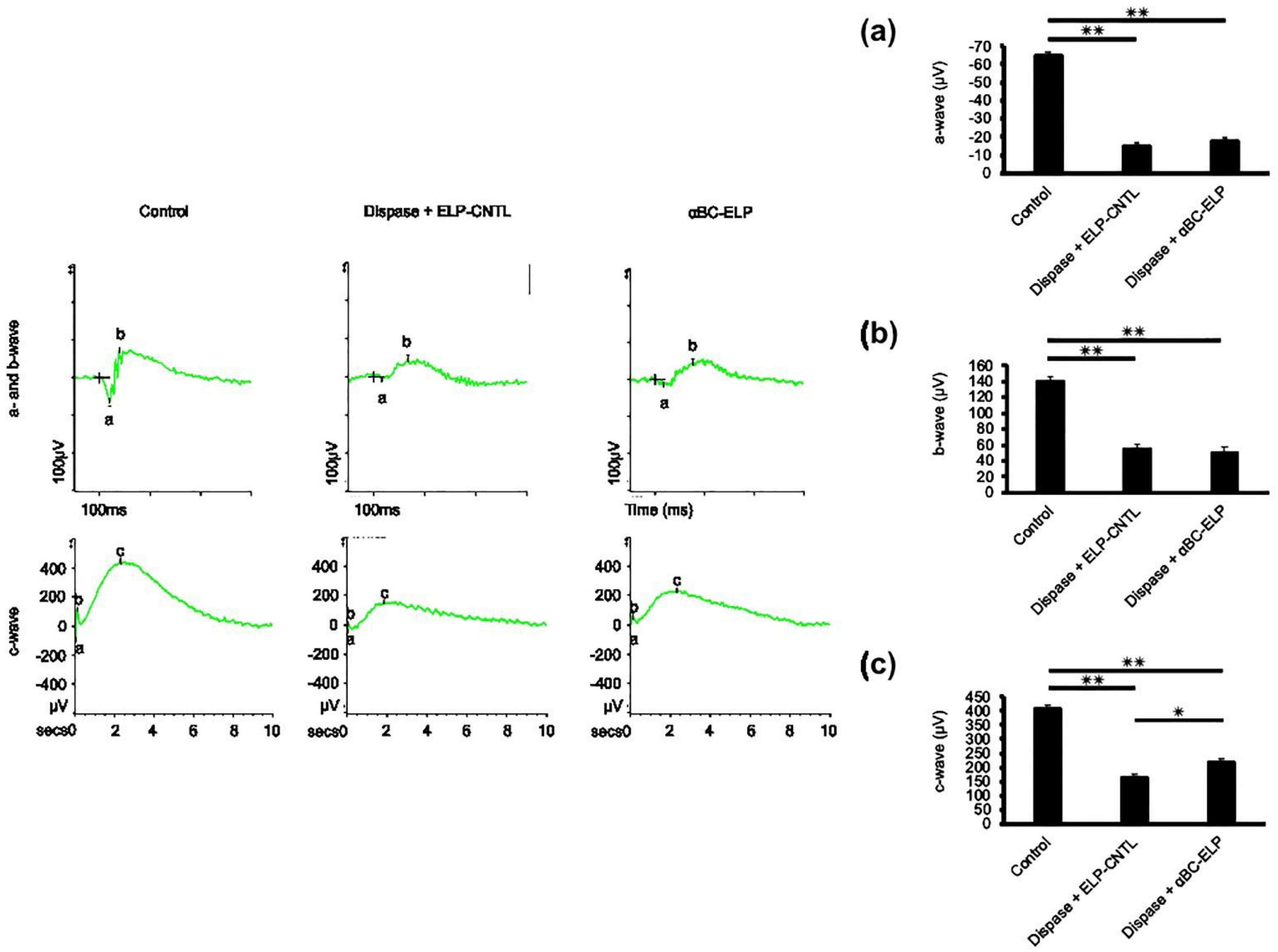
3.8. Evidence for αBC-ELP-Induced Improvement in RPE Cell Function by ERG
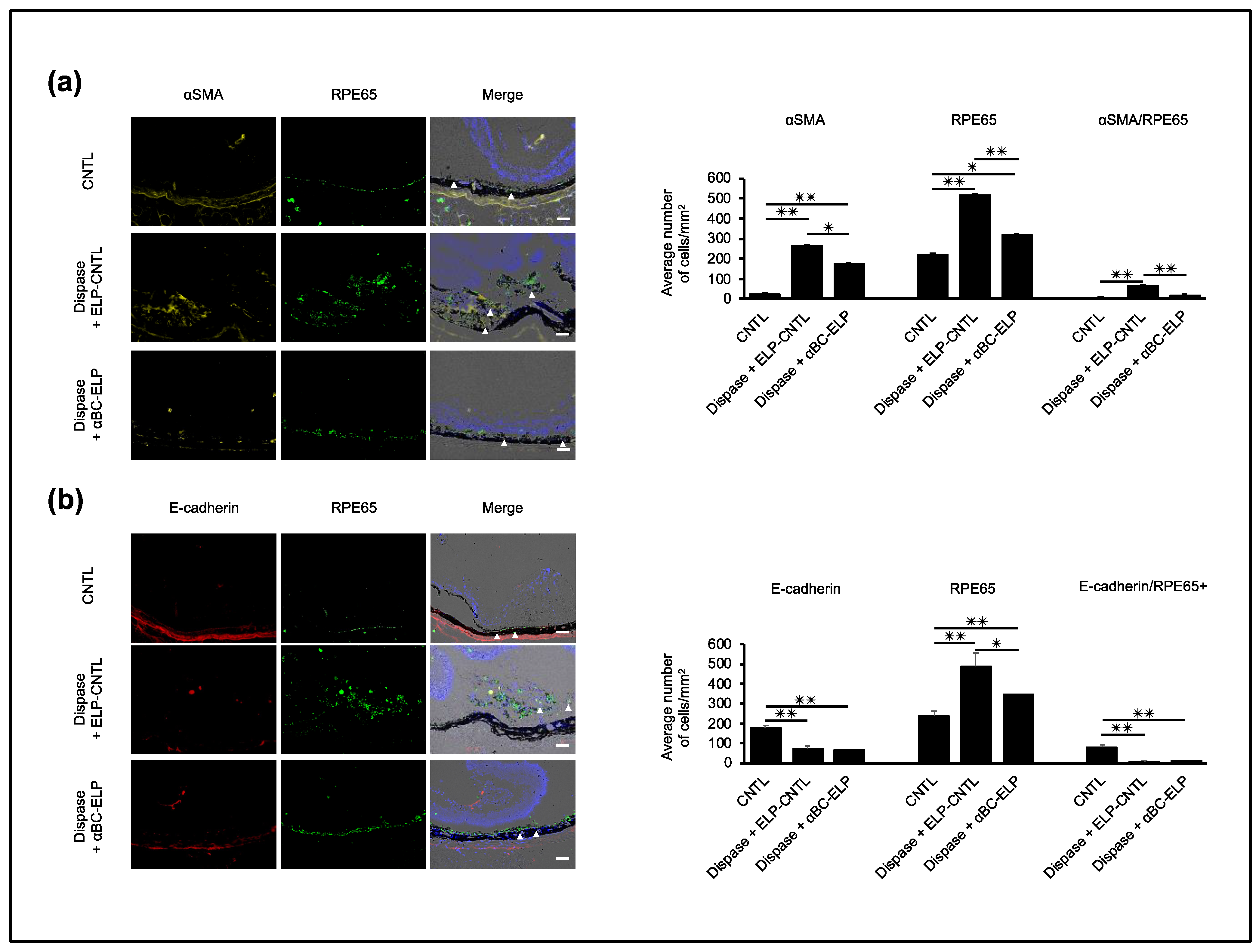
3.9. αBC-ELP Inhibits EMT in Dispase-Induced PVR in Mice
3.10. αBC-ELP Suppressed EMT/MET-Associated Proteins in Dispase-Induced PVR
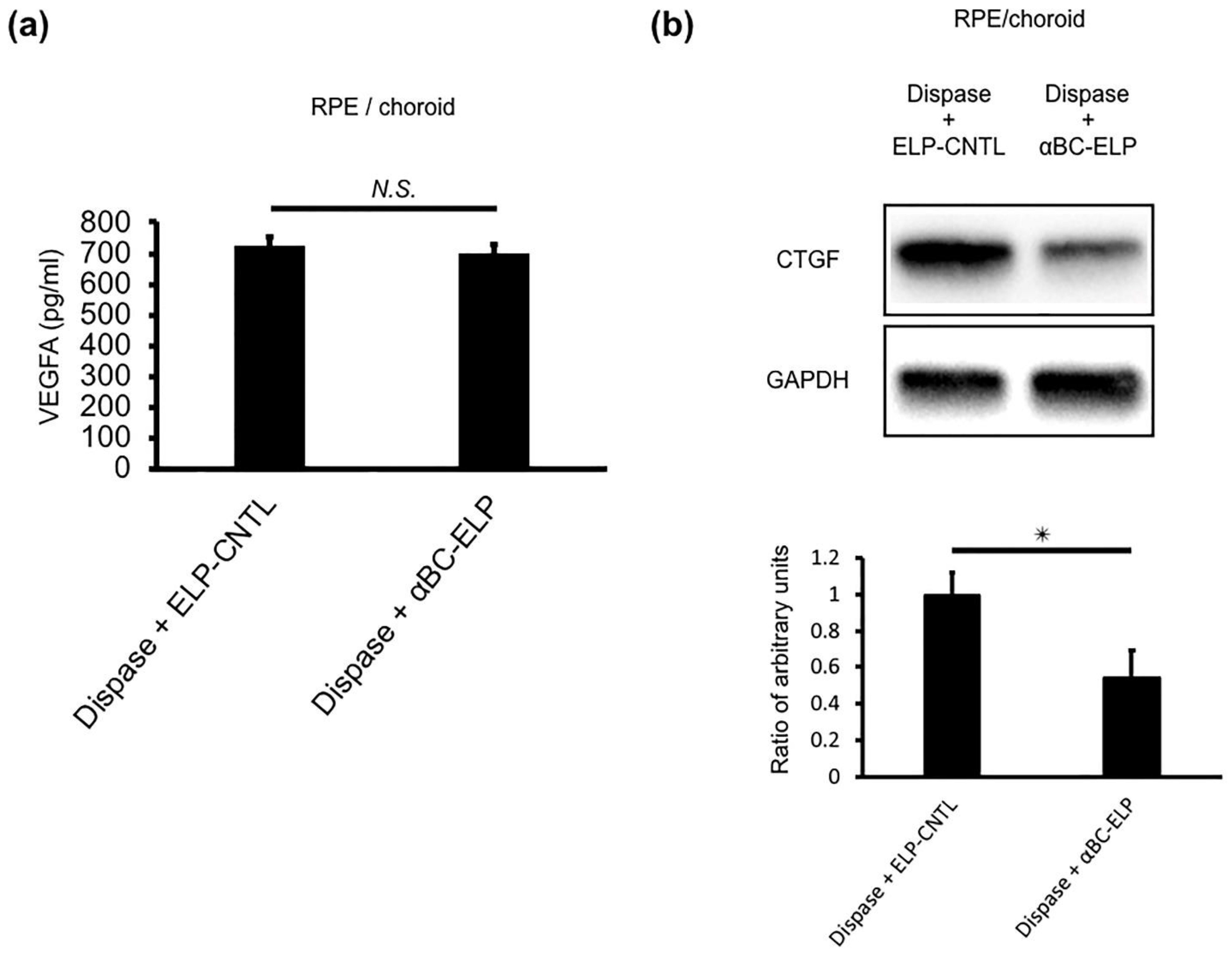
3.11. αBC-ELP Suppressed CTGF in Dispase-Induced PVR in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastor, J.C.; Rojas, J.; Pastor-Idoate, S.; di Lauro, S.; Gonzalez-Buendia, L.; Delgado-Tirado, S. Proliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequences. Prog. Retin. Eye Res. 2016, 51, 125–155. [Google Scholar] [CrossRef]
- Garweg, J.G.; Tappeiner, C.; Halberstadt, M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv. Ophthalmol. 2013, 58, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wladis, E.J.; Falk, N.S.; Iglesias, B.V.; Beer, P.M.; Gosselin, E.J. Analysis of the molecular biologic milieu of the vitreous in proliferative vitreoretinopathy. Retina 2013, 33, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shao, Y.; Li, X. The roles of signaling pathways in epithelial-to-mesenchymal transition of PVR. Mol. Vis. 2015, 21, 706–710. [Google Scholar] [PubMed]
- Ishikawa, K.; Sreekumar, P.G.; Spee, C.; Nazari, H.; Zhu, D.; Kannan, R.; Hinton, D.R. alphaB-Crystallin Regulates Subretinal Fibrosis by Modulation of Epithelial-Mesenchymal Transition. Am. J. Pathol. 2016, 186, 859–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahomi, R.B.; Pantcheva, M.B.; Nagaraj, R.H. alphaB-crystallin is essential for the TGF-beta2-mediated epithelial to mesenchymal transition of lens epithelial cells. Biochem. J. 2016, 473, 1455–1469. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; He, S.; Terasaki, H.; Nazari, H.; Zhang, H.; Spee, C.; Kannan, R.; Hinton, D.R. Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy. Sci. Rep. 2015, 5, 16386. [Google Scholar] [CrossRef] [Green Version]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Novel roles for alpha-crystallins in retinal function and disease. Prog. Retin. Eye Res. 2012, 31, 576–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaung, J.; Jin, M.; Barron, E.; Spee, C.; Wawrousek, E.F.; Kannan, R.; Hinton, D.R. alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol. Vis. 2007, 13, 566–577. [Google Scholar]
- Horwitz, J. Alpha-crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biochim. Biophys. Acta 2016, 1860 Pt B, 258–268. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Chothe, P.; Sharma, K.K.; Baid, R.; Kompella, U.; Spee, C.; Kannan, N.; Manh, C.; Ryan, S.J.; Ganapathy, V.; et al. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2787–2798. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Li, Z.; Wang, W.; Spee, C.; Hinton, D.R.; Kannan, R.; MacKay, J.A. Intra-vitreal alphaB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J. Control. Release 2018, 283, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Wang, M.; Spee, C.; Sadda, S.R.; Kannan, R. Transporter-Mediated Mitochondrial GSH Depletion Leading to Mitochondrial Dysfunction and Rescue with alphaB Crystallin Peptide in RPE Cells. Antioxidants 2020, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Kannan, R.; Kitamura, M.; Spee, C.; Barron, E.; Ryan, S.J.; Hinton, D.R. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE 2010, 5, e12578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreekumar, P.G.; Spee, C.; Ryan, S.J.; Cole, S.P.; Kannan, R.; Hinton, D.R. Mechanism of RPE cell death in alpha-crystallin deficient mice: A novel and critical role for MRP1-mediated GSH efflux. PLoS ONE 2012, 7, e33420. [Google Scholar] [CrossRef] [Green Version]
