Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diet
2.3. Breeding Experiment
2.4. Sample Collection
2.5. Chemical Analysis, RNA Extraction, and Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
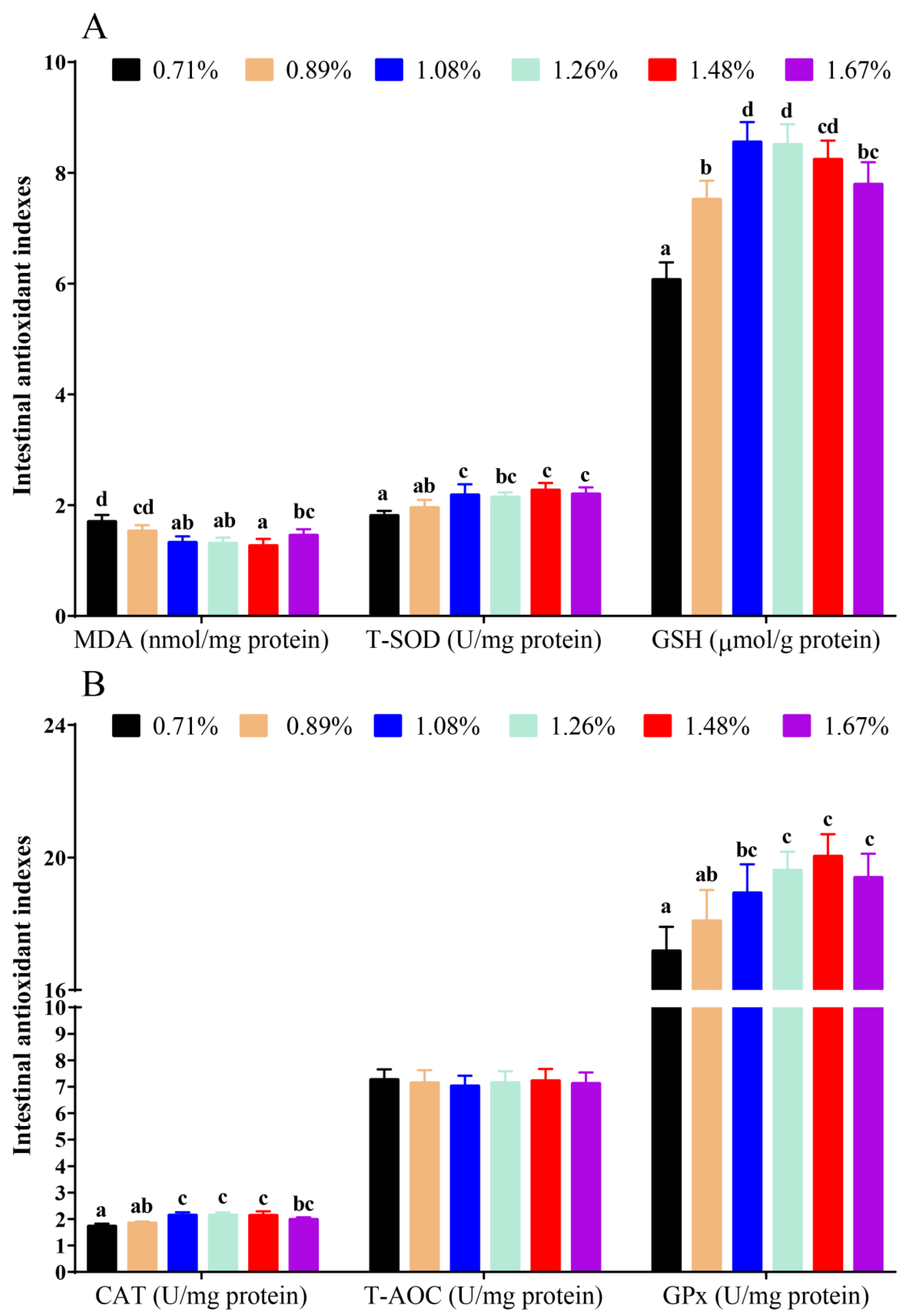
3.1. Intestinal Antioxidant Status
3.2. Intestinal Immune Cytokines
3.3. The mRNA Levels of the Key Genes in the Nrf2-Signalling Pathway
3.4. The mRNA Levels of Inflammatory-Related Genes
3.5. The mRNA Levels of Apoptosis and Necroptosis Genes
3.6. The mRNA Levels of ERS-Related Genes
4. Discussion
4.1. Histidine Deficiency Inhibited Intestinal Antioxidant Capacity
4.2. Histidine Deficiency Suppressed Intestinal Immunocompetence
4.3. Histidine Deficiency Induces Intestinal Endoplasmic-Reticulum Stress Resulting in Inflammatory Response, Apoptosis, and Necroptosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Ran, C.; Teame, T.; Ding, Q.; Hoseinifar, S.H.; Xie, M.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Gatlin, D.M., III; et al. Research progress on gut health of farmers teleost fish: A viewpoint concerning the intestinal mucosal barrier and the impact of its damage. Rev. Fish Biol. Fisher. 2020, 30, 569–586. [Google Scholar] [CrossRef]
- Dawood, M.A. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquacult. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Takahashi, I.; Kiyono, H. Gut as the largest immunologic tissue. J. Parenter. Enter. Nutr. 1999, 23, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Qu, B.; Feng, L.; Jiang, J.; Kuang, S.; Wu, P.; Tang, L.; Tang, W.-N.; Zhang, Y.A.; Zhou, X.-Q.; et al. Histidine prevents cu-induced oxidative stress and the associated decreases in mRNA from encoding tight junction proteins in the intestine of grass carp (Ctenopharyngodon idella). PLoS ONE 2016, 11, e157001. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Maulu, S.; Ren, M.C.; Liang, H.L.; Ge, X.P.; Ji, K.; Yu, H. Dietary lysine levels improved antioxidant capacity and immunity via the TOR and p38 MAPK signaling pathways in grass carp, Ctenopharyngodon idellus fry. Front. Immunol. 2021, 12, 635015. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ji, K.; Ge, X.; Xi, B.; Chen, X. Tributyrin plays an important role in regulating the growth and health status of juvenile blunt snout bream (Megalobrama amblycephala), as evidenced by pathological examination. Front. Immunol. 2021, 12, 652294. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kiyono, H. Vitamin-mediated regulation of intestinal immunity. Front. Immunol. 2013, 4, 189. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Zhou, X.-Q. The regulatory effects of pyridoxine deficiency on the grass carp (Ctenopharyngodon idella) gill barriers immunity, apoptosis, antioxidant, and tight junction challenged with flavobacterium columnar. Fish Shellfish Immunol. 