The Role of Lead and Cadmium in Gynecological Malignancies
Abstract
:1. Introduction
2. Lead
2.1. Lead and Oxidative Stress
2.2. Lead and Carcinogenicity
3. Cadmium
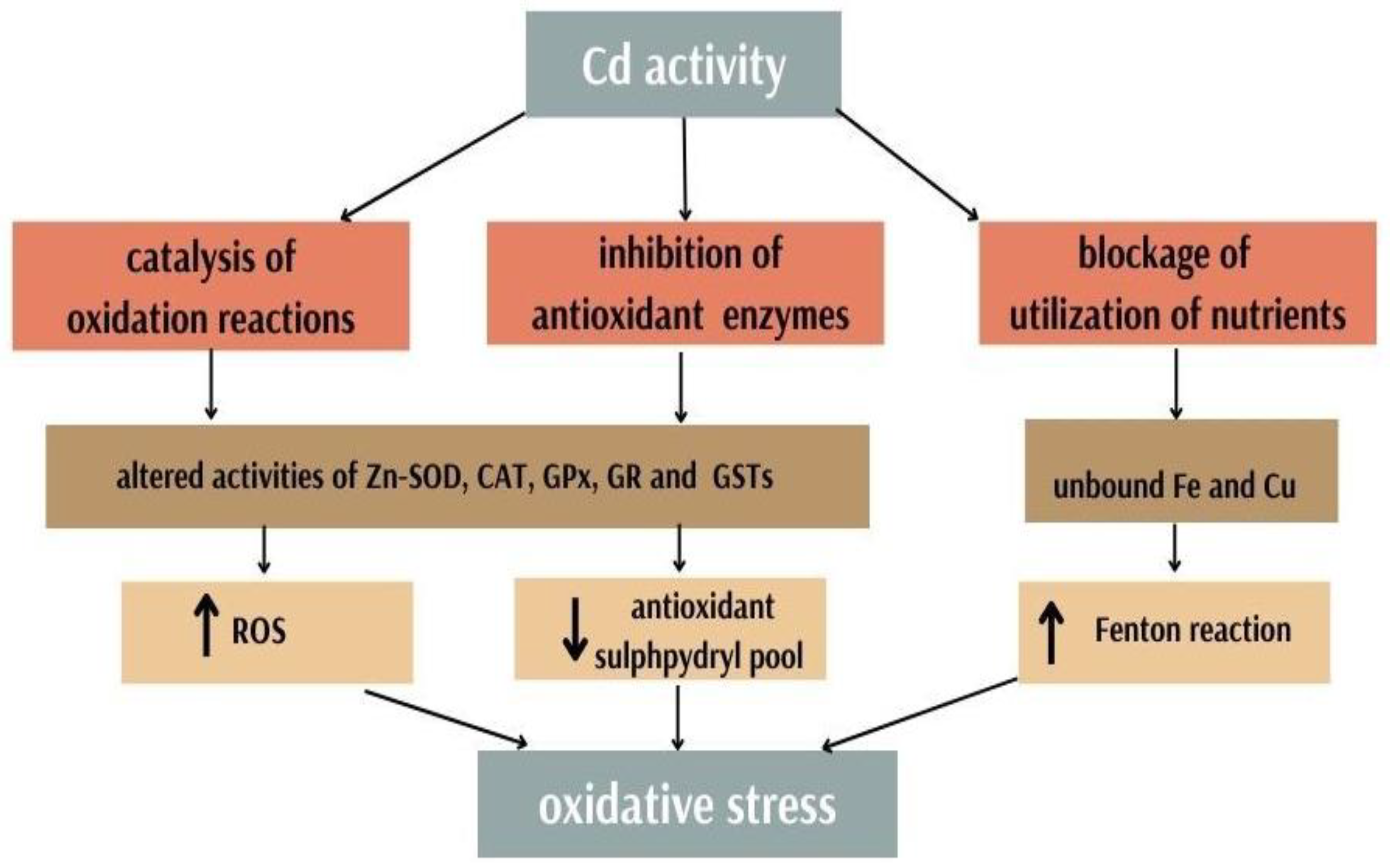
3.1. Cadmium and Zinc
3.2. Cadmium and Metalothioneins
3.3. Cadmium and Antioxidants
3.4. Cadmium and Cancerogecity
4. Lead, Cadmium and Gynaecological Malignancies
4.1. Cervical Cancer
4.2. Endometrial Cancer
4.3. Ovarian Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization; Food and Agriculture Organization of the United Nations; International Atomic Energy Agency. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996; p. 343.
