Characterization and Valorization of ‘Sulmona Red Garlic’ Peels and Small Bulbs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Hydroalcoholic Extraction
2.3. Colorimetric Analysis
2.4. HPLC-DAD Analysis
2.5. Mass-Spectrometry-Based Metabolomics
2.6. Evaluation of Antiradical Activity by DPPH Assay
2.7. Evaluation of Antimicrobial Activity
2.8. Cell Cultures
2.9. MTS Assay
2.10. Analysis of Peroxynitrite Generation
2.11. Western Blot
2.12. Biological Data and Statistical Analysis
3. Results and Discussion
3.1. Hydroalcoholic Extraction
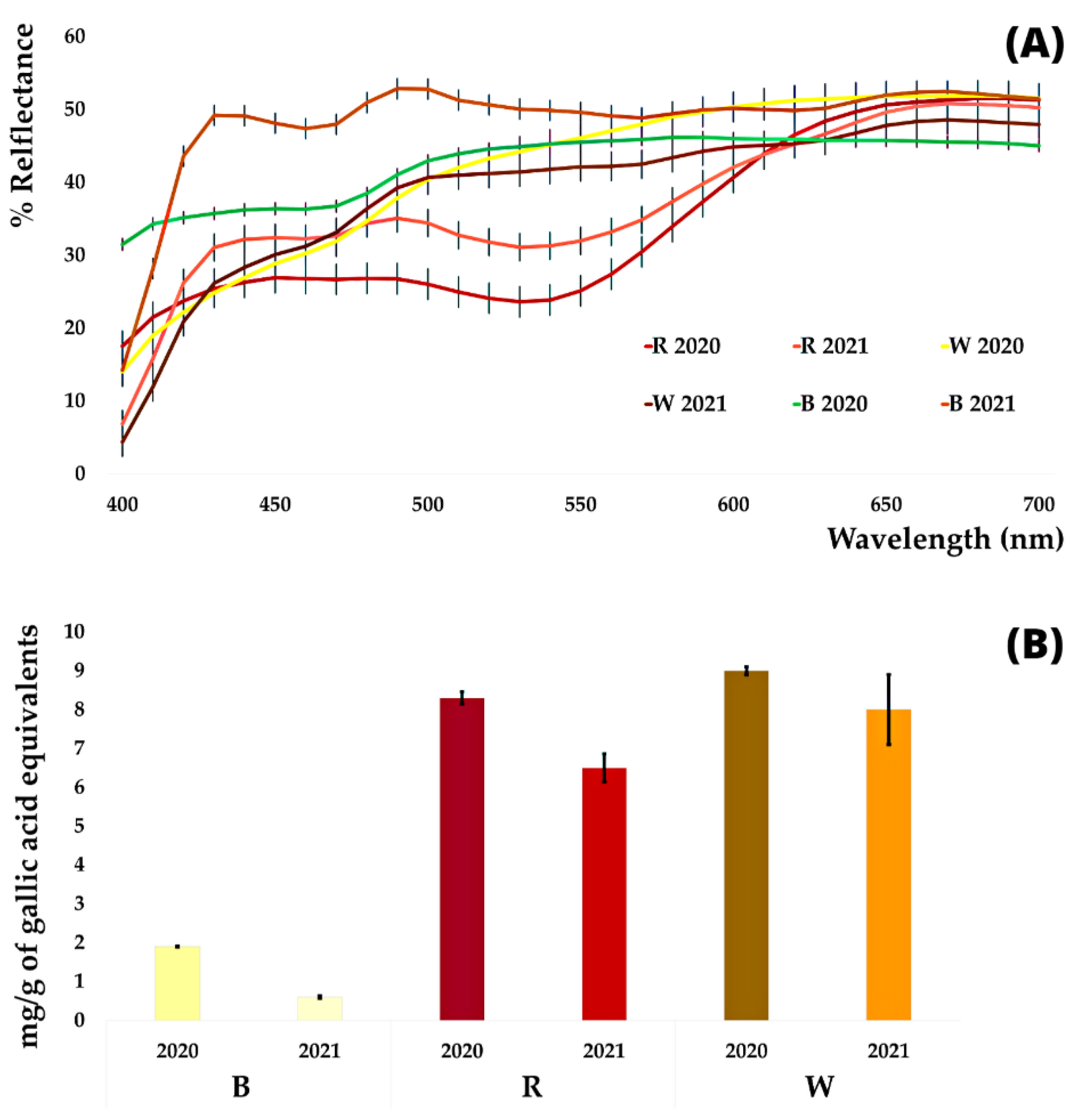
3.2. Colorimetric Analysis
3.3. DPPH Analysis
3.4. HPLC-DAD Analysis
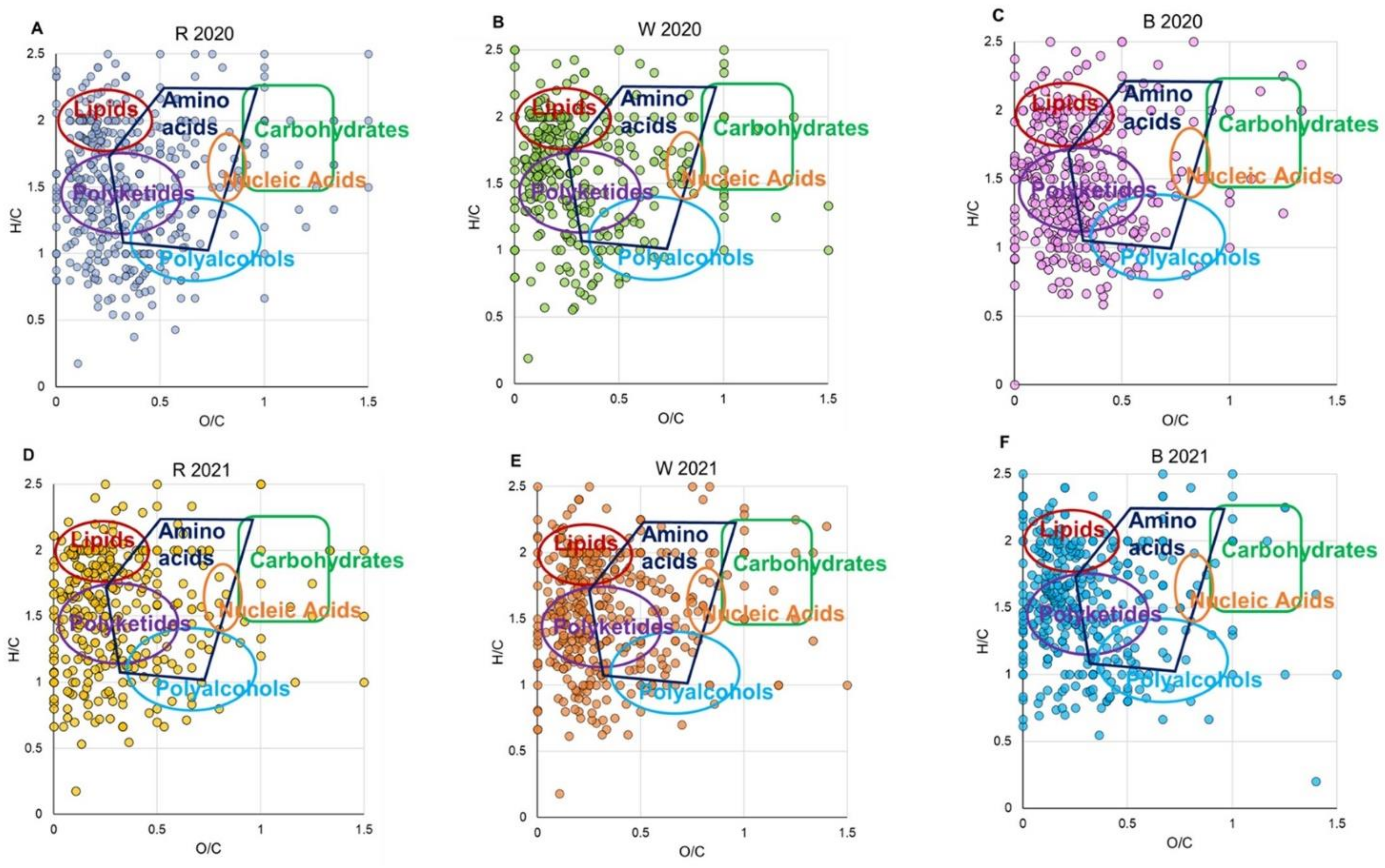
3.5. Principal Component Analysis (PCA)
3.6. ESI FT-ICR Assignment
3.6.1. Amino Acids and Derivatives
3.6.2. Organosulphur Compounds
3.6.3. Organic Acids
3.6.4. Fatty Acids
3.6.5. Miscellaneous
3.6.6. Statistical Analysis: Principal Component Analysis
3.7. Antibacterial Activity
3.8. Biological Evaluation
3.8.1. Effect of Garlic Extracts on Cell Viability
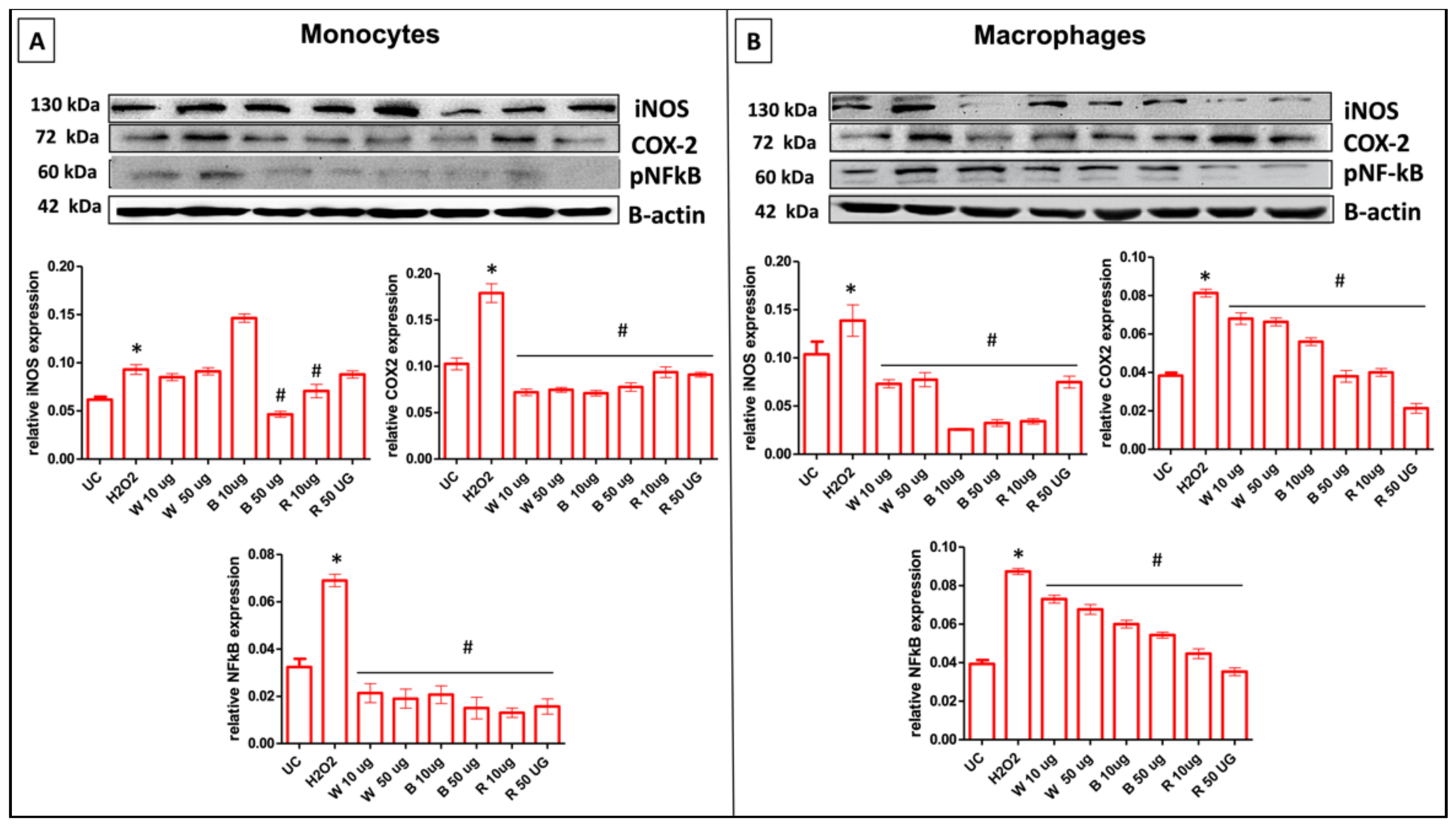
3.8.2. Garlic Extracts Modulate iNOS and COX-2 Protein Expression in H2O2-Stimulated THP-1 Cells
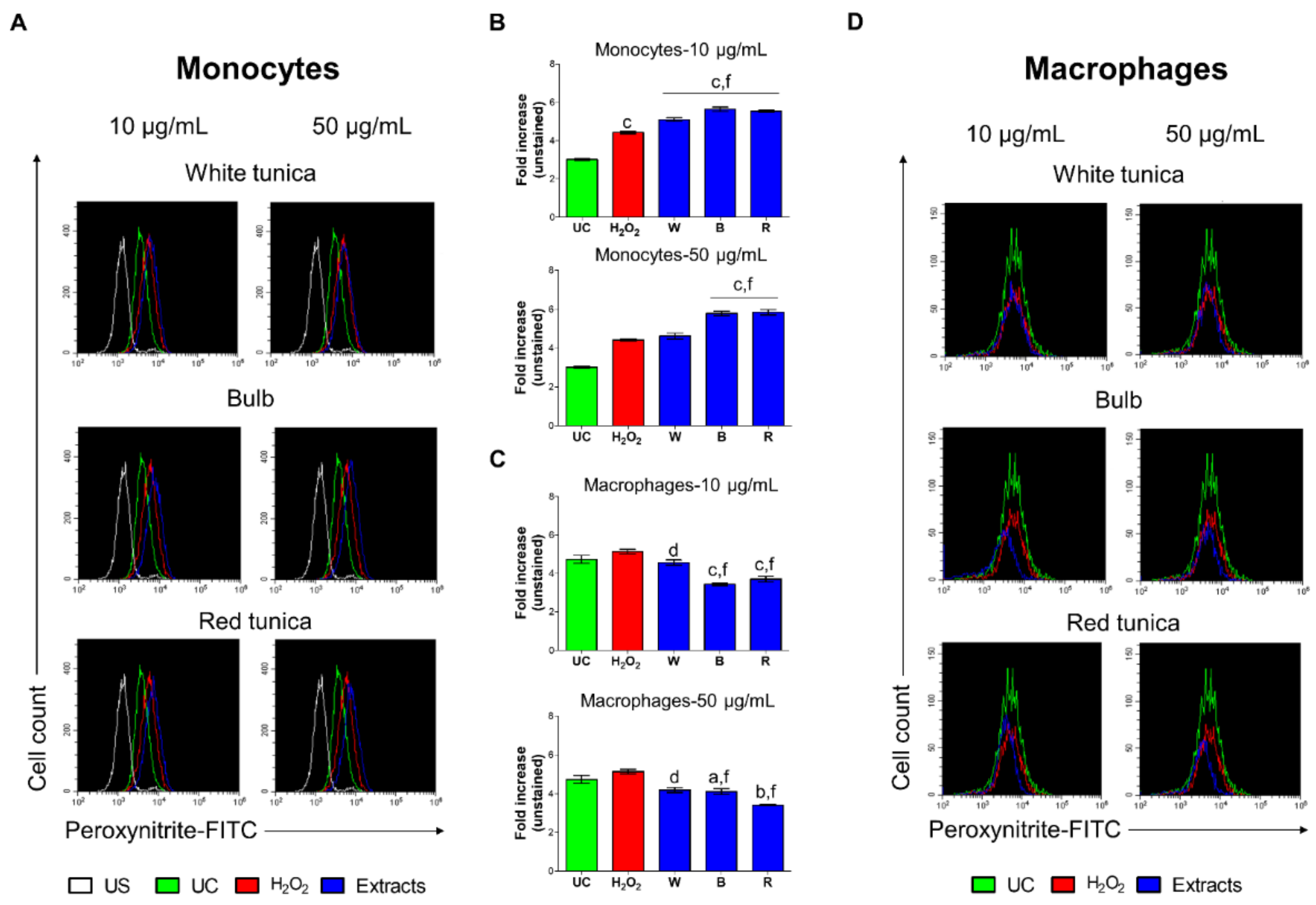
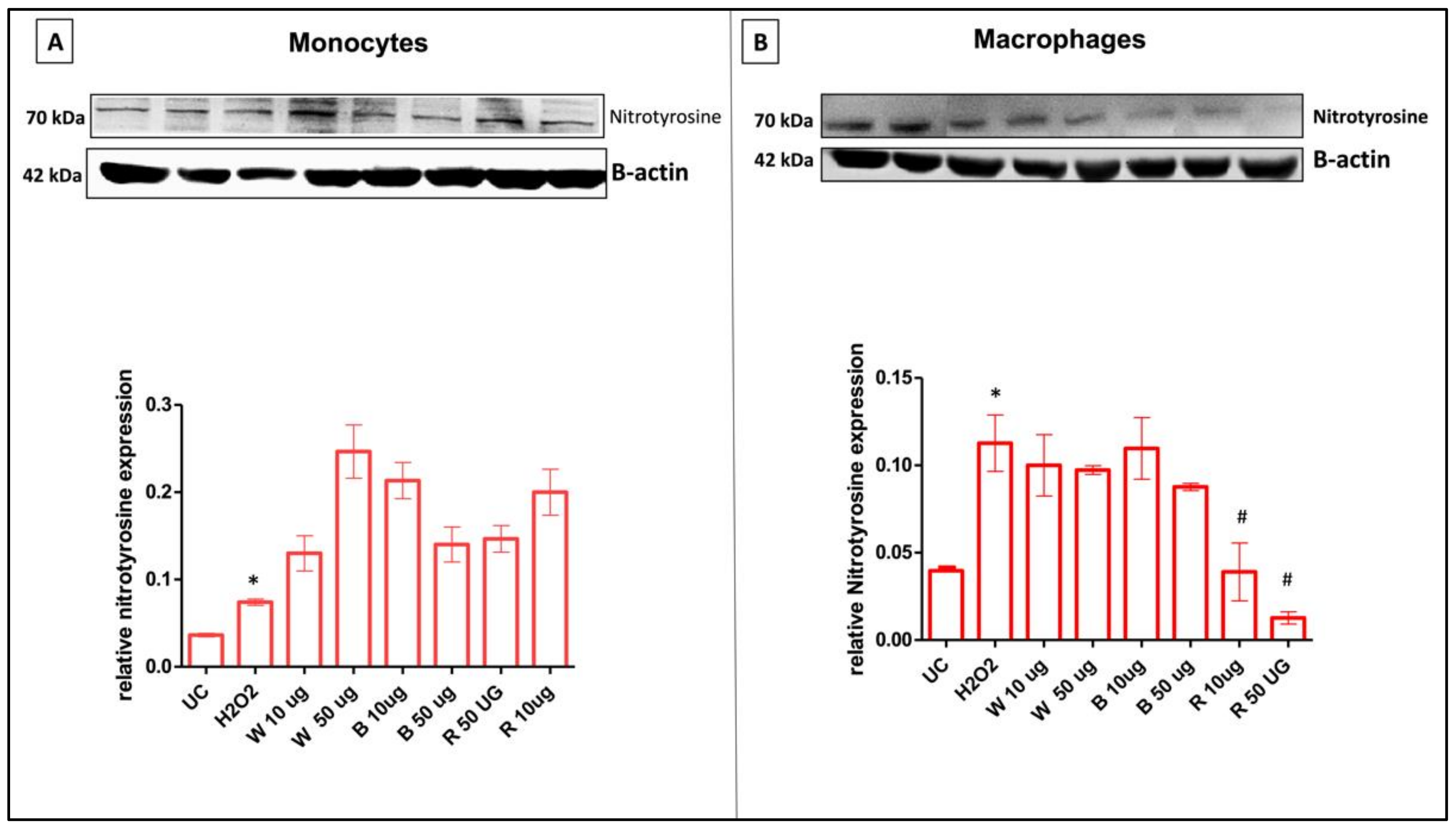
3.8.3. Garlic Extracts Attenuate Nitrosative Stress in H2O2-Stimulated THP-1 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takagi, H. Garlic Allium sativum L. In Onions and Allied Crops; CRC Press: Boca Raton, FL, USA, 2020; pp. 109–146. [Google Scholar]
- Londhe, V.P.; Gavasane, A.T.; Nipate, S.S.; Bandawane, D.D.; Chaudhari, P.D. Role of garlic (Allium sativum) in various diseases: An overview. Angiogenesis 2011, 12, 129–134. [Google Scholar]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2011, 131, 955S–962S. [Google Scholar] [CrossRef] [Green Version]
- Ariga, T.; Seki, T. Antithrombotic and anticancer effects of garlic-derived sulfur compounds: A review. Biofactors 2006, 26, 93–103. [Google Scholar] [CrossRef]
- WHO. Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 1999. [Google Scholar]
