Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction
Abstract
1. Introduction
2. The Physicochemical Characteristics of DON
3. DON-Induced Enterotoxicity in Swine
4. Plant-Derived Polyphenol Application to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine
4.1. Resveratrol
4.2. Oxyresveratrol
4.3. Baicalin
4.4. Ferulic Acid
4.5. Quercetin
4.6. Dihydromyricetin
4.7. Chlorogenic Acid
4.8. Astilbin
4.9. Rosmarinic Acid
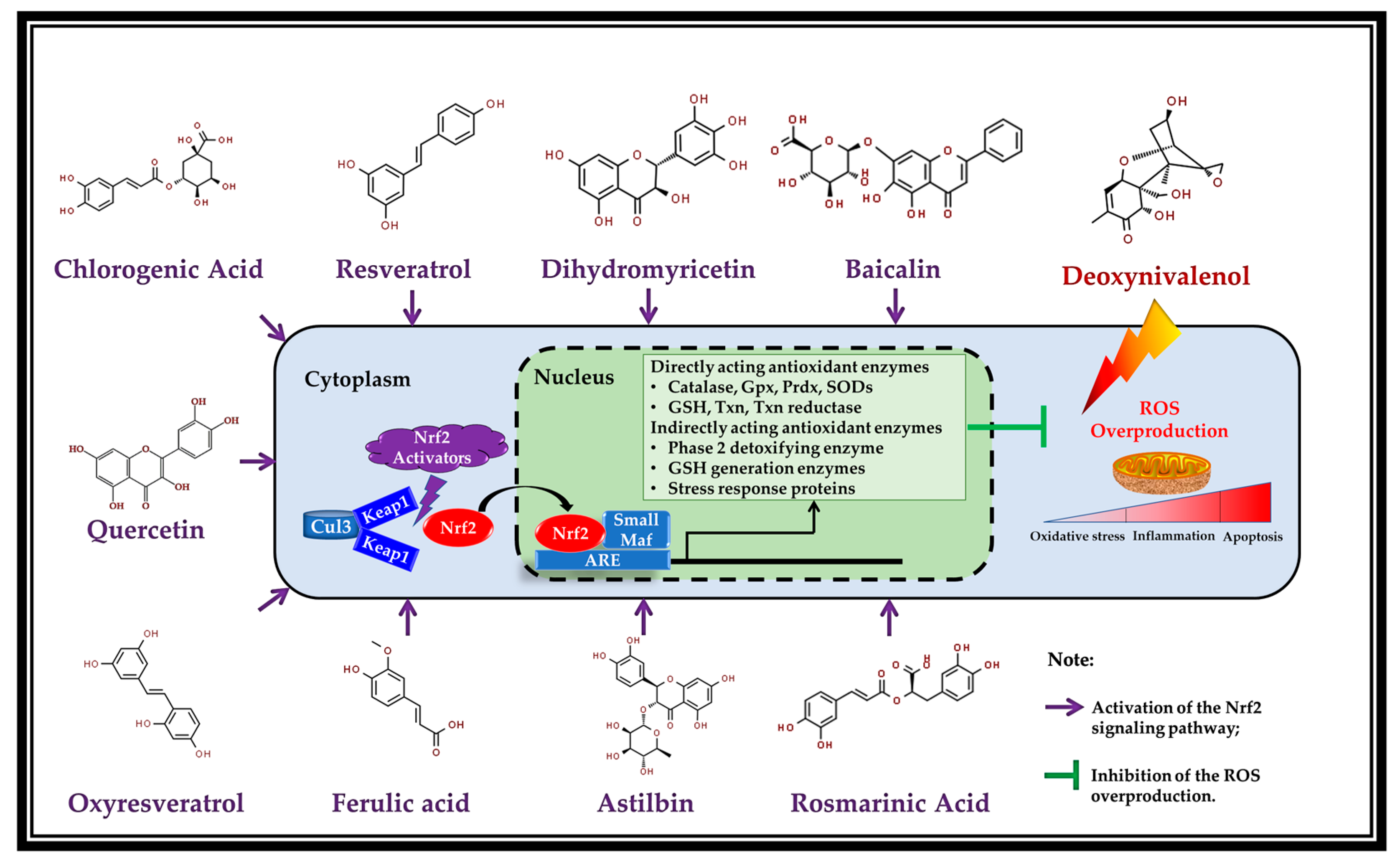
5. Potential Mechanism of Action: Activation of Nrf2 by the Polyphenols
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patience, J.F.; Rossoni-Serão, M.C.; Gutiérrez, N.A. A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.T.; Xie, W.M.; Zhang, N.Y.; Dai, J.F.; Wang, Y.; Rajput, S.A.; Qi, D.S.; et al. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Wellington, M.O.; Bosompem, M.A.; Petracek, R.; Nagl, V.; Columbus, D.A. Effect of long-term feeding of graded levels of deoxynivalenol (DON) on growth performance, nutrient utilization, and organ health in finishing pigs and DON content in biological samples. J. Anim. Sci. 2020, 98, skaa378. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef] [PubMed]
- García, G.R.; Payros, D.; Pinton, P.; Dogi, C.A.; Laffitte, J.; Neves, M.; González Pereyra, M.L.; Cavaglieri, L.R.; Oswald, I.P. Intestinal toxicity of deoxynivalenol is limited by Lactobacillus rhamnosus RC007 in pig jejunum explants. Arch. Toxicol. 2018, 92, 983–993. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, C.; Ye, J.; Lv, Y.; Wang, L.; Chen, Z.; Jiang, Z. Protection of porcine intestinal-epithelial cells from deoxynivalenol-induced damage by resveratrol via the Nrf2 signaling pathway. J. Agric. Food Chem. 2019, 67, 1726–1735. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Pinton, P.; Hupé, J.-F.; Neves, M.; Lippi, Y.; Combes, S.; Castex, M.; Oswald, I.P. Saccharomyces cerevisiae Boulardii reduces the deoxynivalenol-induced alteration of the intestinal transcriptome. Toxins 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.-R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Yang, J.; Wang, X.; Wu, K.; Zhang, B.; Zhang, J.; Yang, A.; Rajput, S.A.; Qi, D. Sodium butyrate protects the intestinal barrier by modulating intestinal host defense peptide expression and gut microbiota after a challenge with deoxynivalenol in weaned piglets. J. Agric. Food Chem. 2020, 68, 4515–4527. [Google Scholar] [CrossRef]
- Tremblay-Franco, M.; Canlet, C.; Pinton, P.; Lippi, Y.; Gautier, R.; Naylies, C.; Neves, M.; Oswald, I.P.; Debrauwer, L.; Alassane-Kpembi, I. Statistical integration of ’omics data increases biological knowledge extracted from metabolomics data: Application to intestinal exposure to the mycotoxin deoxynivalenol. Metabolites 2021, 11, 407. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol induces inflammation in IPEC-J2 cells by activating P38 Mapk and Erk1/2. Toxins 2020, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yu, H.; Yang, Z.; Hu, L.; Liu, Q.; Wang, Y.; Wei, H.-K.; Peng, J. Gly-Pro-Ala peptide and FGSHF3 exert protective effects in DON-induced toxicity and intestinal damage via decreasing oxidative stress. Food Res. Int. 2021, 139, 109840. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular aspects of mycotoxins-a serious problem for human health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Hong, Q.; Li, X.; Lin, Q.; Shen, Z.; Feng, J.; Hu, C. Resveratrol improves intestinal morphology and anti-oxidation ability in deoxynivalenol-challenged piglets. Animals 2022, 12, 311. [Google Scholar] [CrossRef]