- Dou, G.; Sreekumar, P.G.; Spee, C.; He, S.; Ryan, S.J.; Kannan, R.; Hinton, D.R. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic. Biol. Med. 2012, 53, 1111–1122. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Kannan, R.; Spee, C.; Sreekumar, P.G.; Dou, G.; Hinton, D.R. Protection of retina by alphaB crystallin in sodium iodate induced retinal degeneration. PLoS ONE 2014, 9, e98275. [Google Scholar]
- Kase, S.; He, S.; Sonoda, S.; Kitamura, M.; Spee, C.; Wawrousek, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 2010, 115, 3398–3406. [Google Scholar] [CrossRef]
- Wang, W.; Sreekumar, P.G.; Valluripalli, V.; Shi, P.; Wang, J.; Lin, Y.A.; Cui, H.; Kannan, R.; Hinton, D.R.; MacKay, J.A. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J. Control. Release 2014, 191, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Connor, T.B., Jr.; Roberts, A.B.; Sporn, M.B.; Danielpour, D.; Dart, L.L.; Michels, R.G.; de Bustros, S.; Enger, C.; Kato, H.; Lansing, M.; et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J. Clin. Investig. 1989, 83, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.; Heimann, K.; Bartz-schmidt, K.U.; Fontana, A.; Schraermeyer, U.; Thumann, G.; Weller, M. Apoptosis in proliferative vitreoretinal disorders: Possible involvement of TGF-beta-induced RPE cell apoptosis. Exp. Eye Res. 1997, 65, 365–378. [Google Scholar] [CrossRef]
- Kon, C.H.; Occleston, N.L.; Aylward, G.W.; Khaw, P.T. Expression of vitreous cytokines in proliferative vitreoretinopathy: A prospective study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 705–712. [Google Scholar]
- Yu, J.; Peng, R.; Chen, H.; Cui, C.; Ba, J. Elucidation of the pathogenic mechanism of rhegmatogenous retinal detachment with proliferative vitreoretinopathy by proteomic analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8146–8153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.B.; Rud, J.G.; Lin, K.; Xu, L. Nuclear targeting