Recent Overview of Potent Antioxidant Activity of Coordination Compounds
Abstract
:1. Introduction
2. Free Radicals & Antioxidants
3. Mechanism of Free Radicals & Antioxidant
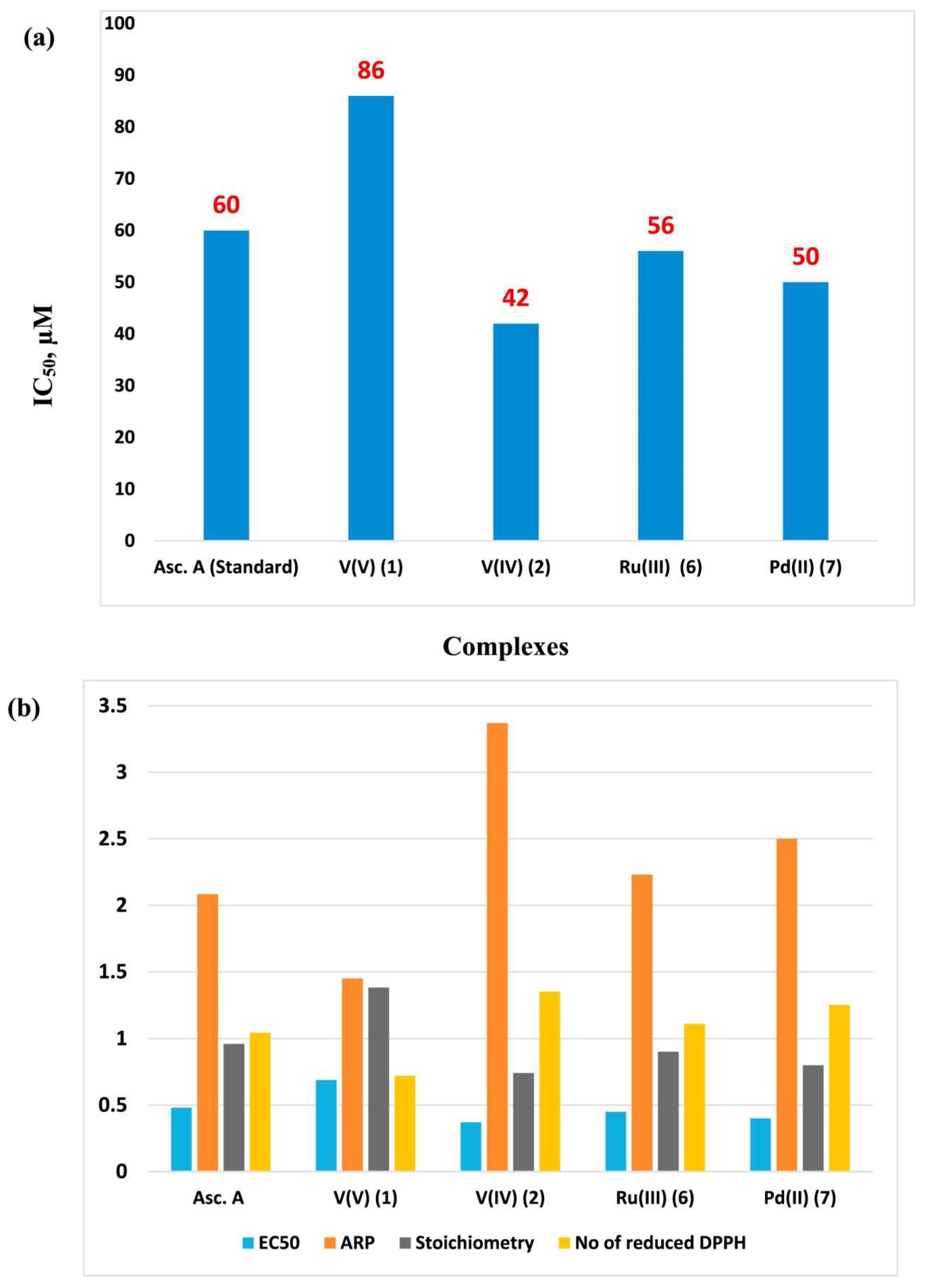
4. Metal Chelates with Antioxidant Properties
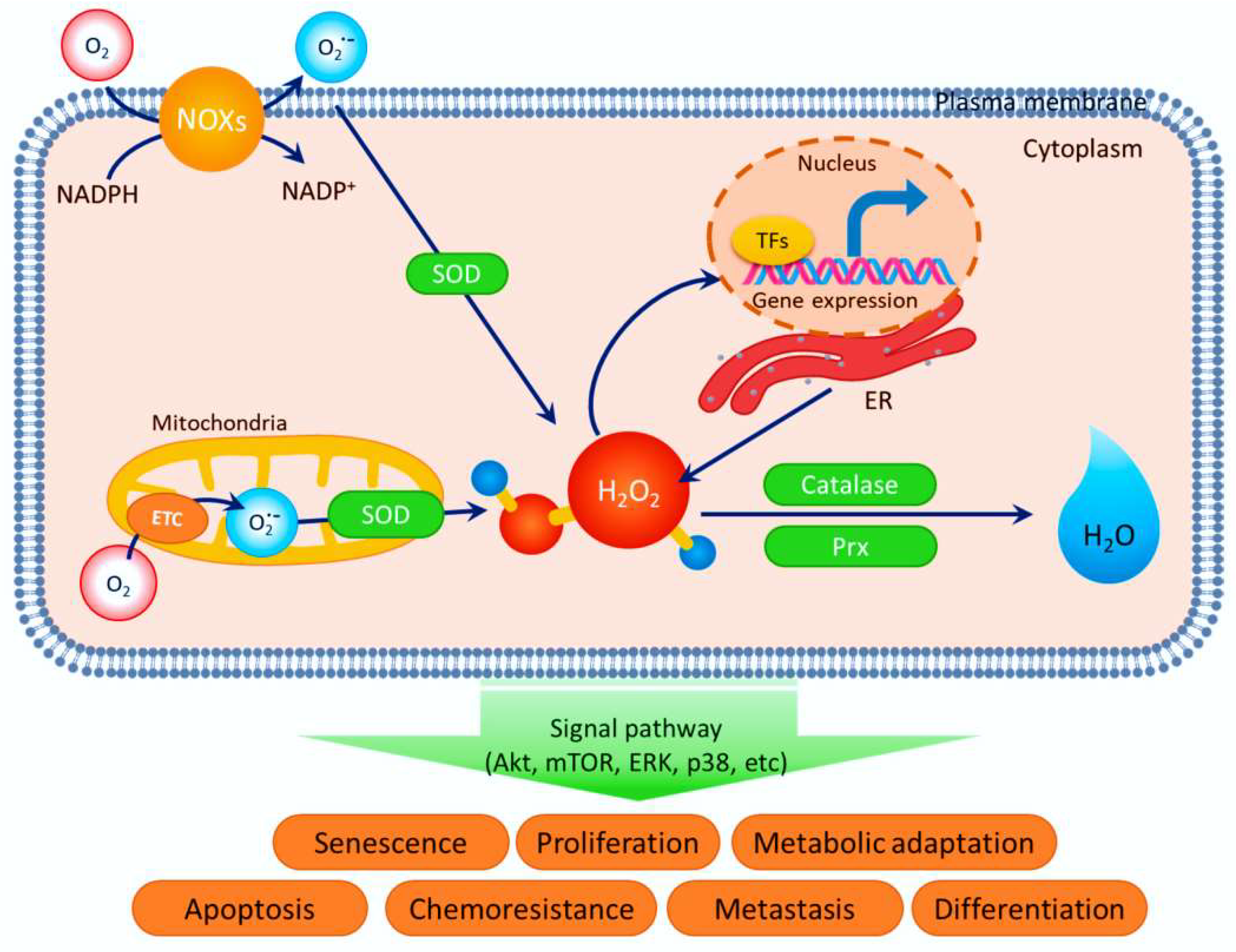
5. Biological Outcomes of Oxidation by ROS
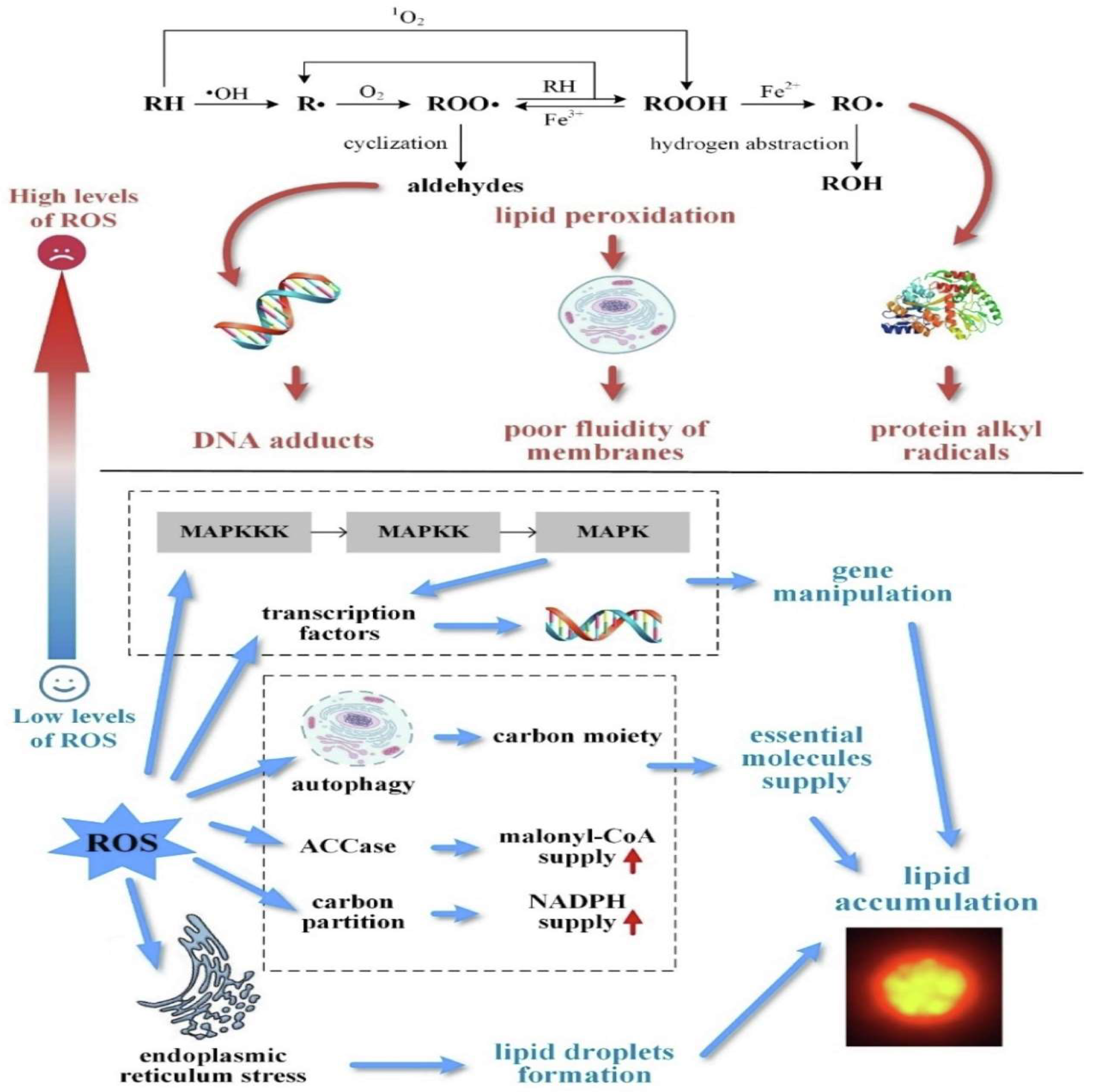
5.1. Reactive Oxygen Species and Lipids