- Bouchard, M.J.; Chorfi, Y.; Létourneau-Montminy, M.P.; Guay, F. Effects of deoxynivalenol and sodium meta-bisulphite on nutrient digestibility in growing pigs. Arch. Anim. Nutr. 2019, 73, 360–373. [Google Scholar] [CrossRef]
- Jo, H.; Kong, C.; Song, M.; Kim, B.G. Effects of dietary deoxynivalenol and zearalenone on apparent ileal digestibility of amino acids in growing pigs. Anim. Feed Sci. Technol. 2016, 219, 77–82. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, L.; Ren, W.; Yin, J.; Duan, J.; Li, T. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)—challenged growing pigs. BMC Vet. Res. 2015, 11, 144. [Google Scholar] [CrossRef]
- Liao, P.; Liao, M.; Li, L.; Tan, B.; Yin, Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol. Res. 2017, 6, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Zou, T.; You, J.; Guan, W. Plant-derived polyphenols in sow nutrition: An update. Anim. Nutr. 2022, in press. [Google Scholar] [CrossRef]
- Xu, X.; Yan, G.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Liu, S.; Zhu, Q.; Lu, F. Astilbin ameliorates deoxynivalenol-induced oxidative stress and apoptosis in intestinal porcine epithelial cells (IPEC-J2). J. Appl. Toxicol. 2020, 40, 1362–1372. [Google Scholar] [CrossRef]
- Long, H.; Xin, Z.; Zhang, F.; Zhai, Z.; Ni, X.; Chen, J.; Yang, K.; Liao, P.; Zhang, L.; Xiao, Z.; et al. The cytoprotective effects of dihydromyricetin and associated metabolic pathway changes on deoxynivalenol treated IPEC-J2 cells. Food Chem. 2021, 338, 128116. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Barna, R.F.; Pászti, E.A.; Babiczky, Á.; Szóládi, Á.; Jerzsele, Á.; Gere, E.P. Beneficial effects of rosmarinic acid on IPEC-J2 cells exposed to the combination of deoxynivalenol and T-2 toxin. Mediat. Inflamm. 2020, 2020, 8880651. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.H.; Wan, M.L.; El-Nezami, H.; Wang, M. Protective capacity of resveratrol, a natural polyphenolic compound, against deoxynivalenol-induced intestinal barrier dysfunction and bacterial translocation. Chem. Res. Toxicol. 2016, 29, 823–833. [Google Scholar] [CrossRef]
- Liao, P.; Li, Y.; Li, M.; Chen, X.; Yuan, D.; Tang, M.; Xu, K. Baicalin alleviates deoxynivalenol-induced intestinal inflammation and oxidative stress damage by inhibiting NF-κB and increasing mTOR signaling pathways in piglets. Food Chem. Toxicol. 2020, 140, 111326. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, C.; Song, B.; Wang, L.; Xiao, H.; Jiang, Z. Resveratrol ameliorates intestinal damage challenged with deoxynivalenol through mitophagy in vitro and in vivo. Front. Vet. Sci. 2022, 8, 807301. [Google Scholar] [CrossRef]
