Apple Polyphenol Diet Extends Lifespan, Slows down Mitotic Rate and Reduces Morphometric Parameters in Drosophila Melanogaster: A Comparison between Three Different Apple Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Apple Polyphenols Extraction
2.3. Total Anthocyanin Assay Method
2.4. HPLC Analysis
2.5. Fruit Fly Strains and Treatments
2.6. Developmental Assay
2.7. Lifespan Assay
2.8. Stress Assay
2.9. Size and Weight of Emerged Offspring
2.10. Mitotic Index
2.11. Climbing Assay
2.12. Statistical Analysis
3. Results
3.1. Apple Polyphenol Extracts Characterization
3.2. Apple Polyphenols Do Not Impact Drosophila Development
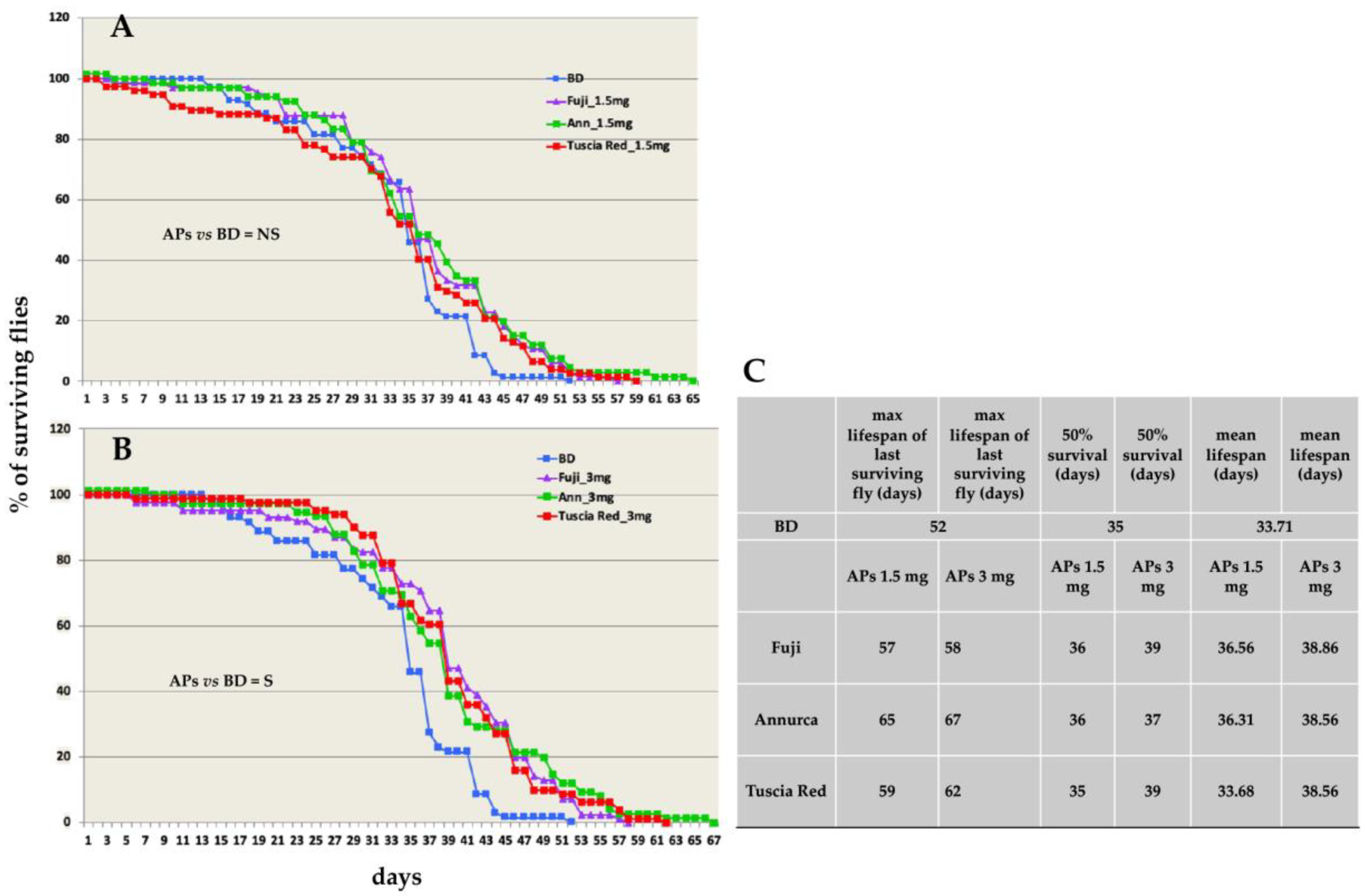
3.3. Apple Polyphenols Extend Adult Fly Lifespan
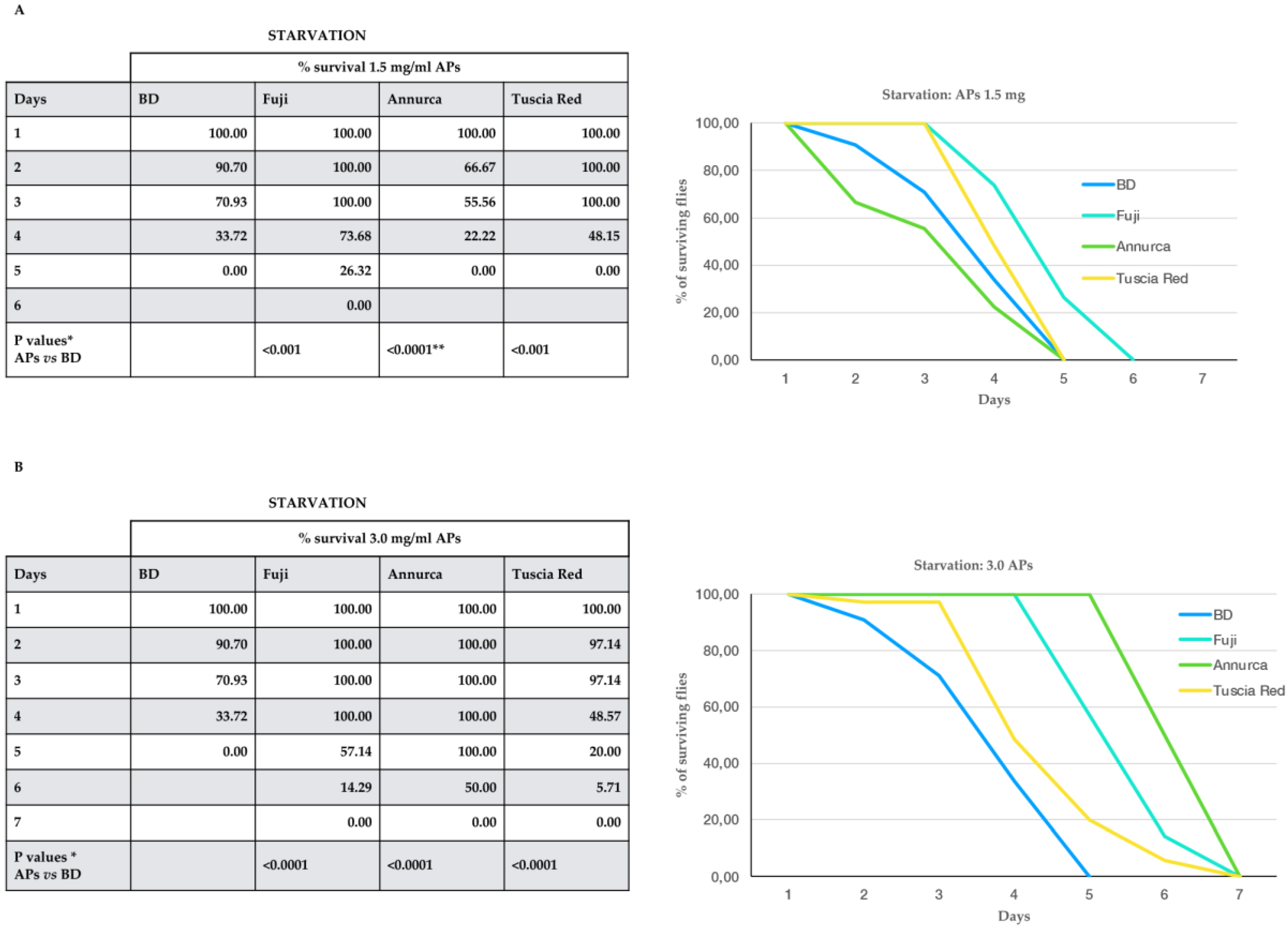
3.4. Apple Polyphenols Ameliorate Fly Resistance to Caloric Restriction
3.5. Apple Polyphenol Effects on Offspring Size and Weight
3.6. Apple Polyphenols Affect Drosophila Mitotic Cell Cycle
3.7. Apple Polyphenol Impact on Drosophila Climbing Ability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carocho, M.; Ferreira, I.C.F.R.; Morales, P.; Soković, M. Antioxidants and Prooxidants: Effects on Health and Aging. Oxid. Med. Cell. Longev. 2018, 2018, 1472708. [Google Scholar] [CrossRef]
- de Groot, H. Reactive oxygen species in tissue injury. Hepatogastroenterology 1994, 4, 328–332. [Google Scholar]
- Grace, P.A. Ischaemia-reperfusion injury. Br. J. Surg. 1994, 81, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- McCord, J.M. Free radicals and inflammation. Science 1974, 185, 529–531. [Google Scholar] [CrossRef]
- Halliwell, B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann. Rheum. Dis. 1995, 54, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Iakovou, E.; Kourti, M. A comprehensive overview of the complex role of oxidative stress in aging, the contributing environmental stressors and emerging antioxidant therapeutic interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef]
- Van Duyn, M.A.; Pivonka, E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: Selected literature. J. Am. Diet Assoc. 2000, 100, 1511–1521. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, Separation, and Detection Methods for Phenolic Acids and Flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I.B.; Dorozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and Free Radical Scavenging Mechanisms of Inhibitory Action of Rutin and Quercetin in Lipid Peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Ohnishi, M.; Morishita, H.; Iwahashi, H.; Toda, S.; Shirataki, Y.; Kimura, M.; Kido, R. Inhibitory effects of chlorogenic acids on linoleic-acid peroxidation and hemolysis. Phitochemistry 1994, 36, 579–583. [Google Scholar] [CrossRef]