of transforming growth factor-beta-activated Smad complexes. J. Biol. Chem. 2005, 280, 21329–21336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, W.; Lan, Y.; Chen, X.; Yang, W.; Yuan, Y.; Tan, J.; Zong, Y.; Jiang, Z. The inhibitory effect of small interference RNA protein kinase C-alpha on the experimental proliferative vitreoretinopathy induced by dispase in mice. Int. J. Nanomed. 2013, 8, 1563–1572. [Google Scholar]
- Soler, M.V.C.; Gallo, J.E.; Dodds, R.A.; Suburo, A.M. A mouse model of proliferative vitreoretinopathy induced by dispase. Exp. Eye Res. 2002, 75, 491–504. [Google Scholar] [CrossRef]
- Kralinger, M.T.; Kieselbach, G.F.; Voigt, M.; Hayden, B.; Hernandez, E.; Fernandez, V.; Parel, J.M. Experimental model for proliferative vitreoretinopathy by intravitreal dispase: Limited by zonulolysis and cataract. Ophthalmologica 2006, 220, 211–216. [Google Scholar] [CrossRef]
- Frenzel, E.M.; Neely, K.A.; Walsh, A.W.; Cameron, J.D.; Gregerson, D.S. A new model of proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2157–2164. [Google Scholar]
- Zhang, W.; Tan, J.; Liu, Y.; Li, W.; Gao, Q.; Lehmann, P.V. Assessment of the innate and adaptive immune system in proliferative vitreoretinopathy. Eye 2012, 26, 872–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Liu, Y.; Li, W.; Gao, Q. Ocular pathogenesis and immune reaction after intravitreal dispase injection in mice. Mol. Vis. 2012, 18, 887–900. [Google Scholar] [PubMed]
- Iribarne, M.; Ogawa, L.; Torbidoni, V.; Dodds, C.M.; Dodds, R.A.; Suburo, A.M. Blockade of endothelinergic receptors prevents development of proliferative vitreoretinopathy in mice. Am. J. Pathol. 2008, 172, 1030–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, S.; Sreekumar, P.G.; Kase, S.; Spee, C.; Ryan, S.J.; Kannan, R.; Hinton, D.R. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: Relevance to age-related macular degeneration. Aging 2009, 2, 28–42. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Reddy, S.T.; Hinton, D.R.; Kannan, R. Mechanisms of RPE senescence and potential role of alphaB crystallin peptide as a senolytic agent in experimental AMD. Exp. Eye Res. 2022, 215, 108918. [Google Scholar] [CrossRef]
- Hombrebueno, J.R.; Luo, C.; Guo, L.; Chen, M.; Xu, H. Intravitreal Injection of Normal Saline Induces Retinal Degeneration in the C57BL/6J Mouse. Transl. Vis. Sci. Technol. 2014, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, C.; Song, X.; Cui, X.; Liu, J.; Ford, N.C.; He, S.; Zhu, G.; Dong, X.; Hanani, M.; et al. BzATP Activates Satellite Glial Cells and Increases the Excitability of Dorsal Root Ganglia Neurons In Vivo. Cells 2022, 11, 2280. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.B.Y.; Chen, W.; Fan, J.; Li, S.; Qu, X.; Chen, Q.; Chen, R.; Zhu, D.; Zhang, J.; et al. Smad4 promotes diabetic nephropathy by modulating glycolysis and OXPHOS. EMBO Rep. 2020, 21, e48781. [Google Scholar] [CrossRef]
- Kobelt, D.; Walther, W.; Stein, U.S. Real-Time Cell Migration Monitoring to Analyze Drug Synergism in the Scratch Assay Using the IncuCyte System. Methods Mol. Biol. 2021, 2294, 133–142. [Google Scholar]
- Rodriguez-Gonzalez, J.C.; Hernandez-Balmaseda, I.; Declerck, K.; Perez-Novo, C.; Logie, E.; Theys, C.; Jakubek, P.; Quinones-Maza, O.L.; Dantas-Cassali, G.; Reis, D.C.D.; et al. Antiproliferative, Antiangiogenic, and Antimetastatic Therapy Response by Mangiferin in a Syngeneic Immunocompetent Colorectal Cancer Mouse Model Involves Changes in Mitochondrial Energy Metabolism. Front. Pharmacol. 2021, 12, 670167. [Google Scholar] [CrossRef]
