Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diet
2.3. Breeding Experiment
2.4. Sample Collection
2.5. Chemical Analysis, RNA Extraction, and Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
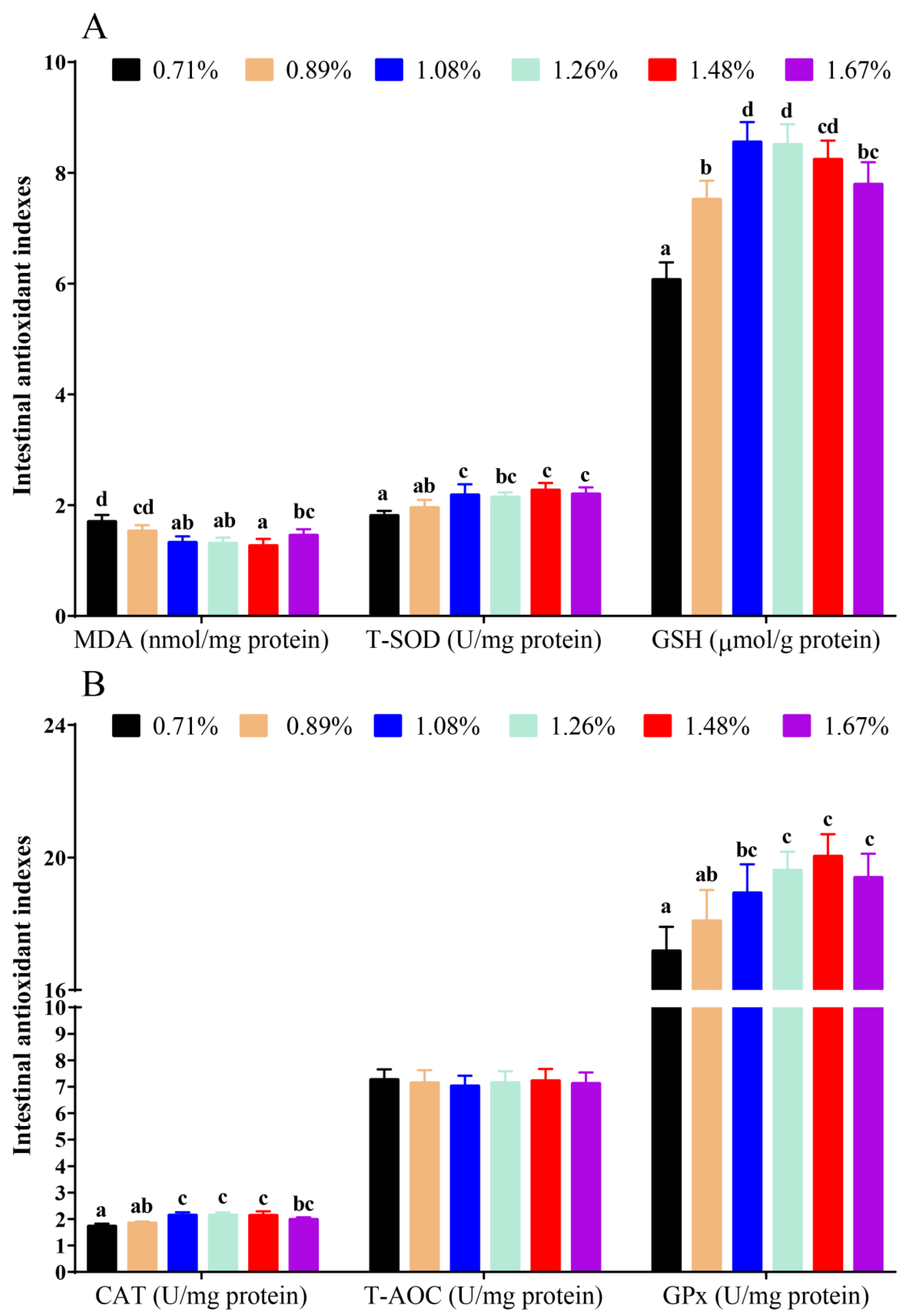
3.1. Intestinal Antioxidant Status
3.2. Intestinal Immune Cytokines
3.3. The mRNA Levels of the Key Genes in the Nrf2-Signalling Pathway
3.4. The mRNA Levels of Inflammatory-Related Genes
3.5. The mRNA Levels of Apoptosis and Necroptosis Genes
3.6. The mRNA Levels of ERS-Related Genes
4. Discussion
4.1. Histidine Deficiency Inhibited Intestinal Antioxidant Capacity
4.2. Histidine Deficiency Suppressed Intestinal Immunocompetence
4.3. Histidine Deficiency Induces Intestinal Endoplasmic-Reticulum Stress Resulting in Inflammatory Response, Apoptosis, and Necroptosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Ran, C.; Teame, T.; Ding, Q.; Hoseinifar, S.H.; Xie, M.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Gatlin, D.M., III; et al. Research progress on gut health of farmers teleost fish: A viewpoint concerning the intestinal mucosal barrier and the impact of its damage. Rev. Fish Biol. Fisher. 2020, 30, 569–586. [Google Scholar] [CrossRef]
- Dawood, M.A. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquacult. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Takahashi, I.; Kiyono, H. Gut as the largest immunologic tissue. J. Parenter. Enter. Nutr. 1999, 23, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Qu, B.; Feng, L.; Jiang, J.; Kuang, S.; Wu, P.; Tang, L.; Tang, W.-N.; Zhang, Y.A.; Zhou, X.-Q.; et al. Histidine prevents cu-induced oxidative stress and the associated decreases in mRNA from encoding tight junction proteins in the intestine of grass carp (Ctenopharyngodon idella). PLoS ONE 2016, 11, e157001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.Y.; Maulu, S.; Ren, M.C.; Liang, H.L.; Ge, X.P.; Ji, K.; Yu, H. Dietary lysine levels improved antioxidant capacity and immunity via the TOR and p38 MAPK signaling pathways in grass carp, Ctenopharyngodon idellus fry. Front. Immunol. 2021, 12, 635015. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ji, K.; Ge, X.; Xi, B.; Chen, X. Tributyrin plays an important role in regulating the growth and health status of juvenile blunt snout bream (Megalobrama amblycephala), as evidenced by pathological examination. Front. Immunol. 2021, 12, 652294. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kiyono, H. Vitamin-mediated regulation of intestinal immunity. Front. Immunol. 2013, 4, 189. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Zhou, X.-Q. The regulatory effects of pyridoxine deficiency on the grass carp (Ctenopharyngodon idella) gill barriers immunity, apoptosis, antioxidant, and tight junction challenged with flavobacterium columnar. Fish Shellfish Immunol. 