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Duffus, J.H. ‘Heavy Metals’—A Meaningless Term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 1999–2001. [Google Scholar] [CrossRef] [Green Version]
- Szyczewski, P.; Siepak, J.; Niedzielski, P.; Sobczyński, T. Research on Heavy Metals in Poland. Pol. J. Environ. Stud. 2009, 18, 755–768. Available online: http://www.pjoes.com/Research-on-Heavy-Metals-in-Poland,88292,0,2.html (accessed on 10 October 2022).
- Callan, A.; Hinwood, A.; Devine, A. Metals in commonly eaten groceries in Western Australia: A market basket survey and dietary assessment. Food Addit. Contam. Part A 2014, 31, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Ojelum, A.L.; Bassey, F.I. A survey of metal profiles in some traditional alcoholic beverages in Nigeria. Food Sci. Nutr. 2014, 2, 724–733. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Kaczmarek, N.; Jurczak, T.; Klimaszyk, P. The multidisciplinary approach to safety and toxicity assessment of microalgae-based food supplements following clinical cases of poisoning. Harmful Algae 2015, 46, 34–42. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Rzymski, P.; Tomczyk, K.; Kozak, L.; Poniedzia1ek, B. Metal accumulation in the human uterus varies by pathology and smoking status. Fertil. Steril. 2016, 105, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Mínguez-Alarcón, L.; Mendiola, J.; Roca, M.; López-Espín, J.J.; Guillén, J.J.; Moreno, J.M.; Moreno-Grau, S.; Martínez-García, M.J.; Vergara-Juárez, N.; Elvira-Rendueles, B.; et al. Correlations between Different Heavy Metals in Diverse Body Fluids: Studies of Human Semen Quality. Adv. Urol. 2012, 2012, 420893. [Google Scholar] [CrossRef] [Green Version]
- Canaz, E.; Kilinc, M.; Sayar, H.; Kiran, G.; Ozyurek, E. Lead, selenium and nickel concentrations in epithelial ovarian cancer, borderline ovarian tumor and healthy ovarian tissues. J. Trace Elem. Med. Biol. 2017, 43, 217–223. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K. Oxidants are a major contributor to aging. Ann. N. Y. Acad. Sci. 1992, 663, 85–96. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.; Alonso, M.; Amiñoso, C.; Anselmo, N.P.; Arjona, D.; Gonzalez-Gomez, P.; Lopez-Marin, I.; de Campos, J.M.; Gutierrez, M.; Isla, A.; et al. Hypermethylation of the DNA repair gene MGMT: Association with TP53 G:C to A:T transitions in a series of 469 nervous system tumors. Mutat. Res. Mol. Mech. Mutagen. 2004, 554, 23–32. [Google Scholar] [CrossRef]
- Park, I.Y.; Sohn, B.H.; Yu, E.; Suh, D.J.; Chung, Y.H.; Lee, J.H.; Surzycki, S.J.; Lee, Y.I. Aberrant Epigenetic Modifications in Hepatocarcinogenesis Induced by Hepatitis B Virus X Protein. Gastroenterology 2007, 132, 1476–1494. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.C.; Anna, C.H.; Foley, J.F.; Stockton, P.S.; Tyson, F.L.; Barrett, J.; Devereux, T.R. Hypermethylation of the p16 (Ink4a) promoter in B6C3F1 mouse primary lung adenocarcinomas and mouse lung cell lines. Carcinog. 2000, 21, 1691–1700. [Google Scholar] [CrossRef] [Green Version]
- González-González, A.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin: A Molecule for Reducing Breast Cancer Risk. Molecules 2018, 23, 336. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Morales, P.; Saceda, M.; Kenney, N.; Kim, N.; Salomon, D.; Gottardis, M.; Solomon, H.; Sholler, P.; Jordan, V.; Martin, M. Effect of Cadmium on Estrogen Receptor Levels and Estrogen-induced Responses in Human Breast Cancer Cells. J. Biol. Chem. 1994, 269, 16896–16901. [Google Scholar] [CrossRef]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M.B. Activation of Estrogen Receptor-by the Heavy Metal Cadmium. Mol. Endocrinol. 2000, 14, 545–553. Available online: https://academic.oup.com/mend/article/14/4/545/2870457 (accessed on 27 October 2022).