2020, 105, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Chen, X.; Yang, H.; Li, X.; Tian, L. Effects of graded levels of histidine on growth performance, digested enzymes activities, erythrocyte osmotic fragility and hypoxia-tolerance of juvenile grass carp Ctenopharyngodon idella. Aquaculture 2016, 452, 388–394. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, B.; Chen, G.F.; Jiang, W.D.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.; Zhou, X. Effects of dietary histidine on antioxidant capacity in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Physiol. Biochem. 2013, 39, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Hsu, C.C.; Lin, M.H.; Liu, K.S.; Yin, M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Feng, L.; Qu, B.; Wu, P.; Kuang, S.Y.; Jiang, L.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-kB signaling in fish. Fish Shellfish Immunol. 2016, 56, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Hultin, H.O. Influence of histidine on lipid peroxidation in sarcoplasmic reticulum. Arch. Biochem. Biophys. 1992, 292, 427–432. [Google Scholar] [CrossRef]
- Wu, P.; Qu, B.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Tang, L.; Zhou, X.-Q.; Liu, Y. Dietary histidine deficiency induced flesh quality loss associated with changes in muscle nutritive composition, antioxidant capacity, Nrf2 and TOR signaling molecules in on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2020, 526, 735399. [Google Scholar] [CrossRef]
- Zheng, Z.; Shang, Y.; Tao, J.; Zhang, J.; Sha, B. Endoplasmic reticulum stress signaling pathways: Activation and diseases. Curr. Protein. Pept. Sci. 2019, 20, 935–943. [Google Scholar] [CrossRef]
- Cao, X.F.; Dai, Y.J.; Liu, M.Y.; Yuan, X.Y.; Wang, C.C.; Huang, Y.Y.; Liu, W.-B.; Jiang, G.-Z. High-fat diet induces aberrant hepatic lipid secretion in blunt snout bream by activating endoplasmic reticulum stress-associated IRE1/XBP1 pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 213–223. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.L.; Cao, L.P.; Feng, W.R.; He, Q.; Xu, P.; Yin, G. Chronic exposure of hydrogen peroxide alters redox state, apoptosis and endoplasmic reticulum stress in common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 229, 105657. [Google Scholar] [CrossRef]
- Xue, S.Q.; Lin, J.W.; Zhou, Q.; Wang, H.T.; Han, Y. Effect of ammonia stress on transcriptome and endoplasmic reticulum stress pathway for common carp (Cyprinus carpio) hepatopancreas. Aquacult. Rep. 2021, 20, 100694. [Google Scholar] [CrossRef]
- Li, H.Y.; Xu, W.J.; Wu, L.Y.; Dong, B.; Jin, J.Y.; Han, D.; Zhu, X.; Yang, Y.; Liu, H.; Xie, S. Differential regulation of endoplasmic reticulum stress-induced autophagy and apoptosis in two strains of gibel carp (Carassius gibelio) exposed to acute waterborne cadmium. Aquat. Toxicol. 2021, 231, 105721. [Google Scholar] [CrossRef]