2020, 105, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Chen, X.; Yang, H.; Li, X.; Tian, L. Effects of graded levels of histidine on growth performance, digested enzymes activities, erythrocyte osmotic fragility and hypoxia-tolerance of juvenile grass carp Ctenopharyngodon idella. Aquaculture 2016, 452, 388–394. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, B.; Chen, G.F.; Jiang, W.D.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.; Zhou, X. Effects of dietary histidine on antioxidant capacity in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Physiol. Biochem. 2013, 39, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Hsu, C.C.; Lin, M.H.; Liu, K.S.; Yin, M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Feng, L.; Qu, B.; Wu, P.; Kuang, S.Y.; Jiang, L.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-kB signaling in fish. Fish Shellfish Immunol. 2016, 56, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Hultin, H.O. Influence of histidine on lipid peroxidation in sarcoplasmic reticulum. Arch. Biochem. Biophys. 1992, 292, 427–432. [Google Scholar] [CrossRef]
- Wu, P.; Qu, B.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Tang, L.; Zhou, X.-Q.; Liu, Y. Dietary histidine deficiency induced flesh quality loss associated with changes in muscle nutritive composition, antioxidant capacity, Nrf2 and TOR signaling molecules in on-growing grass carp (Ctenopharyngodon idella). Aquaculture 2020, 526, 735399. [Google Scholar] [CrossRef]
- Zheng, Z.; Shang, Y.; Tao, J.; Zhang, J.; Sha, B. Endoplasmic reticulum stress signaling pathways: Activation and diseases. Curr. Protein. Pept. Sci. 2019, 20, 935–943. [Google Scholar] [CrossRef]
- Cao, X.F.; Dai, Y.J.; Liu, M.Y.; Yuan, X.Y.; Wang, C.C.; Huang, Y.Y.; Liu, W.-B.; Jiang, G.-Z. High-fat diet induces aberrant hepatic lipid secretion in blunt snout bream by activating endoplasmic reticulum stress-associated IRE1/XBP1 pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 213–223. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.L.; Cao, L.P.; Feng, W.R.; He, Q.; Xu, P.; Yin, G. Chronic exposure of hydrogen peroxide alters redox state, apoptosis and endoplasmic reticulum stress in common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 229, 105657. [Google Scholar] [CrossRef]
- Xue, S.Q.; Lin, J.W.; Zhou, Q.; Wang, H.T.; Han, Y. Effect of ammonia stress on transcriptome and endoplasmic reticulum stress pathway for common carp (Cyprinus carpio) hepatopancreas. Aquacult. Rep. 2021, 20, 100694. [Google Scholar] [CrossRef]
- Li, H.Y.; Xu, W.J.; Wu, L.Y.; Dong, B.; Jin, J.Y.; Han, D.; Zhu, X.; Yang, Y.; Liu, H.; Xie, S. Differential regulation of endoplasmic reticulum stress-induced autophagy and apoptosis in two strains of gibel carp (Carassius gibelio) exposed to acute waterborne cadmium. Aquat. Toxicol. 2021, 231, 105721. [Google Scholar] [CrossRef]