- Brama, M.; Gnessi, L.; Basciani, S.; Cerulli, N.; Politi, L.; Spera, G.; Mariani, S.; Cherubini, S.; D’Abusco, A.S.; Scandurra, R.; et al. Cadmium induces mitogenic signaling in breast cancer cell by an ER-dependent mechanism. Mol. Cell. Endocrinol. 2007, 264, 102–108. [Google Scholar] [CrossRef]
- Brännvall, M.-L.; Bindler, A.R.; Renberg, I.; Emteryd, O.; Bartnicki, J.; Billström, K. The Medieval Metal Industry Was the Cradle of Modern Large-Scale Atmospheric Lead Pollution in Northern Europe. Environ. Sci. Technol. 1999, 33, 4391–4395. [Google Scholar] [CrossRef]
- Kumar, A.; Clark, C.S. Lead loadings in household dust in Delhi, India. Indoor Air 2009, 19, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R. The carcinogenicity of metals in humans. Cancer Causes Control 1997, 8, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Environmental Health Criteria 165 INORGANIC LEAD; WHO: Geneva, Switzerland, 1995.
- Smith, D.R.; Osterloh, J.D.; Flegal, A.R. Use of Endogenous, Stable Lead Isotopes to Determine Release of Lead from the Skeleton. Environ. Health Perspect. 1996, 104, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Todd, A.C. Direct Measurement of Lead in Bone A Promising Biomarker. JAMA 1994, 271, 239. [Google Scholar] [CrossRef] [PubMed]
- Somervaille, L.J.; Chettle, D.R.; Scott, M.C.; Tennant, D.R.; McKiernan, M.J.; Skilbeck, A.; Trethowan, W.N. In vivo tibia lead measurements as an index of cumulative exposure in occupationally exposed subjects. Br. J. Ind. Med. 1988, 45, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Leggett, R.W. An age-specific kinetic model of lead metabolism in humans. Environ. Health Perspect. 1993, 101, 598–616. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Téllez-Rojo, M.M.; Amarasiriwardena, C.; Bellinger, D.; Peterson, K.; Schwartz, J.; Hu, H.; Hernández-Avila, M. Effffect of Breast Milk Lead on Infant Blood Lead Levels at 1 Month of Age. Environ. Health Perspect. 2004, 112, 1381–1385. [Google Scholar] [CrossRef] [Green Version]
- León, O.L.L.; Pacheco, J.M.S. Effects of Lead on Reproductive Health. In Lead Chemistry; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar]
- Tyroler, H.A. Epidemiology of hypertension as a public health problem: An overview as background for evaluation of blood lead-blood pressure relationship. Environ. Health Perspect. 1988, 78, 3–7. [Google Scholar] [CrossRef]
- Goldstein, G.W. Neurologic concepts of lead poisoning in children. Pediatr. Ann. 1992, 21, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Needleman, H.L.; Rabinowitz, M.; Leviton, A.; Linn, S.; Schoenbaum, S. The Relationship Between Prenatal Exposure to Lead and Congenital Anomalies. JAMA 1984, 251, 2956–2959. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Occupational and Environmental Exposure to Lead and Reproductive Health Impairment: An Overview. Indian J. Occup. Environ. Med. 2018, 22, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; White, C.W. Redox systems of the cell: Possible links and implications. Proc. Natl. Acad. Sci. USA 2002, 99, 9617–9618. [Google Scholar] [CrossRef] [Green Version]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- Patrick, L. Lead toxicity part II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. J. Clin. Ther. 2006, 11, 114–127. Available online: https://pubmed.ncbi.nlm.nih.gov/16813461/ (accessed on 12 October 2022).