- Agrawal, R.N.; He, S.; Spee, C.; Cui, J.Z.; Ryan, S.J.; Hinton, D.R. In vivo models of proliferative vitreoretinopathy. Nat. Protoc. 2007, 2, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Pennock, S.; Haddock, L.J.; Mukai, S.; Kazlauskas, A. Vascular endothelial growth factor acts primarily via platelet-derived growth factor receptor alpha to promote proliferative vitreoretinopathy. Am. J. Pathol. 2014, 184, 3052–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Chen, Y.; Khankan, R.; Barron, E.; Burton, R.; Zhu, D.; Ryan, S.J.; Oliver, N.; Hinton, D.R. Connective tissue growth factor as a mediator of intraocular fibrosis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, H.; Li, M.; Wang, F. Mechanisms of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Discov. Med. 2015, 20, 207–217. [Google Scholar] [PubMed]
- Tamiya, S.; Kaplan, H.J. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp. Eye Res. 2016, 142, 26–31. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration. Antioxidants 2021, 10, 661. [Google Scholar]
- Kurnellas, M.P.; Brownell, S.E.; Su, L.; Malkovskiy, A.V.; Rajadas, J.; Dolganov, G.; Chopra, S.; Schoolnik, G.K.; Sobel, R.A.; Webster, J.; et al. Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J. Biol. Chem. 2012, 287, 36423–36434. [Google Scholar] [CrossRef]
- Pasupuleti, N.; Matsuyama, S.; Voss, O.; Doseff, A.I.; Song, K.; Danielpour, D.; Nagaraj, R.H. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010, 1, e31. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Reiser, G. Phosphorylation of Ser45 and Ser59 of alphaB-crystallin and p38/extracellular regulated kinase activity determine alphaB-crystallin-mediated protection of rat brain astrocytes from C2-ceramide- and staurosporine-induced cell death. J. Neurochem. 2011, 118, 354–364. [Google Scholar] [CrossRef]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-beta and reactive oxygen species: A perverse cycle for fibrosis. Redox. Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Abe, Y.; Sakairi, T.; Beeson, C.; Kopp, J.B. TGF-beta1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am. J. Physiol. Ren. Physiol. 