- Xie, L.X.; Chen, S.Q.; Yao, C.R.; Li, D.P.; Li, L.; Tang, R. Nitrite induces endoplasmic reticulum stress and associates apoptosis of liver cells in grass carp (Ctenopharyngodon idella). Aquaculture 2019, 507, 275–281. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sierra, T.; Bellido, B.; Reyes-Fermín, L.M.; Martínez-Klimova, E.; Pedraza-Chaverri, J. Regulation of endoplasmic reticulum stress in models of kidney disease. Adv. Redox. Res. 2021, 3, 100010. [Google Scholar] [CrossRef]
- Liang, H.L.; Xu, G.C.; Xu, P.; Zhu, J.; Li, S.L.; Ren, M.C. Dietary histidine supplementation maintained amino acid homeostasis and reduced hepatic fat accumulation of juvenile largemouth bass, Mieropterus salmoides. Aquacult. Nutr. 2022, 2022, 4034922. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2003. [Google Scholar]
- Liang, H.L.; Ren, M.C.; Habte-Tsion, H.M.; Ge, X.P.; Xie, J.; Mi, H.F.; Xi, B.; Miao, L.; Liu, B.; Zhou, Q.; et al. Dietary arginine affects growth performance, plasma amino acid contents and gene expressions of the TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2016, 461, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.L.; He, G.L.; Jin, T.; Chen, Y.J.; Dai, F.Y.; Luo, L.; Lin, S.-M. High dietary starch impairs intestinal health and microbiota of largemouth bass, Micropterus salmoides. Aquaculture 2021, 534, 736261. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, J.; Chen, F.K.; Tang, X.H.; Liao, L.; Liu, Q.; Luo, J.; Du, Z.; Li, Z.; Luo, W.; et al. High carbohydrate diet induced endoplasmic reticulum stress and oxidative stress, promoted inflammation and apoptosis, impaired intestinal barrier of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 119, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Yu, H.H.; Liang, X.F.; Li, N.; Wang, X.; Li, F.H.; Wu, X.F.; Zheng, Y.H.; Xue, M.; Liang, X.F. Dietary butylated hydroxytoluene improves lipid metabolism, antioxidant and anti-apoptotic response of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2018, 72, 220–229. [Google Scholar] [CrossRef]
- Zhao, L.L.; Liang, J.; Liu, H.; Gong, C.X.; Huang, X.L.; Hu, Y.F.; Liu, Q.; He, Z.; Zhang, X.; Yang, S.; et al. Yinchenhao Decoction ameliorates the high-carbohydrate diet induced suppression of immune response in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 125, 141–151. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.W.; Hou, Y.J.; Guo, T.Y.; Fang, H.H.; Zhang, Y.M.; Liu, Y.; Tian, L.; Niu, J. Effects of dietary oxidized fish oil on growth performance, antioxidant defense system, apoptosis and mitochondrial function of juvenile largemouth bass (Micropterus salmoides). Aquaculture 2019, 500, 347–358. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Li, L.L.; Li, C.J.; Liu, E.G.; Hao, H.; Ling, Q.F. Heat stress-induced endoplasmic reticulum stress promotes liver apoptosis in largemouth bass (Micropterus salmoides). Aquaculture 2022, 546, 737401. [Google Scholar] [CrossRef]