- Xie, L.X.; Chen, S.Q.; Yao, C.R.; Li, D.P.; Li, L.; Tang, R. Nitrite induces endoplasmic reticulum stress and associates apoptosis of liver cells in grass carp (Ctenopharyngodon idella). Aquaculture 2019, 507, 275–281. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sierra, T.; Bellido, B.; Reyes-Fermín, L.M.; Martínez-Klimova, E.; Pedraza-Chaverri, J. Regulation of endoplasmic reticulum stress in models of kidney disease. Adv. Redox. Res. 2021, 3, 100010. [Google Scholar] [CrossRef]
- Liang, H.L.; Xu, G.C.; Xu, P.; Zhu, J.; Li, S.L.; Ren, M.C. Dietary histidine supplementation maintained amino acid homeostasis and reduced hepatic fat accumulation of juvenile largemouth bass, Mieropterus salmoides. Aquacult. Nutr. 2022, 2022, 4034922. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2003. [Google Scholar]
- Liang, H.L.; Ren, M.C.; Habte-Tsion, H.M.; Ge, X.P.; Xie, J.; Mi, H.F.; Xi, B.; Miao, L.; Liu, B.; Zhou, Q.; et al. Dietary arginine affects growth performance, plasma amino acid contents and gene expressions of the TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2016, 461, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.L.; He, G.L.; Jin, T.; Chen, Y.J.; Dai, F.Y.; Luo, L.; Lin, S.-M. High dietary starch impairs intestinal health and microbiota of largemouth bass, Micropterus salmoides. Aquaculture 2021, 534, 736261. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, J.; Chen, F.K.; Tang, X.H.; Liao, L.; Liu, Q.; Luo, J.; Du, Z.; Li, Z.; Luo, W.; et al. High carbohydrate diet induced endoplasmic reticulum stress and oxidative stress, promoted inflammation and apoptosis, impaired intestinal barrier of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 119, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Yu, H.H.; Liang, X.F.; Li, N.; Wang, X.; Li, F.H.; Wu, X.F.; Zheng, Y.H.; Xue, M.; Liang, X.F. Dietary butylated hydroxytoluene improves lipid metabolism, antioxidant and anti-apoptotic response of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2018, 72, 220–229. [Google Scholar] [CrossRef]
- Zhao, L.L.; Liang, J.; Liu, H.; Gong, C.X.; Huang, X.L.; Hu, Y.F.; Liu, Q.; He, Z.; Zhang, X.; Yang, S.; et al. Yinchenhao Decoction ameliorates the high-carbohydrate diet induced suppression of immune response in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 125, 141–151. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.W.; Hou, Y.J.; Guo, T.Y.; Fang, H.H.; Zhang, Y.M.; Liu, Y.; Tian, L.; Niu, J. Effects of dietary oxidized fish oil on growth performance, antioxidant defense system, apoptosis and mitochondrial function of juvenile largemouth bass (Micropterus salmoides). Aquaculture 2019, 500, 347–358. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Li, L.L.; Li, C.J.; Liu, E.G.; Hao, H.; Ling, Q.F. Heat stress-induced endoplasmic reticulum stress promotes liver apoptosis in largemouth bass (Micropterus salmoides). Aquaculture 2022, 546, 737401. [Google Scholar] [CrossRef]