- Castelluccio, C.; Paganga, G.; Melikian, N.; Bolwell, G.P.; Pridham, J.; Sampson, J.; Rice-Evans, C. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher plants. FEBS Lett. 1995, 368, 188–192. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kamil Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB transcription factors that colour our fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, S.; Zhang, Z.; Fang, H.; Xu, H.; Wang, Y.; Chen, X. Malus sieversii: The origin, flavonoid synthesis mechanism, and breeding of red-skinned and red-fleshed apples. Hortic. Res. 2018, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, M.; Latini, G.; Cristofori, V.; Ceccantoni, B.; Luziatelli, F.; Zecchini, M.; Muleo, R.; Ruzzi, M. Polyphenol traits, antimicrobial property and consumer preference of ‘Italian Red Passion’ apple genotypes and cultivar ‘Annurca’. Acta Hortic. 2015, 1106, 185–190. [Google Scholar] [CrossRef]
- Silvestri, C.; Cirilli, M.; Zecchini, M.; Muleo, R.; Ruggieri, A. Consumer acceptance of the new red-fleshed apple variety. J. Food Prod. Mark. 2016, 24, 1–21. [Google Scholar] [CrossRef]
- Harris, S.A.; Robinson, J.P.; Juniper, B.E. Genetic clues to the origin of the apple. Trends Genet. 2002, 18, 426–430. [Google Scholar] [CrossRef]
- Jeibmann, A.; Paulus, W. Drosophila melanogaster as a model organism of brain diseases. Int. J. Mol. Sci. 2009, 10, 407–440. [Google Scholar] [CrossRef]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Models Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Mirzoyan, Z.; Sollazzo, M.; Allocca, M.; Valenza, A.M.; Grifoni, D.; Bellosta, P. Drosophila melanogaster: A Model Organism to Study Cancer. Front. Genet. 2019, 10, 51. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Panchal, K.; Tiwari, A.K. Drosophila melanogaster “a potential model organism” for identification of pharmacological properties of plants/plant-derived components. Biomed. Pharmacother. 2017, 89, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xu, W.; Fan, Y.; Wang, H.-X. Drosophila as an emerging model organism for studies of food-derived antioxidants. Food Res. Int. 2021, 143, 110307. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.L.; Yang, C.P.H.; Lindquist, P.; Anderson, O.R.; Rabino, I. Photocontrol of anthocyanin synthesis. III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef]