- Liang, H.; Ji, K.; Ge, X.; Ren, M.; Liu, B.; Xi, B.; Pan, L. Effects of dietary arginine on antioxidant status and immunity involved in ampk-no signaling pathway in juvenile blunt snout bream. Fish Shellfish Immunol. 2018, 78, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Wen, H.L.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella): Avoid tryptophan deficiency or excess. Food Chem. 2016, 199, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Itoh, K.; Suzuki, T.; Osanai, H.; Nishikawa, K.; Katoh, Y.; Takagi, Y.; Yamamoto, M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells 2002, 7, 807–820. [Google Scholar] [CrossRef]
- Lemire, J.; Milandu, Y.; Auger, C.; Bignucolo, A.; Appanna, V.P.; Appanna, V.D. Histidine is a source of the antioxidant, α-ketoglutarate, in Pseudomonas fuorescens challenged by oxidative stress. FEMS Microbiol. Lett. 2010, 309, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wang, L.; Ding, B.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Kang, P.; Yin, Y.; Wu, G. Alpha-Ketoglutarate and intestinal function. Front. Biosci. (Landmark Ed.) 2011, 16, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Mailloux, R.J.; Puiseux-Dao, S.; Appanna, V.D. Alphaketoglutarate abrogates the nuclear localization of HIF-1alpha in aluminum-exposed hepatocytes. Biochimie 2009, 91, 408–415. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Singh, R.; Brewer, G.; Auger, C.; Lemire, J.; Appanna, V.D. Alpha-ketoglutarate dehydrogenase and glutamate dehydrogenase work in tandem to modulate the antioxidant alpha-ketoglutarate during oxidative stress in Pseudomonas fluorescens. J. Bacteriol. 2009, 191, 3804–3810. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wei, Y.; Wang, C.; Li, J.; Zhao, Z.; Luo, L.; Du, X.; Qiyou, X. Effects of α-ketoglutarate on the growth performance, amino acid metabolism and related gene expression of mirror carp (Cyprinus carpio). Aquac. Nutr. 2017, 23, 926–933. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhao, Y.R.; Jin, B.T.; Liang, J. Effects of dietary alpha-ketoglutarate supplementation on growth and serum biochemical parameters of grass carp (Ctenopharyngodon idella) fingerlings. Isr. J. Aquacult. Bamidgeh. 2016, 68, 1–6. [Google Scholar]
- Lin, X.; Jin, B.T.; Wang, H.Q.; Zhao, Y.R. Effects of diet α-ketoglutarate (AKG) supplementation on the growth performance, antioxidant defense system, intestinal digestive enzymes, and immune response of grass carp (Ctenopharyngodon idellus). Aquacult. Int. 2020, 28, 511–524. [Google Scholar] [CrossRef]