- Beyer, W.N.; Audet, D.J.; Heinz, G.H.; Hoffman, D.J.; Day, D. Relation of Waterfowl Poisoning to Sediment Lead Concentrations in the Coeur d’Alene River Basin; Kluwer Academic Publishers: Idaho City, ID, USA, 2000; pp. 207–218. [Google Scholar]
- Sugawara, E.; Nakamura, K.; Miyake, T.; Fukumura, A.; Seki, Y. Lipid peroxidation and concentration of glutathione in erythrocytes from workers exposed to lead. Br. J. Ind. Med. 1991, 48, 239–2421. [Google Scholar] [CrossRef] [Green Version]
- Hunaiti, A.; Soud, M. Effect of lead concentration on the level of glutathione, glutathione S-transferase, reductase and peroxidase in human blood. Sci. Total Environ. 2000, 248, 45–50. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wang, X.Q.; Oveisi, F.; Rad, B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 2000, 36, 142–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vainio, H.; Hemminki, K.; Wilbourn, J. Data on the carcinogenicity of chemicals in the IARC monographs programme. Carcinogenesis 1985, 6, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Rabbani-Chadegani, A.; Abdosamadi, S.; Fani, N.; Mohammadian, S. A comparison of the effect of lead nitrate on rat liver chromatin, DNA and histone proteins in solution. Arch. Toxicol. 2009, 83, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Rossiello, M.R.; Aresta, A.; Quagliariello, E.; Cavazza, C.; Farber, E. Transitory DNA hypomethylation during liver cell proliferation induced by a single dose of lead nitrate. Arch. Biochem. Biophys. 1991, 286, 212–216. [Google Scholar] [CrossRef]
- Shinozuka, H.; Ohmura, T.; Katyal, S.L.; Zedda, A.I.; Ledda-Columbano, G.M.; Columbano, A. Possible roles of nonparenchymal cells in hepatocyte proliferation induced by lead nitrate and by tumor necrosis factor α. Hepatology 1996, 23, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Choie, D.D.; Richter, G.W. Cell Proliferation in Rat Kidneys After Prolonged Treatment with Lead. Am. J. Pathol. 1972, 68, 359–370. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2032680/ (accessed on 27 October 2022). [PubMed]
- Herbertson, B.M.; King, A.J.; Allen, J. Epithelial cell proliferation in the rat urinary system induced by parenteral injection of lead salts. Br. J. Exp. Pathol. 1987, 68, 167. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2013013/?report=abstract (accessed on 2 November 2022).
- Amaral, A.F.S.; Porta, M.; Silverman, D.T.; Milne, R.L.; Kogevinas, M.; Rothman, N.; Cantor, K.P.; Jackson, B.P.; Pumarega, J.A.; López-Jiménez, T.; et al. Pancreatic cancer risk and levels of trace elements. Gut 2011, 61, 1583–1588. [Google Scholar] [CrossRef] [Green Version]
- Cocco, P.L.; Carta, P.; Belli, S.; Picchiri, G.F.; Flore, M.V. Mortality of Sardinian lead and zinc miners: 1960-88. Occup. Environ. Med. 1994, 51, 674–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Singh, M.K.; Singh, T.B.; Bhartiya, S.K.; Singh, S.P.; Shukla, V.K. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg. 2013, 37, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.A.; Shah, M.H. Comparative Study of Trace Elements in Blood, Scalp Hair and Nails of Prostate Cancer Patients in Relation to Healthy Donors. Biol. Trace Element Res. 2014, 162, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.A.; Shah, M.H. Comparative assessment of selected metals in the scalp hair and nails of lung cancer patients and controls. Biol. Trace Element Res. 2014, 158, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B. Heavy Metals in the Environment; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Yaman, M.; Kaya, G.; Simsek, M. Comparison of trace element concentrations in cancerous and noncancerous human endometrial and ovary tissues. Int. J. Gynecol. Cancer 2007, 17, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: Systematic review and meta-analysis of cohort studies. Leuk. Res. 2015, 45, 782–791. [Google Scholar] [CrossRef] [Green Version]
- Hamer, D.H. Metallothionein. Annu. Rev. Biochem. 1986, 55, 913–951. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.; Williams, D.J.; Moore, M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2002, 137, 65–83. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Cadmium Statistics and Information | U.S. Geological Survey. Available online: https://www.usgs.gov/centers/national-minerals-information-center/cadmium-statistics-and-information (accessed on 10 October 2022).