- Lopez, T.E.; Pham, H.M.; Barbour, J.; Tran, P.; Van Nguyen, B.; Hogan, S.P.; Homo, R.L.; Coskun, V.; Schriner, S.E.; Jafari, M. The impact of green tea polyphenols on development and reproduction in Drosophila melanogaster. J. Funct. Foods 2016, 20, 556–566. [Google Scholar] [CrossRef]
- Fabbretti, F.; Iannetti, I.; Guglielmi, L.; Perconti, S.; Evangelistella, C.; Proietti De Santis, L.; Bongiorni, S.; Prantera, G. Confocal analysis of nuclear lamina behavior during male meiosis and spermatogenesis in Drosophila melanogaster. PLoS ONE 2016, 11, e0151231. [Google Scholar] [CrossRef]
- Volpi, S.; Bongiorni, S.; Fabbretti, F.; Wakimoto, B.T.; Prantera, G. Drosophila rae1 is required for male meiosis and spermatogenesis. J. Cell Sci. 2013, 126, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic Profiles in Eight Apple Cultivars Using High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Vanzani, P.; Rossetto, M.; Rigo, A.; Vrhovsek, U.; Mattivi, F.; D’amato, E.; Scarpa, A.M. Major Phytochemicals in Apple Cultivars: Contribution to Peroxyl Radical Trapping Efficiency. J. Agric. Food Chem. 2005, 53, 3377–3382. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Peng, C.; Zuo, Y.; Kwan, K.M.; Liang, Y.; Ma, K.Y.; Chan, H.Y.E.; Huang, Y.; Yu, H.; Chen, Z.-Y. Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp. Gerontol. 2012, 47, 170–178. [Google Scholar] [CrossRef]
- Peng, C.; Chan, H.Y.E.; Huang, Y.; Yu, H.; Chen, Z.-Y. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. J. Agric. Food Chem. 2011, 59, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Rion, S.; Kawecki, T.J. Evolutionary biology of starvation resistance: What we have learned from Drosophila. J. Evol. Biol. 2007, 20, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W.; Souder, B.M.; Ho, D.H. Metabolic rate and hypoxia tolerance are affected by group interactions and sex in the fruit fly (Drosophila melanogaster): New data and a literature survey. Biol. Open 2017, 6, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Morata, G.; Ripoll, P. Minutes: Mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 1975, 42, 211–221. [Google Scholar] [CrossRef]
- Kongsuwan, K.; Yu, Q.; Vincent, A.; Frisardi, M.C.; Rosbash, M.; Lengyel, J.A.; Merriam, J. A Drosophila Minute gene encodes a ribosomal protein. Nature 1985, 317, 555–558. [Google Scholar] [CrossRef]
- Sæbøe-Larssen, S.; Lyamouri, M.; Merriam, J.; Oksvold, M.P.; Lambertsson, A. Ribosomal Protein Insufficiency and the Minute Syndrome in Drosophila: A Dose-Response Relationship. Genetics 1998, 148, 1215–1224. [Google Scholar] [CrossRef]