- Wan, M.L.; Ling, K.H.; El-Nezami, H.; Wang, M. Oxyresveratrol protective effects against deoxynivalenol-induced intestinal barrier dysfunction and bacterial translocation on porcine intestinal epithelial IPEC-J2 cells. J. Food Bioact. 2018, 1, 116–123. [Google Scholar] [CrossRef]
- Zha, A.; Yuan, D.; Cui, Z.; Qi, M.; Liao, S.; Liao, P.; Tan, B. The evaluation of the antioxidant and intestinal protective effects of baicalin-copper in deoxynivalenol-challenged piglets. Oxid. Med. Cell. Longev. 2020, 2020, 5363546. [Google Scholar] [CrossRef]
- Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Shen, Y.; Wu, S. Protective effects of ferulic acid on deoxynivalenol-induced toxicity in IPEC-J2 cells. Toxins 2022, 14, 275. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Gatt, K.; Jerzsele, Á.; Gere, E.P. The impact of quercetin on a porcine intestinal epithelial cell line exposed to deoxynivalenol. Acta Vet. Hung. 2021, 68, 380–386. [Google Scholar] [CrossRef]
- Xu, X.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Li, M.; Song, A.; Zhu, Q.; Lu, F. Effect of chlorogenic acid on alleviating inflammation and apoptosis of IPEC-J2 cells induced by deoxynivalenol. Ecotoxicol. Environ. Saf. 2020, 205, 111376. [Google Scholar] [CrossRef]
- Qiu, Y.; Nie, X.; Yang, J.; Wang, L.; Zhu, C.; Yang, X.; Jiang, Z. Effect of resveratrol supplementation on intestinal oxidative stress, immunity and gut microbiota in weaned piglets challenged with deoxynivalenol. Antioxidants 2022, 11, 1775. [Google Scholar] [CrossRef]
- Chen, W.; Yeo, S.C.M.; Elhennawy, M.G.A.A.; Lin, H.-S. Oxyresveratrol: A bioavailable dietary polyphenol. J. Funct. Foods 2016, 22, 122–131. [Google Scholar] [CrossRef]
- Hwang, D.; Jo, H.; Ma, S.-H.; Lim, Y.-H. Oxyresveratrol stimulates mucin production in an NAD+-dependent manner in human intestinal goblet cells. Food Chem. Toxicol. 2018, 118, 880–888. [Google Scholar] [CrossRef]
- Hwang, D.; Jo, H.; Hwang, S.; Kim, J.-K.; Kim, I.-H.; Lim, Y.-H. Conditioned medium from LS 174T goblet cells treated with oxyresveratrol strengthens tight junctions in Caco-2 cells. Biomed. Pharmacother. 2017, 85, 280–286. [Google Scholar] [CrossRef]
- Jo, H.; Hwang, D.; Kim, J.-K.; Lim, Y.-H. Oxyresveratrol improves tight junction integrity through the PKC and MAPK signaling pathways in Caco-2 cells. Food Chem. Toxicol. 2017, 108, 203–213. [Google Scholar] [CrossRef]
- Yeom, J.; Ma, S.; Kim, J.-K.; Lim, Y.-H. Oxyresveratrol ameliorates dextran sulfate sodium-induced colitis in rats by suppressing inflammation. Molecules 2021, 26, 2630. [Google Scholar] [CrossRef]
- Hwang, D.; Jo, H.; Kim, J.-K.; Lim, Y.-H. Oxyresveratrol-containing Ramulus mori ethanol extract attenuates acute colitis by suppressing inflammation and increasing mucin secretion. J. Funct. Foods 2017, 35, 146–158. [Google Scholar] [CrossRef]