- Chen, C.-L.; Hsu, L.-I.; Chiou, H.-Y.; Hsueh, Y.-M.; Chen, S.-Y.; Wu, M.-M.; Chen, C.-J.; Blackfoot Disease Study Group. Ingested arsenic, cigarette smoking, and lung cancer risk: A follow-up study in arseniasis-endemic areas in Taiwan. JAMA 2004, 292, 2984–2990. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Toxicological Profile for Cadmium; ATSDR: Atlanta, GA, USA, 2012. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry; International Agency for Research on Cancer; World Health Organization: Geneva, Switzerland, 1993.
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Environmental Health Criteria 134 Cadmium; WHO: Geneva, Switzerland, 1992.
- Hasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Michalczyk, K.; Cymbaluk-Płoska, A. The Role of Zinc and Copper in Gynecological Malignancies. Nutrients 2020, 12, 3732. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D. Homeostasis and Cellular Functions of Zinc. Mater. Werkst. 2002, 33, 764–769. [Google Scholar] [CrossRef]
- Tietz, N.W.; Hirsch, E.F.; Neyman, B. Spectrographic study of trace elements in cancerous and noncancerous human tissues. J. Am. Med. Assoc. 1957, 165, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.B.; Marques, S.F.G.; Almeida, J.A.; Diniz, Y.S.; Faine, L.A.; Ribas, B.O. Toxic mechanism of cadmium exposure on cardiac tissue. Toxic Subst. Mech. 2000, 19, 207–217. [Google Scholar] [CrossRef]
- Ognjanovic, B.; Pavlovic, S.; Maletić, S.D.; Zikić, R.V.; Stajn, A.S.; Radojicić, R.M.; Saicic, Z.; Petrović, V.M. Protective Influence of Vitamin E on Antioxidant Defense System in the Blood of Rats Treated with Cadmium. Physiol. Res. 2003, 52, 563–570. Available online: http://www.biomed.cas.cz/physiolres (accessed on 11 October 2022).
- Wang, T.; Liu, B.; Qin, L.; Wilson, B.; Hong, J.-S. Protective effect of the SOD/catalase mimetic MnTMPyP on inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuronal-glial cultures. J. Neuroimmunol. 2004, 147, 68–72. [Google Scholar] [CrossRef]
- Liaw, K.-Y.; Lee, P.-H.; Wu, F.-C.; Tsai, J.-S.; Lin-Shiau, S.-Y. Zinc, Copper, and Superoxide Dismutase in Hepatocellular Carcinoma. Available online: https://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=00029270&AN=16443985&h=WDjgonjb%2Fw7pLx0LnGUYkMRt9kGl2FBHk9Qb3qe9NOz4EgqVnBYszPnXvBYssnaVz7LM5IV05vw%2FOIofey6KrQ%3D%3D&crl=c (accessed on 14 November 2022).
- Stohs, S.J.; Bagchi, D.; Hassoun, E.; Bagchi, M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 77–88. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.-Q.; Bai, F.-W. Zinc and yeast stress tolerance: Micronutrient plays a big role. J. Biotechnol. 2012, 158, 176–183. [Google Scholar] [CrossRef]
- Burns, K.H. Transposable elements in cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef]
- Hartwig, A. Recent advances in metal carcinogenicity. Pure Appl. Chem. 2000, 72, 973–1066. [Google Scholar] [CrossRef] [Green Version]
- Schrauzer, G.N. The role of trace elements in the etiology of cancer. In Proceedings of the First International Workshop Neuherberg, Federal Republic of Germany, April 1980. 2021, pp. 182–198. Available online: https://www.degruyter.com/document/doi/10.1515/9783112417249-021/html (accessed on 27 October 2022).