- Liang, H.; Ji, K.; Ge, X.; Ren, M.; Liu, B.; Xi, B.; Pan, L. Effects of dietary arginine on antioxidant status and immunity involved in ampk-no signaling pathway in juvenile blunt snout bream. Fish Shellfish Immunol. 2018, 78, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Wen, H.L.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella): Avoid tryptophan deficiency or excess. Food Chem. 2016, 199, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Itoh, K.; Suzuki, T.; Osanai, H.; Nishikawa, K.; Katoh, Y.; Takagi, Y.; Yamamoto, M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells 2002, 7, 807–820. [Google Scholar] [CrossRef]
- Lemire, J.; Milandu, Y.; Auger, C.; Bignucolo, A.; Appanna, V.P.; Appanna, V.D. Histidine is a source of the antioxidant, α-ketoglutarate, in Pseudomonas fuorescens challenged by oxidative stress. FEMS Microbiol. Lett. 2010, 309, 170–177. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Ding, B.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Kang, P.; Yin, Y.; Wu, G. Alpha-Ketoglutarate and intestinal function. Front. Biosci. (Landmark Ed.) 2011, 16, 1186–1196. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Puiseux-Dao, S.; Appanna, V.D. Alphaketoglutarate abrogates the nuclear localization of HIF-1alpha in aluminum-exposed hepatocytes. Biochimie 2009, 91, 408–415. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Singh, R.; Brewer, G.; Auger, C.; Lemire, J.; Appanna, V.D. Alpha-ketoglutarate dehydrogenase and glutamate dehydrogenase work in tandem to modulate the antioxidant alpha-ketoglutarate during oxidative stress in Pseudomonas fluorescens. J. Bacteriol. 2009, 191, 3804–3810. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Wang, C.; Li, J.; Zhao, Z.; Luo, L.; Du, X.; Qiyou, X. Effects of α-ketoglutarate on the growth performance, amino acid metabolism and related gene expression of mirror carp (Cyprinus carpio). Aquac. Nutr. 2017, 23, 926–933. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhao, Y.R.; Jin, B.T.; Liang, J. Effects of dietary alpha-ketoglutarate supplementation on growth and serum biochemical parameters of grass carp (Ctenopharyngodon idella) fingerlings. Isr. J. Aquacult. Bamidgeh. 2016, 68, 1–6. [Google Scholar]
- Lin, X.; Jin, B.T.; Wang, H.Q.; Zhao, Y.R. Effects of diet α-ketoglutarate (AKG) supplementation on the growth performance, antioxidant defense system, intestinal digestive enzymes, and immune response of grass carp (Ctenopharyngodon idellus). Aquacult. Int. 2020, 28, 511–524. [Google Scholar] [CrossRef]