- Galindo, I.; Hernáez, B.; Berná, J.; Fenoll, J.; Cenis, J.L.; Escribano, J.M.; Alonso, C. Comparative inhibitory activity of the stilbenes resveratrol and oxyresveratrol on African swine fever virus replication. Antivir. Res. 2011, 91, 57–63. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Zha, A.; Cui, Z.; Qi, M.; Liao, S.; Chen, L.; Liao, P.; Tan, B. Dietary baicalin zinc supplementation alleviates oxidative stress and enhances nutrition absorption in deoxynivalenol challenged pigs. Curr. Drug Metab. 2020, 21, 614–625. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic acid: A promising therapeutic phytochemical and recent patents advances. Recent. Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, L.Y.; Li, J.L.; Zhang, L.; Gao, F.; Zhou, G.H. Effects of dietary supplementation with ferulic Acid or vitamin e individually or in combination on meat quality and antioxidant capacity of finishing pigs. Asian-Australas J. Anim. Sci. 2015, 28, 374–381. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.; Jimenez-Estrada, I.; Mariscal-Tovar, S.; Lopez-Garcia, K.; Pinelli-Saavedra, A.; Pena-Ramos, E.A.; Muhlia-Almazan, A.; Zamorano-Garcia, L.; Valenzuela-Melendres, M.; Gonzalez-Rios, H. Effects of ferulic acid supplementation on growth performance, carcass traits and histochemical characteristics of muscle fibers in finishing pigs. Animals 2011, 11, 2455. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Yu, J.; Chen, H.; He, J.; Luo, Y.; Zheng, P. Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 2020, 12, 3811. [Google Scholar] [CrossRef]
- Karancsi, Z.; Kovács, D.; Palkovicsné Pézsa, N.; Gálfi, P.; Jerzsele, Á.; Farkas, O. The impact of quercetin and its methylated derivatives 3-o-methylquercetin and rhamnazin in lipopolysaccharide-induced inflammation in porcine intestinal cells. Antioxidants 2022, 11, 1265. [Google Scholar] [CrossRef]
- Zou, Y.; Wei, H.K.; Xiang, Q.-H.; Wang, J.; Zhou, Y.-F.; Peng, J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016, 78, 1487–1494. [Google Scholar] [CrossRef]
- Zou, Y.; Xiang, Q.; Wang, J.; Wei, H.; Peng, J. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livest. Sci. 2016, 192, 33–38. [Google Scholar] [CrossRef]
- Xu, B.; Qin, W.; Xu, Y.; Yang, W.; Chen, Y.; Huang, J.; Zhao, J.; Ma, L. Dietary quercetin supplementation attenuates diarrhea and intestinal damage by regulating gut microbiota in weanling piglets. Oxid. Med. Cell. Longev. 2021, 2021, 6221012. [Google Scholar] [CrossRef]
- Degroote, J.; Vergauwen, H.; Van Noten, N.; Wang, W.; De Smet, S.; Van Ginneken, C.; Michiels, J. The effect of dietary quercetin on the glutathione redox system and small intestinal functionality of weaned piglets. Antioxidants 2019, 8, 312. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin alleviates oxidative damage by activating nuclear factor erythroid 2-related factor 2 signaling in porcine enterocytes. Nutrients 2021, 13, 375. [Google Scholar] [CrossRef]
- Vergauwen, H.; Prims, S.; Degroote, J.; Wang, W.; Casteleyn, C.; van Cruchten, S.; de Smet, S.; Michiels, J.; van Ginneken, C. In vitro investigation of six antioxidants for pig diets. Antioxidants 2016, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of quercetin on proliferation and H₂O₂-induced apoptosis of intestinal porcine enterocyte cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef]