- Heavy Metals in The Environment—Bibudhendra Sarkar—Google Książki. Available online: https://books.google.pl/books?id=OJboWGzbq1EC&pg=PA124&lpg=PA124&dq=P.L.+Goering,+M.P.+Waalkes,+C.D.+Klaassen,+in:+R.A.+Goyer,+M.G.+Cherian+(Eds.),+Handbook+of+Experimental+Pharmacology,+vol.+115,+Toxicology+of+Metals,+Biochemical+Effects,+Springer,+New+York,+1994,+pp.+189%E2%80%93214&source=bl&ots=lSx-SQ82q4&sig=ACfU3U2r7Tfm2d4p8mZAUE97Dzeg0ddNTg&hl=pl&sa=X&ved=2ahUKEwjVocb51r_6AhVmxosKHQlECIIQ6AF6BAgIEAM#v=onepage&q=P.L.%20Goering%2C%20M.P.%20Waalkes%2C%20C.D.%20Klaassen%2C%20in%3A%20R.A.%20Goyer%2C%20M.G.%20Cherian%20(Eds.)%2C%20Handbook%20of%20Experimental%20Pharmacology%2C%20vol.%20115%2C%20Toxicology%20of%20Metals%2C%20Biochemical%20Effects%2C%20Springer%2C%20New%20York%2C%201994%2C%20pp.%20189%E2%80%93214&f=false (accessed on 1 October 2022).
- Kägi, J.H. Overview of metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Elinder, C.G.; Kjellström, T.; Friberg, L.; Linnman, B.L.L. Cadmium in kidney cortex, liver, and pancreas from Swedish autopsies. Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch. Environ. Health Int. J. 1976, 31, 292–302. [Google Scholar] [CrossRef]
- Yang, J.-M.; Arnush, M.; Chen, Q.-Y.; Wu, X.-D.; Pang, B.; Jiang, X.-Z. Cadmium-induced damage to primary cultures of rat Leydig cells. Reprod. Toxicol. 2003, 17, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Price, D.J.; Joshi, J.G. Ferritin. Binding of beryllium and other divalent metal ions. J. Biol. Chem. 1983, 258, 10873–10880. [Google Scholar] [CrossRef]
- Waalkes, M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Verougstraete, V.; Lison, D.; Hotz, P. Cadmium, lung and prostate cancer: A systematic review of recent epidemiological data. J. Toxicol. Environ. Health Part B 2003, 6, 227–256. [Google Scholar] [CrossRef]
- Şaplakoǧlu, U.; Işcan, M. Sister chromatid exchanges in human lymphocytes treated in vitro with cadmium in G(o) and S phase of their cell cycles. Mutat. Res. Toxicol. Environ. Mutagen. 1998, 412, 109–114. [Google Scholar] [CrossRef]
- Pearson, C.; Prozialeck, W. E-Cadherin, beta-Catenin and cadmium carcinogenesis. Med. Hypotheses 2001, 56, 573–581. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501. [Google Scholar] [PubMed]
- Flora, S.J.S.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, F.A.; Rondia, D.J.; Claeys, F.D.; Staessen, J.A.; Lauwerys, R.R.; Bernard, A.M.; Buchet, J.P.; Roels, H.A.; Bruaux, P.J.; Ducoffre, G.M.; et al. Impact of environmental cadmium pollution on cadmium exposure and body burden. Arch. Environ. Health Int. J. 1992, 47, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Passarelli, M.N.; Newcomb, P.A. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup. Environ. Med. 2011, 69, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M.; Couse, J.F.; Korach, K.S. The Multifaceted Mechanisms of Estradiol and Estrogen Receptor Signaling. J. Biol. Chem. 2001, 276, 36869–36872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Metals and breast cancer. J. Mammary Gland. Biol. Neoplasia 2013, 18, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, L. Epidemiology of endocrine-related risk factors for breast cancer. J. Mammary Gland. Biol. Neoplasia 2002, 7, 3–15. [Google Scholar] [CrossRef]
- Safe, S. Cadmium’s disguise dupes the estrogen receptor. Nat. Med. 2003, 9, 1000–1001. [Google Scholar] [CrossRef]
- Darbre, P.D. Metalloestrogens: An emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J. Appl. Toxicol. 2006, 26, 191–197. [Google Scholar] [CrossRef]
- Pettersson, K.; Gustafsson, J. Role of Estrogen Receptor Beta in Estrogen Action. Annu. Rev. Physiol. 2001, 63, 165–192. [Google Scholar] [CrossRef]
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology 2003, 144, 2425–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.D.; Kenney, N.; Stoica, A.; Hilakivi-Clarke, L.; Singh, B.; Chepko, G.; Clarke, R.; Sholler, P.F.; Lirio, A.; Foss, C.; et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat. Med. 2003, 9, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Cervix Uteri. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf (accessed on 27 October 2022).