- Wang, L.S.; Xu, Q.Y.; Wang, C.A.; Li, J.N.; Chen, D.; Zhao, Z.G.; Luo, L.; Du, X. Effects of dietary α-ketoglutarate supplementation on the antioxidant defense system and HSP 70 and HSP 90 gene expression of hybrid sturgeon Acipenser schrenckii ♀ × A. baerii ♂ exposed to ammonia-N stress. Aquac. Res. 2017, 48, 2266–2277. [Google Scholar] [CrossRef]
- Wu, D.; Fan, Z.; Li, J.N.; Zhang, Y.Y.; Wang, C.A.; Xu, Q.Y.; Wang, L. Evaluation of Alpha-Ketoglutarate Supplementation on the Improvement of Intestinal Antioxidant Capacity and Immune Response in Songpu Mirror Carp (Cyprinus carpio) After Infection With Aeromonas hydrophila. Front. Immunol. 2021, 12, 690234. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, Z.; Li, J.N.; Zhang, Y.Y.; Xu, Q.Y.; Wang, L.; Wang, L.S. Low Protein Diets Supplemented With Alpha-Ketoglutarate Enhance the Growth Performance, Immune Response, and Intestinal Health in Common Carp (Cyprinus carpio). Front. Immunol. 2022, 13, 915657. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, H.L.; Mokrani, A.; Ji, K.; Yu, H.; Ge, X.P.; Ren, M.; Xie, J.; Pan, L.; Sun, A. Dietary histidine affects intestinal antioxidant enzyme activities, antioxidant gene expressions and inflammatory factors in juvenile blunt snout bream (Megalobrama amblycephala). Aquacult. Nutr. 2019, 25, 249–259. [Google Scholar] [CrossRef]
- Merle, N.S.; Elizabeth, C.S.; Veronique, F.B.; Roumenina, L.T. Complement system part I-molecular mechanisms of activation and regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Thom, V.; Arumugam, T.V.; Tim, M.; Gelderblom, M. Therapeutic potential of intravenous immunoglobulin in acute brain injury. Front. Immunol. 2017, 8, 875. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Ogawa, E.; Kurohane, K.; Konishi, H.; Mochizuki, N.; Manabe, K.; Imai, Y. Adjuvant effect of short chain triacylglycerol tributyrin on a mouse contact hypersensitivity model. Toxicol. Lett. 2018, 284, 56–62. [Google Scholar] [CrossRef]
- Dodds, M.W.; Law, S.K. The phylogeny and evolution of the thioester bond-containing proteins C3, C4 and alpha 2-macroglobulin. Immunol. Rev. 1998, 166, 15–26. [Google Scholar] [CrossRef]
- Li, M.Q.; Baumeister, P.; Roy, B.; Phan, T.; Foti, D.; Luo, S.Z.; Lee, A.S. AFT6 as a transcription activator of the endoplasmic reticulum stress element: Thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol. Cell. Biol. 2000, 20, 5096–5106. [Google Scholar] [CrossRef]
- Gass, J.N.; Gifford, N.M.; Brewer, J.W. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J. Biol. Chem. 2002, 277, 49047–49054. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Brewer, J.W.; Diehl, J.A.; Hendershot, L.M. Two distinct stress signaling pathways converge upon the chop promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002, 318, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, M.T.; Meise, R.; Aasland, D.; Berte, N.; Kitzinger, R.; Krämer, O.H.; Kaina, B.; Christmann, M. Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-jun-mediated induction of the BH3-only protein BIM. Oncotarget 2015, 6, 33755–33768. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; Sanket, M.; et al. Endoplasmic reticulum stress signalling-from basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Wang, Y.; Tan, R. Anti-Neuroinflammatory effect of Alantolactone through the suppression of the NF-κB and MAPK signaling pathways. Cells 2019, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liang, H.; Ren, M.; Ge, X.; Mi, H.; Pan, L.; Yu, H. The immunoreaction and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) involves the PI3K/Akt/Nrf2 and NF-κB signal pathways in response to dietary methionine levels. Fish shellfish Immun. 2020, 105, 126–134. [Google Scholar] [CrossRef] [PubMed]






| Ingredient | Diet 1 (%) | Diet 2 (%) | Diet 3 (%) | Diet 4 (%) | Diet 5 (%) | Diet 6 (%) |
|---|---|---|---|---|---|---|
| Fish meal 2 | 30 | 30 | 30 | 30 | 30 | 30 |
| Rapeseed meal 2 | 8 | 8 | 8 | 8 | 8 | 8 |
| Soybean meal 2 | 10 | 10 | 10 | 10 | 10 | 10 |
| Wheat meal 2 | 16 | 16 | 16 | 16 | 16 | 16 |
| Fish oil | 5 | 5 | 5 | 5 | 5 | 5 |
| Sleeve-fish ointment | 2 | 2 | 2 | 2 | 2 | 2 |
| Amino acid mixes 3 | 13.21 | 13.21 | 13.21 | 13.21 | 13.21 | 13.21 |
| Choline chloride | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Vitamin premix 4 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mineral premix 4 | 1 | 1 | 1 | 1 | 1 | 1 |
| Monocalcium phosphate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Microcrystalline cellulose | 3.18 | 3.18 | 3.18 | 3.18 | 3.18 | 3.18 |
| Rice bran | 7 | 7 | 7 | 7 | 7 | 7 |
| Ethoxylquinine | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Glycine | 1 | 0.8 | 0.6 | 0.4 | 0.2 | 0 |
| L-histidine | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1 |
| Compositional analysis (dry matter) | ||||||
| Crude protein (%) | 46.93 | 47.01 | 47.11 | 46.83 | 46.91 | 47.03 |
| Crude lipid (%) | 11.02 | 10.98 | 10.94 | 11.01 | 10.97 | 10.99 |
| Energy (MJ/kg) | 18.87 | 18.85 | 18.83 | 18.85 | 18.91 | 18.88 |
| Histidine levels (%) | 0.71 | 0.89 | 1.08 | 1.26 | 1.48 | 1.67 |
| Gene Name | Forward Sequence | Reverse Sequence | Amplification Efficiency (%) | Source |
|---|---|---|---|---|
| nrf2 | CCACACGTGACTCTGATTTCTC | TCCTCCATGACCTTGAAGCAT | 102.5 | [27] |
| keap1 | GCACCTAACCGTGGAACTCAA | CCAGTTTTAGCCAGTCATTGTTCC | 99.8 | |
| cat | TGGTGTTCACGGATGAGATGG | GGAGAAGCGGACAGCAATAGG | 98.6 | |
| sod | CCACCAGAGGTCTCACAGCA | CCACTGAACCGAAGAAGGACT | 101.2 | |
| gpx | CCCTGCAATCAGTTTGGACA | TTGGTTCAAAGCCATTCCCT | 102.5 | [28] |
| nf-κb | CCACTCAGGTGTTGGAGCTT | TCCAGAGCACGACACACTTC | 100.8 | XP_027136364.1 |
| tnf-α | CTTCGTCTACAGCCAGGCATCG | TTTGGCACACCGACCTCACC | 99.9 | [29] |
| il-8 | CGTTGAACAGACTGGGAGAGATG | AGTGGGATGGCTTCATTATCTTGT | 103.6 | |