- Batiha, G.E.-S.; Beshbishy, A.M.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; El-Hack, M.E.A.; Taha, A.E.; Abd-Elhakim, Y.M.; Devkota, H.P. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef] [Green Version]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Khubber, S.; Hashemifesharaki, R.; Mohammadi, M.; Gharibzahedi, S.M.T. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020, 19, 124. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Foschi, M.; Aloia, R.; Maggi, M.A.; Rossi, L.; Ruggieri, F. A focus on red garlic from Italian production, was explored by multivariate statistical analysis on ICP-OES data. Food Chem. 2019, 275, 333–338. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F.; D’Archivio, A.A. Geographical discrimination of red garlic (Allium sativum L.) using fast and non-invasive Attenuated Total Reflectance-Fourier Transformed Infrared (ATR-FTIR) spectroscopy combined with chemometrics. J. Food Compost. Anal. 2020, 86, 103351. [Google Scholar] [CrossRef]
- Biancolillo, A.; Aloia, R.; Rossi, L.; D’Archivio, A.A. Organosulfur volatile profiles in Italian red garlic (Allium sativum L.) varieties investigated by HS-SPME/GC-MS and chemometrics. Food Control 2022, 131, 108477. [Google Scholar] [CrossRef]
- Azzini, E.; Durazzo, A.; Foddai, M.S.; Temperini, O.; Venneria, E.; Valentini, S.; Maiani, G. Phytochemicals content in Italian garlic bulb (Allium sativum L.) varieties. J. Food Res. 2014, 3, 26. [Google Scholar] [CrossRef]
- Pianezze, S.; Perini, M.; Bontempo, L.; Ziller, L.; D’Archivio, A.A. Geographical discrimination of garlic (Allium Sativum L.) based on Stable isotope ratio analysis coupled with statistical methods: The Italian case study. Food Chem. Toxicol. 2019, 134, 110862. [Google Scholar] [CrossRef]
- Ferioli, F.; Giambanelli, E.; D’Alessandro, V.; D’Antuono, L.F. Comparison of two extraction methods (high pressure extraction vs. maceration) for the total and relative amount of hydrophilic and lipophilic organosulfur compounds in garlic cloves and stems. An application to the Italian ecotype “Aglio Rosso di Sulmona” (Sulmona Red Garlic). Food Chem. 2020, 312, 126086. [Google Scholar] [CrossRef] [PubMed]