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Gustafsson, L.; Pontén, J.; Zack, M.; Adami, H.-O. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control 1997, 8, 755–763. [Google Scholar] [CrossRef]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int. J. Cancer 2005, 118, 1481–1495. [Google Scholar] [CrossRef]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: Collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int. J. Cancer 2006, 119, 1108–1124. [Google Scholar] [CrossRef]
- Smith, J.S.; Green, J.; de Gonzalez, A.B.; Appleby, P.; Peto, J.; Plummer, M.; Franceschi, S.; Beral, V. Cervical cancer and use of hormonal contraceptives: A systematic review. Lancet 2003, 361, 1159–1167. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Wadhwa, S.K.; Kazi, T.G.; Afridi, H.I.; Talpur, F.N. Interaction between carcinogenic and anti-carcinogenic trace elements in the scalp hair samples of different types of Pakistani female cancer patients. Clin. Chim. Acta 2015, 439, 178–184. [Google Scholar] [CrossRef]
- Balasubramaniyan, N.; Subramanian, S.; Sekar, N.; Bhuvarahamurthy, V.; Govindasamy, S. Involvement of plasma copper, zinc and cadmium in human carcinoma of uterine cervix. Med. Oncol. 1994, 11, 147–148. [Google Scholar] [CrossRef]
- Zhang, J.; Nazeri, S.A.; Sohrabi, A. Lead (Pb) exposure from outdoor air pollution: A potential risk factor for cervical intraepithelial neoplasia related to HPV genotypes. Environ. Sci. Pollut. Res. 2021, 29, 26969–26976. [Google Scholar] [CrossRef] [PubMed]
- Corpus Uteri Source: Globocan 2020. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/24-Corpus-uteri-fact-sheet.pdf (accessed on 27 October 2022).
- Hill, H.A.; Eley, J.W.; Harlan, L.C.; Greenberg, R.S.; Ii, R.J.B.; Chen, V.W. Racial differences in endometrial cancer survival: The black/white cancer survival study. Obstet. Gynecol. 1996, 88, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Clement, P.B.; Young, R.H. Endometrioid carcinoma of the uterine corpus: A review of its pathology with emphasis on recent advances and problematic aspects. Adv. Anat. Pathol. 2002, 9, 145–184. [Google Scholar] [CrossRef]
- Neven, P.; De Muylder, X.; Van Belle, Y.; Van-Hooff, I.; Vanderick, G. Longitudinal hysteroscopic follow-up during tamoxifen treatment. Lancet 1998, 351, 36. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; La Vecchia, C.; Negri, E.; Fedele, L.; Balotta, F. Reproductive factors and risk of endometrial cancer. Am. J. Obstet. Gynecol. 1991, 164, 522–527. [Google Scholar] [CrossRef]
- Van Gorp, T.; Neven, P. Endometrial safety of hormone replacement therapy: Review of literature. Maturitas 2002, 42, 93–104. [Google Scholar] [CrossRef]
- Anderson, G.L.; Judd, H.L.; Kaunitz, A.M.; Barad, D.H.; Beresford, S.A.A.; Pettinger, M.; Liu, J.; McNeeley, S.G.; Lopez, A.M.; Women’s Health Initiative Investigators. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: The Women’s Health Initiative randomized trial. JAMA 2003, 290, 1739–1748. [Google Scholar] [CrossRef] [Green Version]
- Beral, V.; Bull, D.; Reeves, G.; Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005, 365, 1543–1551. [Google Scholar] [CrossRef]
- Hinkula, M.; Pukkala, E.; Kyyrönen, P.; Kauppila, A. Grand multiparity and incidence of endometrial cancer: A population-based study in Finland. Int. J. Cancer 2002, 98, 912–915. [Google Scholar] [CrossRef]