- Przystupski, D.; Niemczura, M.J.; Górska, A.; Supplitt, S.; Kotowski, K.; Wawryka, P.; Rozborska, P.; Woźniak, K.; Michel, O.; Kiełbik, A.; et al. In Search of Panacea—Review of Recent Studies Concerning Nature-Derived Anticancer Agents. Nutrients 2019, 11, 1426. [Google Scholar] [CrossRef] [PubMed]
- Visanji, J.M.; Thompson, D.G.; Padfield, P.J. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006, 237, 130–136. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Halder, B.; Das Gupta, S.; Gomes, A. Black tea polyphenols induce human leukemic cell cycle arrest by inhibiting Akt signaling: Possible involvement of Hsp90, Wnt/beta-catenin signaling and FOXO1. FEBS J. 2012, 279, 2876–2891. [Google Scholar] [CrossRef]
- Delmas, D.; Solary, E.; Latruffe, N. Resveratrol, a phytochemical inducer of multiple cell death pathways: Apoptosis, autophagy and mitotic catastrophe. Curr. Med. Chem. 2011, 18, 1100–1121. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Guan, X.; Grün, C.; Zhou, Z.; Schepers, U.; Nick, P. Gallic acid induces mitotic catastrophe and inhibits centrosomal clustering in HeLa cells. Toxicol. In Vitro 2015, 30, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Mosieniak, G.; Sliwinska, M.A.; Przybylska, D.; Grabowska, W.; Sunderland, P.; Bielak-Zmijewska, A.; Sikora, E. Curcumin-treated cancer cells show mitotic disturbances leading to growth arrest and induction of senescence phenotype. Int. J. Biochem. Cell Biol. 2016, 74, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Chang, J.C.; Lin, W.Y.; Li, C.C.; Hsieh, M.; Chen, H.W.; Wang, T.S.; Wu, W.T.; Liu, C.S.; Liu, K.L. Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Free Radic. Biol. Med. 2018, 115, 309–317. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Z.; Liu, D.; Li, X.; Rehman, R.-U.; Wang, H.; Wu, Z. Apple phlorizin attenuates oxidative stress in Drosophila melanogaster. J. Food Biochem. 2019, 43, e12744. [Google Scholar]
- Yang, J.; Dungrawala, H.; Hua, H.; Manukyan, A.; Abraham, L.; Lane, W.; Mead, H.; Wright, J.; Schneider, B.L. Cell size and growth rate are major determinants of replicative lifespan. Cell Cycle 2011, 10, 144–155. [Google Scholar] [CrossRef]
- Jiménez, J.; Bru, S.; Ribeiro, M.; Clotet, J. Live fast, die soon: Cell cycle progression and lifespan in yeast cells. Microb. Cell 2015, 2, 62–67. [Google Scholar] [CrossRef]
| Compound | Fuji | Tuscia Red | Annurca |
|---|---|---|---|
| Total Polyphenol (mg GAE/100 g fw) | 101.7 ± 6.8 a | 316.8 ± 28.4 b | 183.6 ± 39.7 c |
| Anthocyanin content (mg Cy3Glu/100 g fw) | N.D. | 4.234 ± 0.643 d | 0.256 ± 0.078 e |
| Compounds (mg/Kg fw) | Apple Variety | |
|---|---|---|