- Maccelli, A.; Cesa, S.; Cairone, F.; Secci, D.; Menghini, L.; Chiavarino, B.; Fornarini, S.; Crestoni, M.E.; Locatelli, M. Metabolic profiling of different wild and cultivated Allium species based on high-resolution mass spectrometry, high performance liquid chromatography-photodiode array detector, and color analysis. J. Mass Spectrom. 2020, 55, e4525. [Google Scholar] [CrossRef]
- Kallel, F.; Chaabouni, S.E. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: A review. Environ. Prog. Sustain. 2017, 36, 1765–1777. [Google Scholar] [CrossRef]
- Garzoli, S.; Cairone, F.; Carradori, S.; Mocan, A.; Menghini, L.; Paolicelli, P.; Ak, G.; Zengin, G.; Cesa, S. Effects of processing on polyphenolic and volatile composition and fruit quality of clery strawberries. Antioxidants 2020, 9, 632. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective effects induced by a hydroalcoholic Allium sativum extract in isolated mouse heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef]
- Corinti, D.; Crestoni, M.E.; Fornarini, S.; Pieper, M.; Niehaus, K.; Giampà, M. An integrated approach to study novel properties of a MALDI matrix (4-maleicanhydridoproton sponge) for MS imaging analyses. Anal. Bioanal. Chem. 2019, 411, 953–964. [Google Scholar] [CrossRef] [Green Version]
- Cairone, F.; Petralito, S.; Scipione, L.; Cesa, S. Study on Extra Virgin Olive Oil: Quality evaluation by anti-radical activity, color analysis, and polyphenolic HPLC-DAD analysis. Foods 2021, 10, 1808. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iarlori, C.; Gambi, D.; Lugaresi, A.; Patruno, A.; Felaco, M.; Salvatore, M.; Speranza, L.; Reale, M. Reduction of free radicals in multiple sclerosis: Effect of glatiramer acetate (Copaxone®). Mult. Scler. 2008, 14, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratianni, F.; Ombra, M.N.; Cozzolino, A.; Riccardi, R.; Spigno, P.; Tremonte, P.; Coppola, R.; Nazzaro, F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.). J. Funct. Foods 2016, 21, 240–248. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.W. Extraction and characterization of cellulose microfibers from agricultural wastes of onion and garlic. J. Nat. Fibers 2018, 15, 465–473. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, D.; Li, M.; Hu, X.; Zhao, G. One new pathway for Allium discoloration. Food Chem. 2010, 119, 548–553. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical characteristics and antimicrobial activity of Australian grown garlic (Allium sativum L.) cultivars. Foods 2019, 8, 358. [Google Scholar] [CrossRef] [Green Version]
- Carradori, S.; Cairone, F.; Garzoli, S.; Fabrizi, G.; Iazzetti, A.; Giusti, A.M.; Menghini, L.; Uysal, S.; Ak, G.; Zengin, G.; et al. Phytocomplex characterization and biological evaluation of powdered fruits and leaves from Elaeagnus Angustifolia. Molecules 2020, 25, 2021. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Eghdami, A.; Sohi, S.M.H.; Asli, D.E.; Houshmandfar, A. Antioxidant activity of methanolic and hydroalcohlic extracts of garlic plant. Adv. Environ. Biol. 2011, 5, 1575–1578. [Google Scholar]
- Bar, M.; Binduga, U.E.; Szychowski, K.A. Methods of isolation of active substances from garlic (Allium sativum L.) and its impact on the composition and biological properties of garlic extracts. Antioxidants 2022, 11, 1345. [Google Scholar] [CrossRef]
- Yoo, M.; Lee, S.; Lee, S.; Seog, H.; Shin, D. Validation of high performance liquid chromatography methods for determination of bioactive sulfur compounds in garlic bulbs. Food Sci. Biotechnol. 2010, 19, 1619–1626. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ryu, K.; Yoshida, J.; Ide, N.; Kodera, Y.; Sasaoka, T.; Rosen, R.T. Identification of six phenylpropanoids from garlic skin as major antioxidants. J. Agr. Food Chem. 2003, 51, 7313–7317. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Altamirano, J.C.; Camargo, A.B. Multi-phytochemical determination of polar and non-polar garlic bioactive compounds in different food and nutraceutical preparations. Food Chem. 2021, 337, 127648. [Google Scholar] [CrossRef]