2013, 305, F1477–F1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Gomez-Duran, A.; Saretzki, G.; Jin, S.; Tilgner, K.; Melguizo-Sanchis, D.; Anyfantis, G.; Al-Aama, J.; Vallier, L.; Chinnery, P.; et al. The mitochondrial protein CHCHD2 primes the differentiation potential of human induced pluripotent stem cells to neuroectodermal lineages. J. Cell Biol. 2016, 215, 187–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [Green Version]
- Kanow, M.A.; Giarmarco, M.M.; Jankowski, C.S.; Tsantilas, K.; Engel, A.L.; Du, J.; Linton, J.D.; Farnsworth, C.C.; Sloat, S.R.; Rountree, A.; et al. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. eLife 2017, 6, e28899. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.T.; Kannan, R.; Felszeghy, S.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. The Regulation of NFE2L2 (NRF2) Signalling and Epithelial-to-Mesenchymal Transition in Age-Related Macular Degeneration Pathology. Int. J. Mol. Sci. 2019, 20, 5800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef]
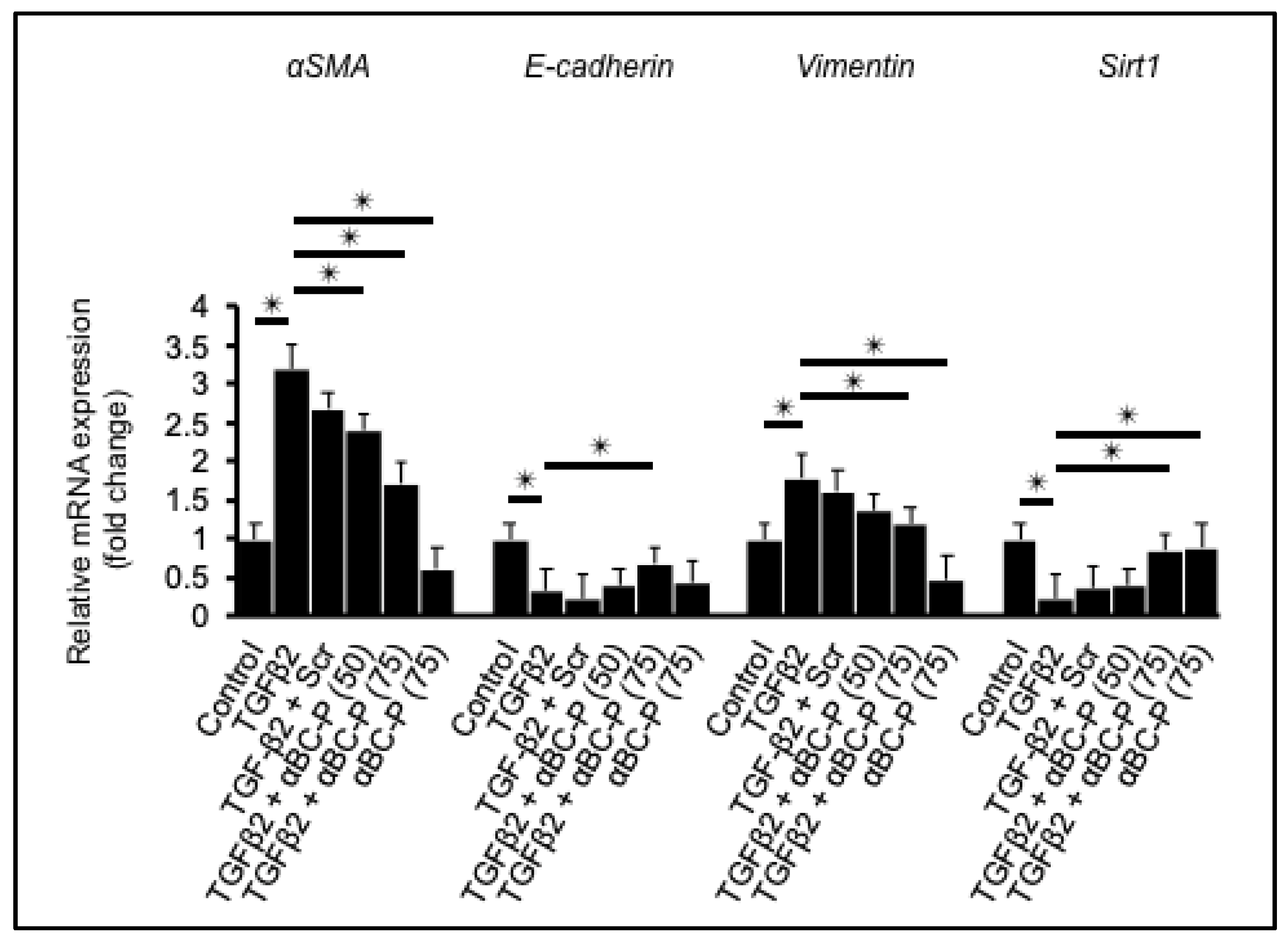
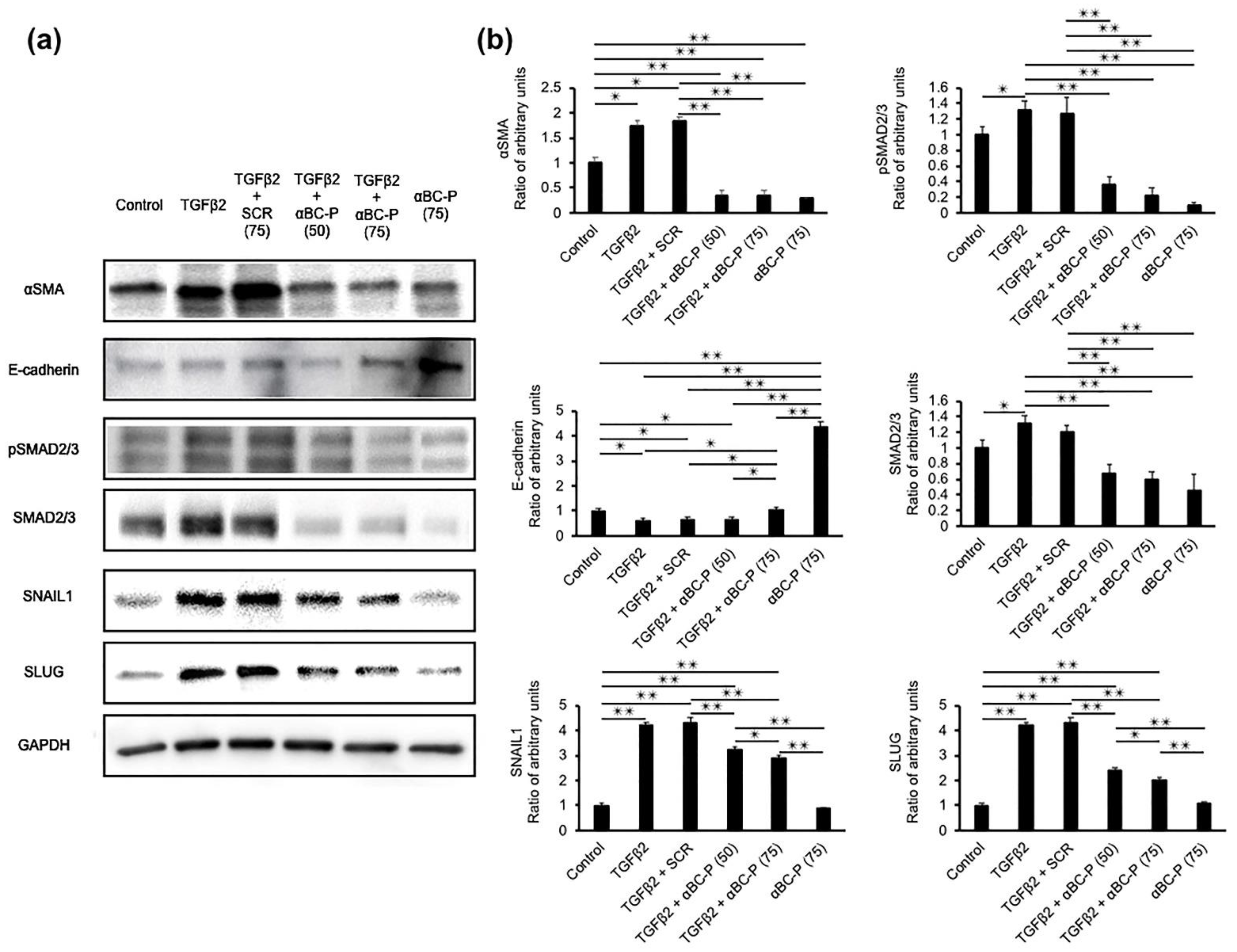
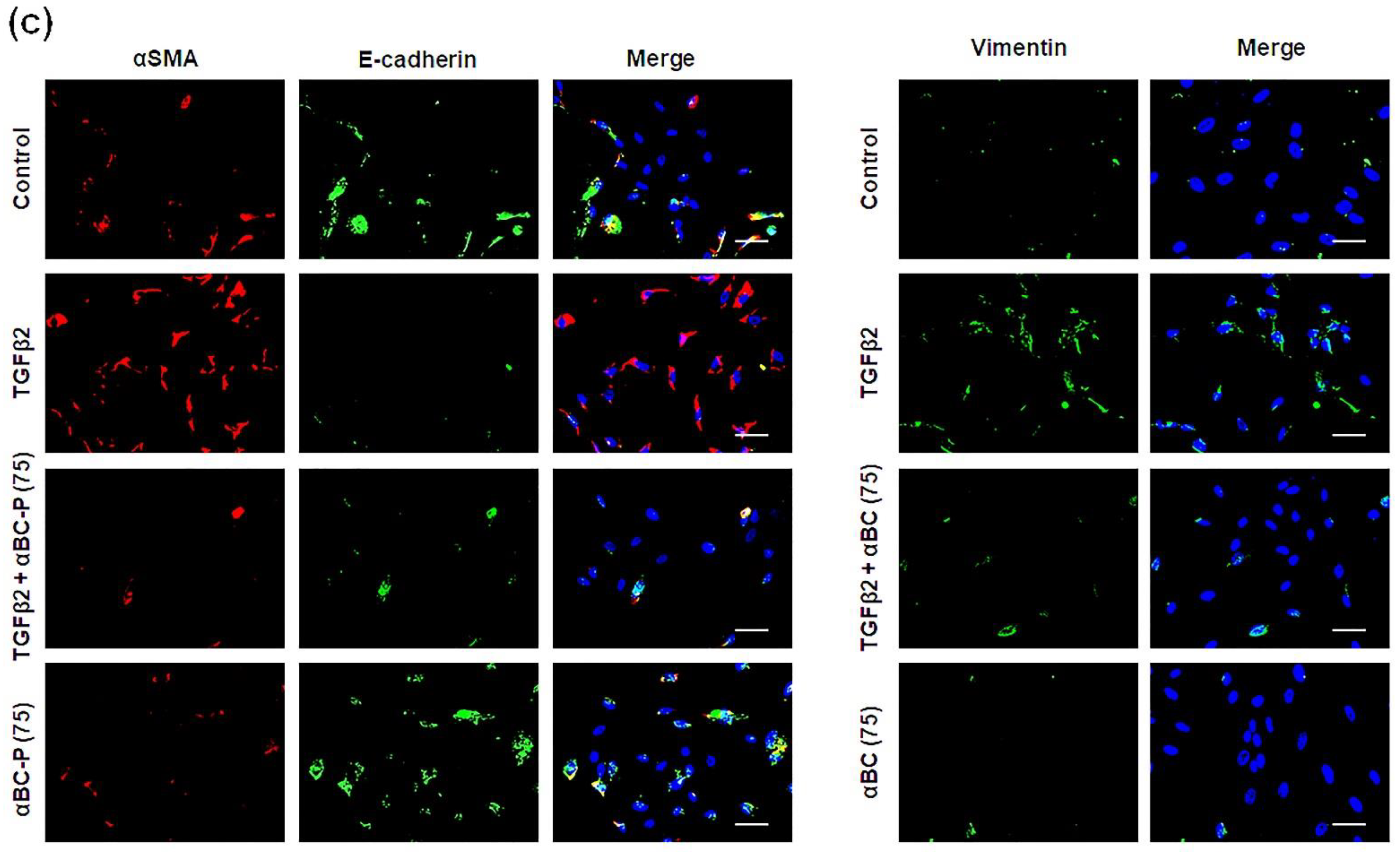

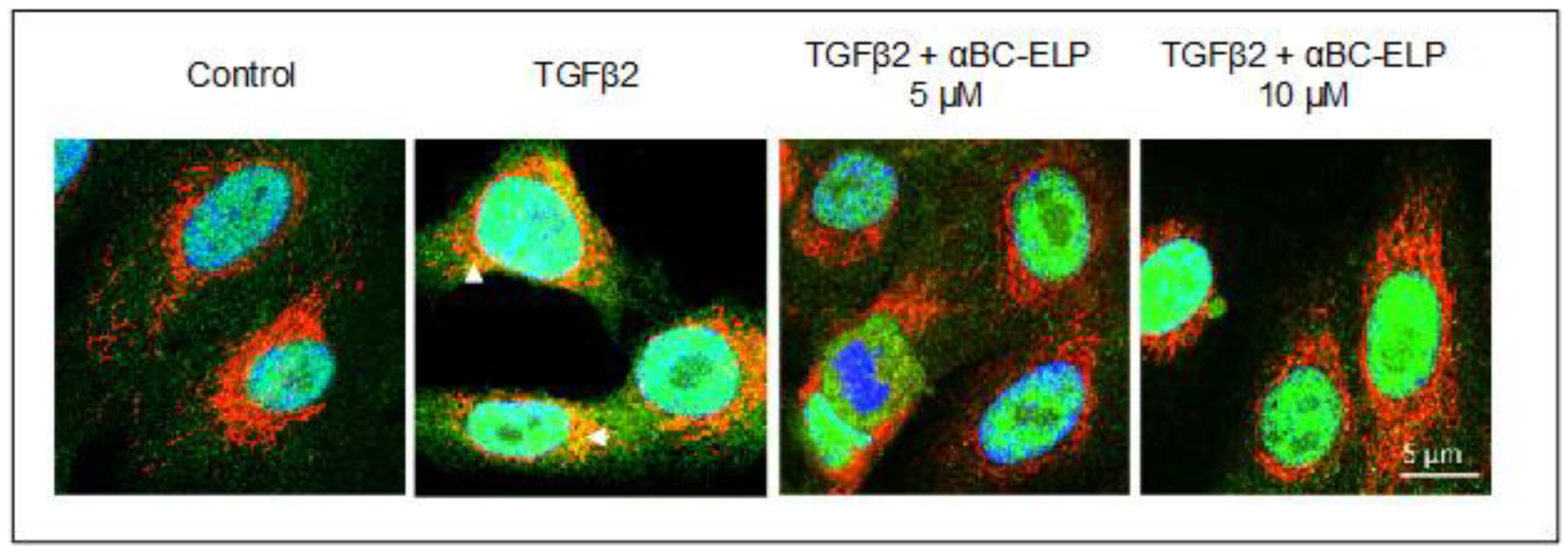


| Target Molecule | Accession Number | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| αSMA | NM_001613 | 5′-TCTGTAAGGCCGGCTTTGC-3′ | 5′-TGTCCCATTCCCACCATCA-3′ |
| Vimentin | NM_003380 | 5′-TGAGTACCGGAGACAGGTGCAG-3′ | 5′-TAGCAGCTTCAACGGCAAAGTTC-3′ |
| E-cadherin | NM_004360 | 5′-ATTTTTCCCTCACACCCGAT-3′ | 5′-TCCCAGGCGTAGACCAAGA-3′ |
| Sirt1 | NM_012238 | 5′-TCCTGGACAATTCCAGCCATCTCT-3′ | 5′-TTCCAGCGTTATGTTCTGGGT-3′ |
| GAPDH | NM_002046 | 5′-ACAGTCGCCGCATCTTCTT-3′ | 5′-CTTGATTTTGGAGGGATCTCGC-3′ |
| Target Molecule | Antibody Type | Source | Dilution |
|---|---|---|---|