- Wang, L.S.; Xu, Q.Y.; Wang, C.A.; Li, J.N.; Chen, D.; Zhao, Z.G.; Luo, L.; Du, X. Effects of dietary α-ketoglutarate supplementation on the antioxidant defense system and HSP 70 and HSP 90 gene expression of hybrid sturgeon Acipenser schrenckii ♀ × A. baerii ♂ exposed to ammonia-N stress. Aquac. Res. 2017, 48, 2266–2277. [Google Scholar] [CrossRef]
- Wu, D.; Fan, Z.; Li, J.N.; Zhang, Y.Y.; Wang, C.A.; Xu, Q.Y.; Wang, L. Evaluation of Alpha-Ketoglutarate Supplementation on the Improvement of Intestinal Antioxidant Capacity and Immune Response in Songpu Mirror Carp (Cyprinus carpio) After Infection With Aeromonas hydrophila. Front. Immunol. 2021, 12, 690234. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, Z.; Li, J.N.; Zhang, Y.Y.; Xu, Q.Y.; Wang, L.; Wang, L.S. Low Protein Diets Supplemented With Alpha-Ketoglutarate Enhance the Growth Performance, Immune Response, and Intestinal Health in Common Carp (Cyprinus carpio). Front. Immunol. 2022, 13, 915657. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, H.L.; Mokrani, A.; Ji, K.; Yu, H.; Ge, X.P.; Ren, M.; Xie, J.; Pan, L.; Sun, A. Dietary histidine affects intestinal antioxidant enzyme activities, antioxidant gene expressions and inflammatory factors in juvenile blunt snout bream (Megalobrama amblycephala). Aquacult. Nutr. 2019, 25, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Merle, N.S.; Elizabeth, C.S.; Veronique, F.B.; Roumenina, L.T. Complement system part I-molecular mechanisms of activation and regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thom, V.; Arumugam, T.V.; Tim, M.; Gelderblom, M. Therapeutic potential of intravenous immunoglobulin in acute brain injury. Front. Immunol. 2017, 8, 875. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, K.; Ogawa, E.; Kurohane, K.; Konishi, H.; Mochizuki, N.; Manabe, K.; Imai, Y. Adjuvant effect of short chain triacylglycerol tributyrin on a mouse contact hypersensitivity model. Toxicol. Lett. 2018, 284, 56–62. [Google Scholar] [CrossRef]
- Dodds, M.W.; Law, S.K. The phylogeny and evolution of the thioester bond-containing proteins C3, C4 and alpha 2-macroglobulin. Immunol. Rev. 1998, 166, 15–26. [Google Scholar] [CrossRef]
- Li, M.Q.; Baumeister, P.; Roy, B.; Phan, T.; Foti, D.; Luo, S.Z.; Lee, A.S. AFT6 as a transcription activator of the endoplasmic reticulum stress element: Thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol. Cell. Biol. 2000, 20, 5096–5106. [Google Scholar] [CrossRef] [Green Version]
- Gass, J.N.; Gifford, N.M.; Brewer, J.W. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J. Biol. Chem. 2002, 277, 49047–49054. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Brewer, J.W.; Diehl, J.A.; Hendershot, L.M. Two distinct stress signaling pathways converge upon the chop promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002, 318, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, M.T.; Meise, R.; Aasland, D.; Berte, N.; Kitzinger, R.; Krämer, O.H.; Kaina, B.; Christmann, M. Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-jun-mediated induction of the BH3-only protein BIM. Oncotarget 2015, 6, 33755–33768. [Google Scholar] [CrossRef] [Green Version]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; Sanket, M.; et al. Endoplasmic reticulum stress signalling-from basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Li, J.; Wang, Y.; Tan, R. Anti-Neuroinflammatory effect of Alantolactone through the suppression of the NF-κB and MAPK signaling pathways. Cells 2019, 8, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, K.; Liang, H.; Ren, M.; Ge, X.; Mi, H.; Pan, L.; Yu, H. The immunoreaction and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) involves the PI3K/Akt/Nrf2 and NF-κB signal pathways in response to dietary methionine levels. Fish shellfish Immun. 2020, 105, 126–134. [Google Scholar] [CrossRef] [PubMed]






| Ingredient | Diet 1 (%) | Diet 2 (%) | Diet 3 (%) | Diet 4 (%) | Diet 5 (%) | Diet 6 (%) |
|---|---|---|---|---|---|---|
| Fish meal 2 | 30 | 30 | 30 | 30 | 30 | 30 |
| Rapeseed meal 2 | 8 | 8 | 8 | 8 | 8 | 8 |
| Soybean meal 2 | 10 | 10 | 10 | 10 | 10 | 10 |
| Wheat meal 2 | 16 | 16 | 16 | 16 | 16 | 16 |
| Fish oil | 5 | 5 | 5 | 5 | 5 | 5 |
| Sleeve-fish ointment | 2 | 2 | 2 | 2 | 2 | 2 |
| Amino acid mixes 3 | 13.21 | 13.21 | 13.21 | 13.21 | 13.21 | 13.21 |
| Choline chloride | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Vitamin premix 4 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mineral premix 4 | 1 | 1 | 1 | 1 | 1 | 1 |
| Monocalcium phosphate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Microcrystalline cellulose | 3.18 | 3.18 | 3.18 | 3.18 | 3.18 | 3.18 |
| Rice bran | 7 | 7 | 7 | 7 | 7 | 7 |
| Ethoxylquinine | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Glycine | 1 | 0.8 | 0.6 | 0.4 | 0.2 | 0 |
| L-histidine | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1 |
| Compositional analysis (dry matter) | ||||||
| Crude protein (%) | 46.93 | 47.01 | 47.11 | 46.83 | 46.91 | 47.03 |
| Crude lipid (%) | 11.02 | 10.98 | 10.94 | 11.01 | 10.97 | 10.99 |
| Energy (MJ/kg) | 18.87 | 18.85 | 18.83 | 18.85 | 18.91 | 18.88 |
| Histidine levels (%) | 0.71 | 0.89 | 1.08 | 1.26 | 1.48 | 1.67 |
| Gene Name | Forward Sequence | Reverse Sequence | Amplification Efficiency (%) | Source |
|---|---|---|---|---|
| nrf2 | CCACACGTGACTCTGATTTCTC | TCCTCCATGACCTTGAAGCAT | 102.5 | [27] |
| keap1 | GCACCTAACCGTGGAACTCAA | CCAGTTTTAGCCAGTCATTGTTCC | 99.8 | |
| cat | TGGTGTTCACGGATGAGATGG | GGAGAAGCGGACAGCAATAGG | 98.6 | |
| sod | CCACCAGAGGTCTCACAGCA | CCACTGAACCGAAGAAGGACT | 101.2 | |
| gpx | CCCTGCAATCAGTTTGGACA | TTGGTTCAAAGCCATTCCCT | 102.5 | [28] |
| nf-κb | CCACTCAGGTGTTGGAGCTT | TCCAGAGCACGACACACTTC | 100.8 | XP_027136364.1 |
| tnf-α | CTTCGTCTACAGCCAGGCATCG | TTTGGCACACCGACCTCACC | 99.9 | [29] |
| il-8 | CGTTGAACAGACTGGGAGAGATG | AGTGGGATGGCTTCATTATCTTGT | 103.6 | |