- Spano, M.; Maccelli, A.; Di Matteo, G.; Ingallina, C.; Biava, M.; Crestoni, M.E.; Bardaud, J.X.; Giusti, A.M.; Mariano, A.; Scotto D’Abusco, A.; et al. Metabolomic profiling of fresh goji (Lycium barbarum L.) berries from two cultivars grown in central Italy: A multi-methodological approach. Molecules 2021, 26, 5412. [Google Scholar] [CrossRef]
- Corinti, D.; Maccelli, A.; Crestoni, M.E.; Cesa, S.; Quaglio, D.; Botta, B.; Ingallina, C.; Mannina, L.; Tintaru, A.; Chiavarino, B.; et al. IR ion spectroscopy in a combined approach with MS/MS and IM-MS to discriminate epimeric anthocyanin glycosides (cyanidin 3-O-glucoside and -galactoside). Int. J. Mass Spectrom. 2019, 444, 116179. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Wang, X.; Yao, H. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res. Int. 2006, 39, 833–839. [Google Scholar] [CrossRef]
- Chilicka, K.C.; Rogowska, A.M.; Szyguła, R.; Dzieńdziora-Urbińska, I.; Taradaj, J. A comparison of the effectiveness of azelaic and pyruvic acid peels in the treatment of female adult acne: A randomized controlled trial. Sci. Rep. 2020, 10, 12612. [Google Scholar] [CrossRef] [PubMed]
- Varin, L. Flavonoid sulfation: Phytochemistry, enzymology and molecular biology. In Phenolic Metabolism in Plants; Springer: Boston, MA, USA, 1992; pp. 233–254. [Google Scholar]
- Guglielmi, P.; Mathew, B.; Secci, D.; Carradori, S. Chalcones: Unearthing their therapeutic possibility as monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2020, 205, 112650. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Fornasari, E.; Di Stefano, A.; Cerasa, L.S.; Marinelli, L.; Baldassarre, L.; Sozio, P.; Turkez, H.; Franceschelli, S.; Ferrone, A.; et al. Synthesis of a novel cyclic prodrug of S-allyl-glutathione able to attenuate LPS-induced ROS production through the inhibition of MAPK pathways in U937 cells. Mol. Pharm. 2015, 12, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J. Dev. Physiol. 1991, 15, 53–59. [Google Scholar] [PubMed]