- Liu, C.M.; Yang, W.; Ma, J.Q.; Yang, H.X.; Feng, Z.J.; Sun, J.M.; Cheng, C.; Jiang, H. Dihydromyricetin inhibits lead-induced cognitive impairments and inflammation by the adenosine 5’-monophosphate-activated protein kinase pathway in mice. J. Agric. Food Chem. 2018, 66, 7975–7982. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Zheng, P. Dihydromyricetin improves meat quality and promotes skeletal muscle fiber type transformations via AMPK signaling in growing-finishing pigs. Food Funct. 2022, 13, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Chen, X.; Chen, D.; Yu, B.; Zheng, P.; He, J.; Chen, H.; Yan, H.; Luo, Y.; Huang, Z. Dihydromyricetin enhances intestinal antioxidant capacity of growing-finishing pigs by activating ERK/Nrf2/HO-1 signaling pathway. Antioxidants 2022, 11, 704. [Google Scholar] [CrossRef]
- Wei, C.; Chen, X.; Chen, D.; He, J.; Zheng, P.; Chen, H.; Yan, H.; Yu, B.; Luo, Y.; Huang, Z. Effects of dietary dihydromyricetin supplementation on intestinal barrier and humoral immunity in growing-finishing pigs. Anim. Biotechnol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, B.; Chen, D.; Mao, X.; Zheng, P.; Luo, J.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Huang, Z.; Yan, H.; et al. Chlorogenic acid attenuates oxidative stress-induced intestinal mucosa disruption in weaned pigs. Front. Vet. Sci. 2022, 9, 806253. [Google Scholar] [CrossRef]
- Chen, J.; Xie, H.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Luo, Y.; Luo, J.; He, J. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. ACS Omega 2018, 3, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef]
- Nakahara, T.; Nishitani, Y.; Nishiumi, S.; Yoshida, M.; Azuma, T. Astilbin from Engelhardtia chrysolepis enhances intestinal barrier functions in Caco-2 cell monolayers. Eur. J. Pharmacol. 2017, 804, 46–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Zhao, J.; Deng, W.; Li, K.; Liu, H. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides Oliver leaf on growth performance and quality and oxidative status of meat in finishing pigs fed diets containing fresh or oxidized corn oil. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1116–1125. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Nrf2: A main responsive element in cells to mycotoxin-induced toxicity. Arch. Toxicol. 2021, 95, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Barber, A.J.; Spagnuolo, C.; Russo, G.L.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sánchez, E. Nrf2 as molecular target for polyphenols: A novel therapeutic strategy in diabetic retinopathy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 293–312. [Google Scholar] [CrossRef]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Bao, M.; Liang, M.; Sun, X.; Mohyuddin, S.G.; Chen, S.; Wen, J.; Yong, Y.; Ma, X.; Yu, Z.; Ju, X.; et al. Baicalin alleviates LPS-induced oxidative stress via NF-κB and Nrf2-HO1 signaling pathways in IPEC-J2 cells. Front. Vet. Sci. 2021, 8, 808233. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, L.; Chen, X.; Zhang, H.; Xue, H.; Lu, Y.; Tang, J.; Lu, Y. Orthosiphon stamineus and rosmarinic acid reduce heat stress in laying hens. Livest. Sci. 2020, 240, 104124. [Google Scholar] [CrossRef]