| Annurca | Tuscia Red | |
| Cholorogenic acid | 1439.2 ± 225.7 | 2427.5 ± 574.2 |
| Coumaryl-quinic acid | 203.4 ± 69.6 | 491.2 ± 160.9 * |
| Other hydroxyl-cinnamics | 39.4 ± 15.4 | 68.4 ± 28.8 |
| Total hydroxyl-cinnamics | 1682.1± 310.7 | 2987.1 ± 763.9 |
| Catechin | 3.7 ± 1.2 | 17.2 ± 7.3 * |
| Epicatechin | 107.5 ± 24.6 | 317.8 ± 110.2 * |
| Procyanidin B1 | 71.4 ± 21.4 | 194.7± 56.8 * |
| Procyanidin B2 | 146.5 ± 30.7 | 498.8 ± 169.0 * |
| Other procyanidins | 81.4 ± 2.2 | 465.8 ± 33.7 * |
| Toal flavanols and procyanidins | 410.5 ± 80.2 | 1494.3 ± 377.0 * |
| Cyanidin 3′-5′ diglucoside | 0.0 | 12.6 ± 6.4 ** |
| Cyanidin 3′-galactoside | 0.0 | 64.1 ± 27.6 ** |
| Other cyanidins | 0.0 | 6.6 ± 0.3 ** |
| Total anthocyanin | 0.0 | 83.3 ± 34.2 ** |
| Quercitin 3′ glucoside | 2.8 ± 2.3 | 11.5 ± 6.6 |
| Quercitin 3′ ramnoside | 5.5 ± 4.0 | 11.6 ± 6.9 |
| Total flavonols | 8.4 ± 6.4 | 23.1 ± 13. 0 |
| 3′ hydroxyl-phloretin-glucoside | 52.5 ± 10.2 | 42.2 ± 11.8 |
| 3′ phloretin-xyloglucoside | 119.5 ± 42.4 * | 23.5 ± 12.8 |
| Phlorizin | 31.4 ± 16.9 | 62.1 ± 27.0 |
| Total di-hydro chalcones | 203.4 ± 69.5 | 127.8 ± 41.7 |
| Total polyphenols | 2326.2 ± 466.7 | 4617.9 ± 1166.3 * |
| A | ||||
|---|---|---|---|---|
| BD | Fuji | Tuscia Red | Annurca | |
| Larval length | 3.9 | 3.7 | 3.8 | 2.9 |
| p values * APs vs. BD | <<0.001 | <0.001 | <<0.001 | |
| B | ||||
| BD | Fuji | Tuscia Red | Annurca | |
| Larval length | 3.9 | 3.0 | 2.7 | 2.6 |
| p values * APs vs BD | <<0.001 | <<0.001 | <<0.001 |
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Weight (g) * | ||||||||
| BD | Fuji | Tuscia Red | Annurca | |||||
| females | males | females | males | females | males | females | males | |
| Average | 0.0085 | 0.0055 | 0.0070 s | 0.0048 ns | 0.0079 ns | 0.0055 ns | 0.0062 s | 0.0052 ns |
| SD | 0.0010 | 0.0014 | 0.0014 | 0.0008 | 0.0018 | 0.0009 | 0.0009 | 0.0015 |
| B | ||||||||
| Weight (g) * | ||||||||
| BD | Fuji | Tuscia Red | Annurca | |||||
| females | males | females | males | females | males | females | males | |
| Average | 0.0085 | 0.0055 | 0.0069 s | 0.0053 ns | 0.0071 s | 0.0052 ns | 0.0069 s | 0.0049 ns |
| SD | 0.0010 | 0.0014 | 0.0014 | 0.0016 | 0.0005 | 0.0014 | 0.0010 | 0.0004 |
| A | ||||
|---|---|---|---|---|
| APs 1.5 mg | Mitotic Divisions | Optical Fields | Mitotic Index | p APs vs. BD |
| BD | 216 | 200 | 1.08 | |
| Fuji | 51 | 220 | 0.23 | <<0.001 |
| Annurca | 79 | 308 | 0.26 | <<0.001 |
| Tuscia Red | 88 | 306 | 0.29 | <<0.001 |
| B | ||||
| APs 3 mg | Mitotic Divisions | Optical Fields | Mitotic Index | pAPs vs. BD |