- Singiri, J.R.; Swetha, B.; Ben-Natan, A.; Grafi, G. What worth the garlic peel. Int. J. Mol. Sci. 2022, 23, 2126. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.Z.; Irfan, A.; Zahoor, A.F.; Zafar, M.; Maria, A.; Chand, A.J.; Ashfaq, S. Determination of antimicrobial and antioxidant potential of agro-waste peels. An. Acad. Bras. Cienc. 2020, 92, e20181103. [Google Scholar] [CrossRef] [PubMed]


| ESI (+) FT-ICR MS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Putative Annotation | Adduct | Formula (M) | Theoretical m/z a | ppm b R2020 | ppm b W2020 | ppm b B2020 | ppm b R2021 | ppm b W2021 | ppm b B2021 |
| Carbohydrates | |||||||||
| Hexose | [M+Na]+ | C6H12O6 | 203.05261 | −1.4 | 0.2 | - | - | 0.0 | - |
| [M+K]+ | C6H12O6 | 219.02655 | 4.3 | −0.4 | - | - | −0.6 | - | |
| Mannitol | [M+Na]+ | C6H14O6 | 205.06814 | - | 0.6 | - | - | 1.5 | - |
| Lactose | [M+Na]+ | C12H22O11 | 365.10543 | −1.3 | - | - | - | 2.0 | - |
| Amino acids | |||||||||
| Glycine | [M+Na]+ | C2H5NO2 | 98.02139 | - | −1.4 | - | −2.9 | - | - |
| Leucine/ Isoleucine | [M+H]+ | C6H13NO2 | 132.10191 | 1.0 | −1.3 | - | - | - | - |
| [M+Na]+ | C6H13NO2 | 154.08385 | −4.6 | 3.4 | - | - | - | - | |
| Valine | [M+H]+ | C5H11NO2 | 118.08626 | 2.2 | 2.4 | - | - | - | - |
| Asparagine | [M+K]+ | C4H8N2O3 | 171.01665 | −2.9 | - | - | 4.3 | 0.7 | - |
| Phenylalanine | [M+H]+ | C9H11NO2 | 166.08626 | 1.1 | - | −2.7 | −1.4 | - | - |
| Lysine | [M+H]+ | C6H14N2O2 | 147.11280 | 0.2 | −0.5 | 3.8 | - | - | - |
| Arginine | [M+H]+ | C6H14N4O2 | 175.11895 | 0.3 | 0.1 | −0.2 | 0.0 | - | −0.2 |
| [M+K]+ | C6H14N4O2 | 213.07484 | 0.4 | - | - | - | 2.1 | −4.3 | |
| Allysine | [M+H]+ | C6H11NO3 | 146.08079 | - | - | - | - | - | 2.6 |
| [M+Na]+ | C6H11NO3 | 168.06337 | - | - | −1.5 | - | - | - | |
| Organic acids | |||||||||
| Cysteic acid | [M+H]+ | C3H7NO5S | 170.01141 | 4.3 | - | - | 2.1 | −4.1 | - |
| Coumaric acid | [M+H]+ | C9H8O3 | 165.05462 | −1.4 | 3.2 | 4.6 | 1.1 | −4.2 | 3.1 |
| Ferulic acid | [M+H]+ | C10H10O4 | 195.06519 | 4.3 | 3.6 | 3.9 | - | 3.5 | - |