| αSMA (ab7817) | Mouse monoclonal | Abcam | 1:1000 |
| E-cadherin (24E10) | Rabbit monoclonal | Cell Signaling | 1:1000 |
| pSMAD2/3 ((S465/467)/(S423/425) | Rabbit monoclonal | Cell Signaling | 1:1000 |
| SMAD2/3 (D7G7) | Rabbit monoclonal | Cell Signaling | 1:1000 |
| Fibronectin (EP5) | Mouse monoclonal | Santa Cruz Biotechnology | 1:1000 |
| Vimentin (3CB2) | Mouse monoclonal | Santa Cruz Biotechnology | 1:1000 |
| SNAIL (C15D3) | Rabbit monoclonal | Cell Signaling | 1:1000 |
| SLUG (G-18) | Goat polyclonal | Santa Cruz Biotechnology | 1:1000 |
| CTGF (ab6992) | Rabbit monoclonal | Abcam | 1:1000 |
| GAPDH (MAB374) | Mouse monoclonal | EMD Millipore | 1:1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, I.; Sreekumar, P.G.; Spee, C.; MacKay, A.J.; Ip, M.; Kannan, R. Mechanisms of Epithelial-Mesenchymal Transition and Prevention of Dispase-Induced PVR by Delivery of an Antioxidant αB Crystallin Peptide. Antioxidants 2022, 11, 2080. https://doi.org/10.3390/antiox11102080
Wada I, Sreekumar PG, Spee C, MacKay AJ, Ip M, Kannan R. Mechanisms of Epithelial-Mesenchymal Transition and Prevention of Dispase-Induced PVR by Delivery of an Antioxidant αB Crystallin Peptide. Antioxidants. 2022; 11(10):2080. https://doi.org/10.3390/antiox11102080
Chicago/Turabian StyleWada, Iori, Parameswaran G Sreekumar, Christine Spee, Andrew J MacKay, Michael Ip, and Ram Kannan. 2022. "Mechanisms of Epithelial-Mesenchymal Transition and Prevention of Dispase-Induced PVR by Delivery of an Antioxidant αB Crystallin Peptide" Antioxidants 11, no. 10: 2080. https://doi.org/10.3390/antiox11102080
APA StyleWada, I., Sreekumar, P. G., Spee, C., MacKay, A. J., Ip, M., & Kannan, R. (2022). Mechanisms of Epithelial-Mesenchymal Transition and Prevention of Dispase-Induced PVR by Delivery of an Antioxidant αB Crystallin Peptide. Antioxidants, 11(10), 2080. https://doi.org/10.3390/antiox11102080