| il-1β | CGTGACTGACAGCAAAAAGAGG | GATGCCCAGAGCCACAGTTC | 103.4 | |
| il-10 | CGGCACAGAAATCCCAGAGC | CAGCAGGCTCACAAAATAAACATCT | 101.1 | |
| tgf-β1 | GCTCAAAGAGAGCGAGGATG | TCCTCTACCATTCGCAATCC | 98.5 | |
| hepcidin1 | CATTCACCGGGGTGCAA | CCTGATGTGATTTGGCATCATC | 99.4 | [28] |
| cox2 | CACTGGGTCGTGTCACTTT | TGATTCTCCTCCTTGCTGT | 101.3 | [30] |
| cd80 | TCTTCATCGTGGTAATAATAGG | TGTGGTGTCTTCAGGGTCT | 98.9 | |
| cd83 | CACTGTTGTGCCTTGCTG | GGAGCCTCTTTGACCTTGT | 99.8 | |
| ikbα | CCCCAACTACAGTGGACAAA | AAGGTCAAGGAGGCAACG | 103.1 | |
| caspase 3 | GAGGCGATGGACAAGAGTCA | CACAGACGAATGAAGCGTGG | 99.8 | XM_038713063.1 |
| bcl-xl | CATCCTCCTTGGCTCTGG | GGGTCTGTTTGCCTTTGG | 103.5 | [31] |
| caspase 8 | GAGACAGACAGCAGACAACCA | TTCCATTTCAGCAAACACATC | 101.8 | [28] |
| caspase 9 | CTGGAATGCCTTCAGGAGACGGG | GGGAGGGGCAAGACAACAGGGTG | 99.7 | |
| bcl-2 | CGCCATCCACAGAGTCCT | CCGGAACAGTTCGTCTATCACC | 101.1 | [30] |
| bax | ACTTTGGATTACCTGCGGGA | TGCCAGAAATCAGGAGCAGA | 101.9 | |
| mlkl | CCCAAGCCTCAGTTCCTC | TTTCTTCGGTCTGGTGCA | 102.1 | |
| tnrf1a | GCATACCCAGAATGTGAGA | CATAACCGCCACGACTAA | 99.3 | |
| ripk3 | GTTTAGGGCAGGAGGTGA | TTCTGAGTTTCCCAATGTTT | 99.7 | |
| eif2α | CCTCGTTTGTCCGTCTGTATC | GCTGACTCTGTCGGCCTTG | 101.2 | [28] |
| traf2 | CTGCCAAACCTTAATCCTT | ACAGACTTACAGCCCACTTC | 99.5 | |
| xbp1 | ACACCCTCGACACGAAAGA | AGAATGCCCAGTAGCAAATC | 98.9 | [30] |
| grp78 | TTGCCGATGACGACGAAA | CAATCAGACGCTCACCCT | 102.1 | |
| chopα | GATGAGCAGCCTAAGCCACG | AACAGGTCAGCCAAGAAGTCG | 101.5 | |
| perk | CCACCGCAGAGCAGATGTAA | TGCTGGAGTCATCCTACCGA | 102.7 | [32] |
| ask1 | CAACTACGCCTTCATCCCGT | GGTCCCAACAGCATCTCGAA | 99.7 | |
| ire1 | CTGCCAGATCCGCATACACT | GGTGTCCACTCTTGAAGGCA | 98.5 | |
| atf6 | GACGCCCCGCATAAGAGTAA | GCAGACTTGAGGAGAGCTGG | 101.6 | |
| jnk1 | TGCACTACCTGAGCCACTTG | TGTGCTTCCTGGCTGATGTT | 100.3 | XM_038735152.1 |
| atf4 | GCGGACATTTGTGTTGCACT | CTGTCCTGCCAGGTGATGAA | 99.2 | XM_038712790.1 |
| gapdh | ACTGTCACTCCTCCATCTT | CACGGTTGCTGTATCCAA | AZA04761.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Xu, P.; Xu, G.; Zhang, L.; Huang, D.; Ren, M.; Zhang, L. Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants 2022, 11, 2399. https://doi.org/10.3390/antiox11122399
Liang H, Xu P, Xu G, Zhang L, Huang D, Ren M, Zhang L. Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants. 2022; 11(12):2399. https://doi.org/10.3390/antiox11122399
Chicago/Turabian StyleLiang, Hualiang, Pao Xu, Gangchun Xu, Lin Zhang, Dongyu Huang, Mingchun Ren, and Lu Zhang. 2022. "Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides)" Antioxidants 11, no. 12: 2399. https://doi.org/10.3390/antiox11122399
APA StyleLiang, H., Xu, P., Xu, G., Zhang, L., Huang, D., Ren, M., & Zhang, L. (2022). Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants, 11(12), 2399. https://doi.org/10.3390/antiox11122399