| BD | 216 | 200 | 1.08 | |
| Fuji | 56 | 290 | 0.19 | <<0.001 |
| Annurca | 31 | 200 | 0.15 | <<0.001 |
| Tuscia Red | 37 | 200 | 0.18 | <<0.001 |
| A | |||
|---|---|---|---|
| APs 1.5 mg | T10% | T20% | T30% |
| BD | 85 | 90 | 100 |
| Fuji | 80 | 95 | 100 |
| Annurca | 40 (*) | 95 | 100 |
| Tuscia Red | 90 | 90 | 100 |
| B | |||
| APs 3.0 mg | T10% | T20% | T30% |
| BD | 85 | 90 | 100 |
| Fuji | 35 (**) | 90 | 100 |
| Annurca | 5 (***) | 50 (*) | 65 (*) |
| Tuscia Red | 70 | 100 | 100 |
| Annurca | Fuji | Tuscia Red | ||||
|---|---|---|---|---|---|---|
| 1.5 | 3.0 | 1.5 | 3.0 | 1.5 | 3.0 | |
| Development timing | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean lifespan | 0 | + | 0 | + | 0 | + |
| Resistance to starvation | – | + | + | + | + | + |
| Larval size | – | – | – | – | – | – |
| Weight (females) | – | – | – | – | 0 | – |
| Mitotic index | – – | – – | – – | – – | – – | – – |
| Climbing ability | – | – | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongiorni, S.; Arisi, I.; Ceccantoni, B.; Rossi, C.; Cresta, C.; Castellani, S.; Forgione, I.; Rinalducci, S.; Muleo, R.; Prantera, G. Apple Polyphenol Diet Extends Lifespan, Slows down Mitotic Rate and Reduces Morphometric Parameters in Drosophila Melanogaster: A Comparison between Three Different Apple Cultivars. Antioxidants 2022, 11, 2086. https://doi.org/10.3390/antiox11112086
Bongiorni S, Arisi I, Ceccantoni B, Rossi C, Cresta C, Castellani S, Forgione I, Rinalducci S, Muleo R, Prantera G. Apple Polyphenol Diet Extends Lifespan, Slows down Mitotic Rate and Reduces Morphometric Parameters in Drosophila Melanogaster: A Comparison between Three Different Apple Cultivars. Antioxidants. 2022; 11(11):2086. https://doi.org/10.3390/antiox11112086
Chicago/Turabian StyleBongiorni, Silvia, Ivan Arisi, Brunella Ceccantoni, Cristina Rossi, Camilla Cresta, Simona Castellani, Ivano Forgione, Sara Rinalducci, Rosario Muleo, and Giorgio Prantera. 2022. "Apple Polyphenol Diet Extends Lifespan, Slows down Mitotic Rate and Reduces Morphometric Parameters in Drosophila Melanogaster: A Comparison between Three Different Apple Cultivars" Antioxidants 11, no. 11: 2086. https://doi.org/10.3390/antiox11112086
APA StyleBongiorni, S., Arisi, I., Ceccantoni, B., Rossi, C., Cresta, C., Castellani, S., Forgione, I., Rinalducci, S., Muleo, R., & Prantera, G. (2022). Apple Polyphenol Diet Extends Lifespan, Slows down Mitotic Rate and Reduces Morphometric Parameters in Drosophila Melanogaster: A Comparison between Three Different Apple Cultivars. Antioxidants, 11(11), 2086. https://doi.org/10.3390/antiox11112086