| Azelaic acid | [M+Na]+ | C9H16O4 | 211.09408 | −1.6 | −0.3 | −1.2 | −0.8 | −1.3 | |
| Ascorbic acid | [M+Na]+ | C6H8O6 | 199.02174 | - | - | - | −2.2 | - | −2.9 |
| Fatty acids | |||||||||
| Decenedioic acid | [M+Na]+ | C10H16O4 | 223.09408 | −2.8 | - | - | - | −0.9 | −2.4 |
| Palmitic acid | [M+Na]+ | C16H32O2 | 279.22945 | −0.3 | - | - | - | - | - |
| Palmitoleic acid | [M+Na]+ | C16H30O2 | 277.21380 | 1.7 | 0.1 | - | - | - | - |
| Oleic acid | [M+H]+ | C18H34O2 | 283.26316 | 2.4 | - | - | - | - | - |
| Myristic acid | [M+Na]+ | C14H28O2 | 251.19807 | - | 0.3 | - | - | - | - |
| [M+K]+ | C14H28O2 | 267.17237 | - | - | - | −1.1 | - | 3.4 | |
| Myristoleic acid | [M+Na]+ | C14H26O2 | 249.18250 | −0.8 | −1.2 | - | - | - | - |
| Other compounds | |||||||||
| Purpurogalin | [M+H]+ | C11H8O5 | 221.04445 | 2.0 | - | - | - | - | - |
| Rosinidin | [M+H]+ | C17H15O6 | 316.09414 | 2.9 | - | - | - | - | - |
| Apiole | [M+H]+ | C12H14O4 | 223.09572 | - | 3.4 | 3.4 | 2.0 | - | - |
| [M+Na]+ | C12H14O4 | 245.07843 | 3.4 | −2.0 | - | - | 0.1 | −2.1 | |
| Gingerol | [M+Na]+ | C21H34O4 | 373.23484 | - | - | - | 0.2 | −1.9 | - |
| ESI (−) FT-ICR MS | |||||||||
| Putative Annotation | Adduct | Formula (M) | Theoretical m/z | ppm R2020 | ppm W2020 | ppm B2020 | ppm R2021 | ppm W2021 | ppm B2021 |
| Carbohydrates | |||||||||
| Hexose | [M-H]− | C6H12O6 | 179.05599 | 4.3 | −0.4 | - | - | −0.6 | - |
| Amino acids | |||||||||
| Asparagine | [M-H]− | C4H8N2O3 | 131.04622 | - | 2.4 | - | - | - | - |
| Fatty acid | |||||||||
| Octenoic acid | [M-H]− | C8H14O2 | 141.09210 | 3.0 | - | - | - | - | - |
| Palmitic acid | [M-H]− | C16H32O2 | 255.23295 | −0.4 | −0.2 | −1.1 | 2.8 | - | 1.0 |
| Palmitoleic acid | [M-H]− | C16H30O2 | 253.21719 | - | - | - | - | 0.5 | - |
| Oleic acid | [M-H]− | C18H34O2 | 281.24860 | 1.1 | - | 1.2 | −0.3 | −0.1 | −2.0 |
| Myristic acid | [M-H]− | C14H28O2 | 227.20176 | - | - | - | - | −0.5 | - |
| [M+Cl]− | C14H28O2 | 263.17833 | 2.1 | 0.4 | - | −2.1 | 1.9 | - | |
| Stearic acid | [M-H]− | C18H36O2 | 283.26425 | −1.2 | −0.4 | −0.5 | 0.9 | - | - |