- Wang, S.-W.; Xu, Y.; Weng, Y.-Y.; Fan, X.-Y.; Bai, Y.-F.; Zheng, X.-Y.; Lou, L.-J.; Zhang, F. Astilbin ameliorates cisplatin-induced nephrotoxicity through reducing oxidative stress and inflammation. Food Chem. Toxicol. 2018, 114, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Lee, J.-H.; Jegal, K.H.; Cho, I.J.; Kim, Y.W.; Kim, S.C. Oxyresveratrol abrogates oxidative stress by activating ERK–Nrf2 pathway in the liver. Chem. Biol. Interact. 2016, 245, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [PubMed]

| Polyphenols | In Vivo/In Vitro Model | Polyphenol Doses and Duration | DON Doses and Duration | Main Findings (Polyphenol + DON Treatment vs. DON Treatment) | References |
|---|---|---|---|---|---|
| Resveratrol | Piglets | 300 mg/kg, 21 days (Cotreatment with DON) | 2.65 mg/kg, 21 days |
| [21] |
| |||||
| |||||
| Resveratrol | Piglets | 300 mg/kg, 28 days (Cotreatment with DON) | 3.8 mg/kg, 28 days |
| [31] |
| |||||
| |||||
| |||||
| |||||
| Resveratrol | Piglets | 300 mg/kg, 28 days (Cotreatment with DON) | 3.8 mg/kg, 28 days |
| [34] |
| |||||
| Resveratrol | IPEC-J2 cells | 15 µM, 24 h (pretreatment) | 0.5 μg/mL, 24 h |
| [34] |
| |||||
| Resveratrol | IPEC-J2 cells | 15 µM, 24 h (pretreatment) | 0.5 μg/mL, 24 h |
| [10] |
| |||||
| |||||
| Resveratrol | IPEC-J2 cells | 50 µM, 12 h (1 h pretreatment and 11 h cotreatment with DON) | 4 μM, 11 h |
| [32] |
| |||||
| |||||
| |||||
| Oxyresveratrol | IPEC-J2 cells | 25 µM, 12 (1 h pretreatment and 11 h cotreatment with DON) | 4 μM, 12 h |
| [35] |
| |||||
| Baicalin | Piglets | 0.1%, 14 days (Cotreatment with DON) | 4 mg/kg, 14 days |
| [33] |
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Baicalin-copper | Piglets | 5 g/kg, 14 days (Cotreatment with DON) | 4 mg/kg, 14 days |
| [36] |
| Ferulic acid | IPEC-J2 cells | 60 µM, 12 h (pretreatment) | 40 µM, 12 h |
| [37] |
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Quercetin | IPEC-J2 cells | 20 μM, 24 h (pretreatment) | 1 μM, 1 h |
| [38] |
| |||||
| Dihydromyricetin | IPEC-J2 cells | 40 μM, 24 h (cotreatment with DON) | 250 ng/mL, 24 h |
| [29] |
| |||||
| |||||
| |||||
| Chlorogenic acid | IPEC-J2 cells | 40 µg/mL, 1 h (Pretreatment) | 0.5 µg/mL, 6 h |
| [39] |
| |||||
| |||||
| |||||
| |||||
| Astilbin | IPEC-J2 cells | 20 μg/mL, 6 h (cotreatment with DON) | 0.5 μg/mL, 6 h |
| [28] |
| |||||
| |||||
| |||||
| |||||
| |||||
| Rosmarinic acid | IPEC-J2 cells | 50 μM, 24 h (pretreatment) | 1 μM DON and 5 nmol/L T-2, 48 and 72 h |
| [30] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants 2022, 11, 2379. https://doi.org/10.3390/antiox11122379
Chen J, Huang Z, Cao X, Chen X, Zou T, You J. Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants. 2022; 11(12):2379. https://doi.org/10.3390/antiox11122379
Chicago/Turabian StyleChen, Jun, Zhouyin Huang, Xuehai Cao, Xingping Chen, Tiande Zou, and Jinming You. 2022. "Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction" Antioxidants 11, no. 12: 2379. https://doi.org/10.3390/antiox11122379
APA StyleChen, J., Huang, Z., Cao, X., Chen, X., Zou, T., & You, J. (2022). Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants, 11(12), 2379. https://doi.org/10.3390/antiox11122379