| il-1β | CGTGACTGACAGCAAAAAGAGG | GATGCCCAGAGCCACAGTTC | 103.4 | |
| il-10 | CGGCACAGAAATCCCAGAGC | CAGCAGGCTCACAAAATAAACATCT | 101.1 | |
| tgf-β1 | GCTCAAAGAGAGCGAGGATG | TCCTCTACCATTCGCAATCC | 98.5 | |
| hepcidin1 | CATTCACCGGGGTGCAA | CCTGATGTGATTTGGCATCATC | 99.4 | [28] |
| cox2 | CACTGGGTCGTGTCACTTT | TGATTCTCCTCCTTGCTGT | 101.3 | [30] |
| cd80 | TCTTCATCGTGGTAATAATAGG | TGTGGTGTCTTCAGGGTCT | 98.9 | |
| cd83 | CACTGTTGTGCCTTGCTG | GGAGCCTCTTTGACCTTGT | 99.8 | |
| ikbα | CCCCAACTACAGTGGACAAA | AAGGTCAAGGAGGCAACG | 103.1 | |
| caspase 3 | GAGGCGATGGACAAGAGTCA | CACAGACGAATGAAGCGTGG | 99.8 | XM_038713063.1 |
| bcl-xl | CATCCTCCTTGGCTCTGG | GGGTCTGTTTGCCTTTGG | 103.5 | [31] |
| caspase 8 | GAGACAGACAGCAGACAACCA | TTCCATTTCAGCAAACACATC | 101.8 | [28] |
| caspase 9 | CTGGAATGCCTTCAGGAGACGGG | GGGAGGGGCAAGACAACAGGGTG | 99.7 | |
| bcl-2 | CGCCATCCACAGAGTCCT | CCGGAACAGTTCGTCTATCACC | 101.1 | [30] |
| bax | ACTTTGGATTACCTGCGGGA | TGCCAGAAATCAGGAGCAGA | 101.9 | |
| mlkl | CCCAAGCCTCAGTTCCTC | TTTCTTCGGTCTGGTGCA | 102.1 | |
| tnrf1a | GCATACCCAGAATGTGAGA | CATAACCGCCACGACTAA | 99.3 | |
| ripk3 | GTTTAGGGCAGGAGGTGA | TTCTGAGTTTCCCAATGTTT | 99.7 | |
| eif2α | CCTCGTTTGTCCGTCTGTATC | GCTGACTCTGTCGGCCTTG | 101.2 | [28] |
| traf2 | CTGCCAAACCTTAATCCTT | ACAGACTTACAGCCCACTTC | 99.5 | |
| xbp1 | ACACCCTCGACACGAAAGA | AGAATGCCCAGTAGCAAATC | 98.9 | [30] |
| grp78 | TTGCCGATGACGACGAAA | CAATCAGACGCTCACCCT | 102.1 | |
| chopα | GATGAGCAGCCTAAGCCACG | AACAGGTCAGCCAAGAAGTCG | 101.5 | |
| perk | CCACCGCAGAGCAGATGTAA | TGCTGGAGTCATCCTACCGA | 102.7 | [32] |
| ask1 | CAACTACGCCTTCATCCCGT | GGTCCCAACAGCATCTCGAA | 99.7 | |
| ire1 | CTGCCAGATCCGCATACACT | GGTGTCCACTCTTGAAGGCA | 98.5 | |
| atf6 | GACGCCCCGCATAAGAGTAA | GCAGACTTGAGGAGAGCTGG | 101.6 | |
| jnk1 | TGCACTACCTGAGCCACTTG | TGTGCTTCCTGGCTGATGTT | 100.3 | XM_038735152.1 |
| atf4 | GCGGACATTTGTGTTGCACT | CTGTCCTGCCAGGTGATGAA | 99.2 | XM_038712790.1 |
| gapdh | ACTGTCACTCCTCCATCTT | CACGGTTGCTGTATCCAA | AZA04761.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Xu, P.; Xu, G.; Zhang, L.; Huang, D.; Ren, M.; Zhang, L. Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants 2022, 11, 2399. https://doi.org/10.3390/antiox11122399
Liang H, Xu P, Xu G, Zhang L, Huang D, Ren M, Zhang L. Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants. 2022; 11(12):2399. https://doi.org/10.3390/antiox11122399
Chicago/Turabian StyleLiang, Hualiang, Pao Xu, Gangchun Xu, Lin Zhang, Dongyu Huang, Mingchun Ren, and Lu Zhang. 2022. "Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides)" Antioxidants 11, no. 12: 2399. https://doi.org/10.3390/antiox11122399
APA StyleLiang, H., Xu, P., Xu, G., Zhang, L., Huang, D., Ren, M., & Zhang, L. (2022). Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants, 11(12), 2399. https://doi.org/10.3390/antiox11122399