| Lauric acid | [M-H]− | C12H24O2 | 199.17035 | 2.4 | - | −0.7 | - | −2.4 | - |
| Organic acids | |||||||||
| Quinic acid | [M-H]− | C7H12O6 | 191.05611 | 3.3 | - | - | - | - | - |
| Malonic acid | [M+Cl]− | C3H4O4 | 138.98036 | 4.5 | - | - | - | - | - |
| Oxalacetic Acid | [M-H]− | C4H4O5 | 130.99856 | - | - | - | - | - | 0.3 |
| Ferulic acid | [M+Cl]− | C10H10O4 | 229.02731 | - | 2.1 | - | - | - | - |
| Malic acid | [M-H]− | C4H6O5 | 133.01389 | - | - | - | 2.7 | - | - |
| Other compounds | |||||||||
| Bergapten | [M-H]− | C12H8O4 | 215.03587 | - | - | - | −4.1 | 0.6 | - |
| Chamazulene | [M+Cl]− | C14H16 | 219.09499 | - | - | - | - | −1.8 | 0.0 |
| β-Tocopherol | [M-H]− | C28H48O2 | 415.35786 | - | - | - | - | - | 0.7 |
| Extract | Ion Type | Identified MF | |||
|---|---|---|---|---|---|
| 2020 | 2021 | ||||
| White tunica | ESI (+) | 270 | 341 | 322 | 414 |
| ESI (−) | 106 | 121 | |||
| Red tunica | ESI (+) | 362 | 417 | 267 | 340 |
| ESI (−) | 106 | 101 | |||
| Bulb | ESI (+) | 282 | 374 | 292 | 375 |
| ESI (−) | 116 | 104 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasalvia, A.; Cairone, F.; Cesa, S.; Maccelli, A.; Crestoni, M.E.; Menghini, L.; Carradori, S.; Marinacci, B.; Gallorini, M.; Elsallabi, O.; et al. Characterization and Valorization of ‘Sulmona Red Garlic’ Peels and Small Bulbs. Antioxidants 2022, 11, 2088. https://doi.org/10.3390/antiox11112088
Lasalvia A, Cairone F, Cesa S, Maccelli A, Crestoni ME, Menghini L, Carradori S, Marinacci B, Gallorini M, Elsallabi O, et al. Characterization and Valorization of ‘Sulmona Red Garlic’ Peels and Small Bulbs. Antioxidants. 2022; 11(11):2088. https://doi.org/10.3390/antiox11112088
Chicago/Turabian StyleLasalvia, Alba, Francesco Cairone, Stefania Cesa, Alessandro Maccelli, Maria Elisa Crestoni, Luigi Menghini, Simone Carradori, Beatrice Marinacci, Marialucia Gallorini, Osama Elsallabi, and et al. 2022. "Characterization and Valorization of ‘Sulmona Red Garlic’ Peels and Small Bulbs" Antioxidants 11, no. 11: 2088. https://doi.org/10.3390/antiox11112088