- Deligeoroglou, E.; Michailidis, E.; Creatsas, G. Oral contraceptives and reproductive system cancer. Ann. N. Y. Acad. Sci. 2003, 997, 199–208. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Feskanich, D.; De Vivo, I.; Hunter, D.J.; Barbieri, R.L.; Rosner, B.; Colditz, G.; Hankinson, S.E. Smmoking and the risk of endometrial cancer: Results from the Nurses’ Health Study. Int. J. Cancer 2005, 114, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Lesko, S.M.; Rosenberg, L.; Kaufman, D.W.; Helmrich, S.P.; Miller, D.R.; Strom, B.; Schottenfeld, D.; Rosenshein, N.B.; Knapp, R.C.; Lewis, J.; et al. Cigarette smoking and the risk of endometrial cancer. N. Engl. J. Med. 1985, 313, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Wynn, R.M. The Human Endometrium. In Biology of the Uterus; Springer: Boston, MA, USA, 1989; pp. 289–331. [Google Scholar] [CrossRef]
- Eriksen, K.T.; Halkjær, J.; Sørensen, M.; Meliker, J.R.; McElroy, J.A.; Tjønneland, A.; Raaschou-Nielsen, O. Dietary cadmium intake and risk of breast, endometrial and ovarian cancer in danish postmenopausal women: A prospective cohort study. PLoS ONE 2014, 9, e100815. [Google Scholar] [CrossRef]
- Adams, S.V.; Quraishi, S.M.; Shafer, M.M.; Passarelli, M.N.; Freney, E.P.; Chlebowski, R.T.; Luo, J.; Meliker, J.R.; Mu, L.; Neuhouser, M.L.; et al. Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the women’s health initiative. Environ. Health Perspect. 2014, 122, 594–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akesson, A.; Julin, B.; Wolk, A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: A population-based prospective cohort study. Cancer Res. 2008, 68, 6435–6441. [Google Scholar] [CrossRef] [Green Version]
- McElroy, J.A.; Kruse, R.L.; Guthrie, J.; Gangnon, R.E.; Robertson, J.D. Cadmium exposure and endometrial cancer risk: A large midwestern U.S. population-based case-control study. PLoS ONE 2017, 12, e0179360. [Google Scholar] [CrossRef] [Green Version]
- Wieder-Huszla, S.; Chudecka-Głaz, A.; Cymbaluk-Płoska, A.; Karakiewicz, B.; Bosiacki, M.; Chlubek, D.; Jurczak, A. Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage-Preliminary Study. Nutrients 2022, 14, 2368. [Google Scholar] [CrossRef]
- Mittal, K.; Sebenik, M.; Irwin, C.; Yan, Z.; Popiolek, D.; Curtin, J.; Palazzo, J. Preresence of endometrial adenocarcinoma in situ in complex atypical endometrial hyperplasia is associated with increased incidence of endometrial carcinoma in subsequent hysterectomy. Mod. Pathol. 2008, 22, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Ovary. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf (accessed on 27 October 2022).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Pietragalla, A.; Arcieri, M.; Marchetti, C.; Scambia, G.; Fagotti, A. Ovarian cancer predisposition beyond BRCA1 and BRCA2 genes. Int. J. Gynecol. Cancer 2020, 30, 1803–1810. [Google Scholar] [CrossRef]
- La Vecchia, C. Ovarian cancer: Epidemiology and risk factors. Eur. J. Cancer Prev. 2017, 26, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Julin, B.; Wolk, A.; Åkesson, A. Dietary cadmium exposure and risk of epithelial ovarian cancer in a prospective cohort of Swedish women. Br. J. Cancer 2011, 105, 441–444. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtak, G.; Kozłowski, M.; Kwiatkowski, S.; Cymbaluk-Płoska, A. The Role of Lead and Cadmium in Gynecological Malignancies. Antioxidants 2022, 11, 2468. https://doi.org/10.3390/antiox11122468
Furtak G, Kozłowski M, Kwiatkowski S, Cymbaluk-Płoska A. The Role of Lead and Cadmium in Gynecological Malignancies. Antioxidants. 2022; 11(12):2468. https://doi.org/10.3390/antiox11122468
Chicago/Turabian StyleFurtak, Gabriela, Mateusz Kozłowski, Sebastian Kwiatkowski, and Aneta Cymbaluk-Płoska. 2022. "The Role of Lead and Cadmium in Gynecological Malignancies" Antioxidants 11, no. 12: 2468. https://doi.org/10.3390/antiox11122468