5.2. Reactive Oxygen Species and Proteins
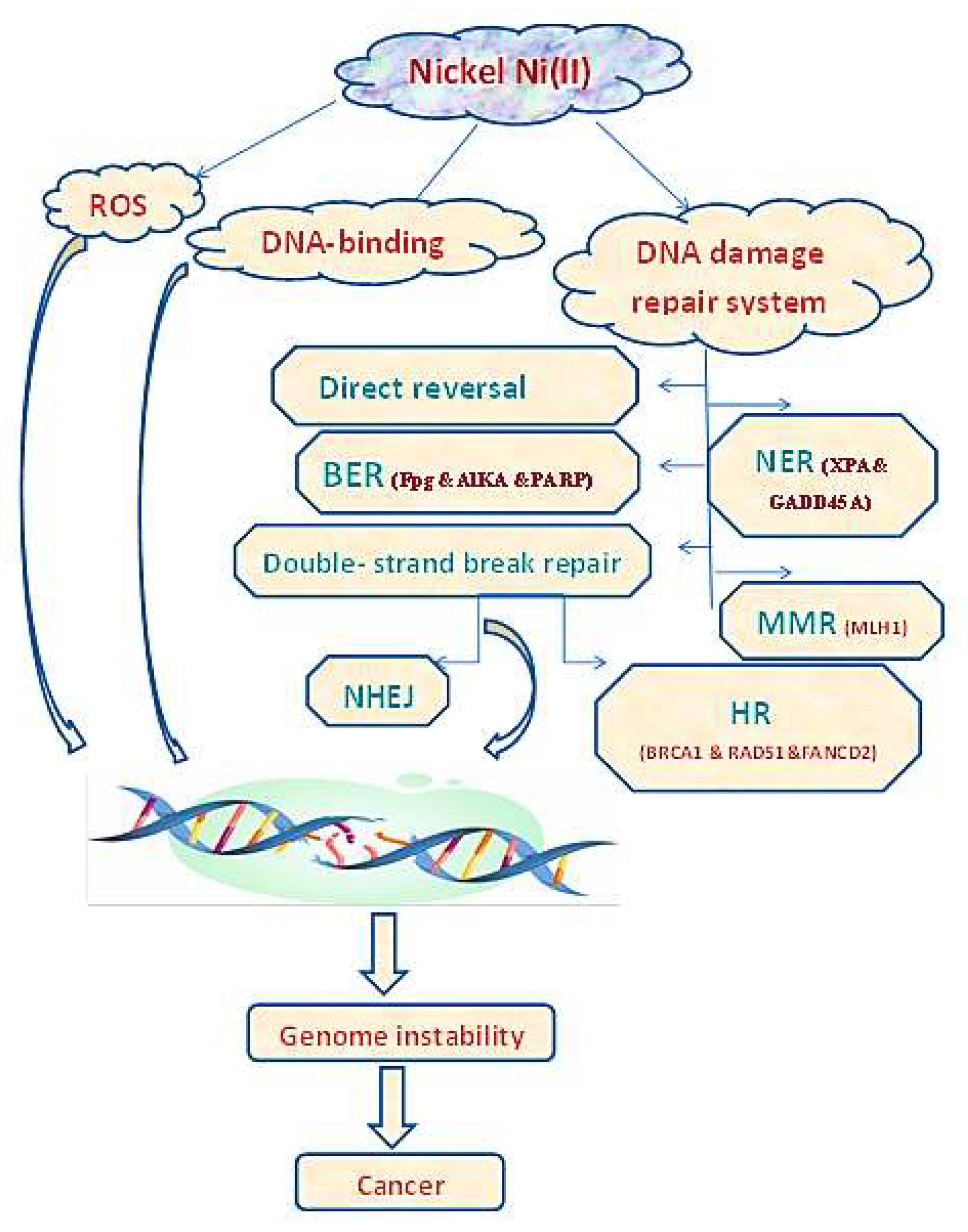
5.3. ROS and DNA
6. Main In Vitro Assays to Assess Antioxidant Activity
6.1. DPPH Radical Assay
UV–Vis Detection
6.2. Lipid Peroxidation
Lipid Peroxidation—EPR Detection
6.3. Ferric Reducing Antioxidant Power (FRAP)
6.4. Cupric Reducing Antioxidant Capacity (CUPRAC)
6.5. H2O2 Scavenging Activity
6.6. ABTS Assay
7. Overview of Metal Chelates as an Antioxidant
8. Conclusions and Future Trends
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiologicalignaling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Dreher, D.; Junod, A.F. Role of Oxygen Free Radicals in Cancer Development. Eur. J. Cancer 1996, 32, 30–38. [Google Scholar] [CrossRef]
- Vendemiale, G.; Grattagliano, I.; Altomare, E. An update on the role of free radicals and antioxidant defense in human disease. Int. J. Clin. Lab. Res. 1999, 29, 49–55. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Spitz, D.R.; Hauer-Jensen, M. Ionizing Radiation-Induced Responses: Where Free Radical Chemistry Meets Redox Biology and Medicine. Antioxid. Redox Signal. 2014, 20, 1407–1409. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin. Chim. Acta 2019, 491, 85–90. [Google Scholar] [CrossRef]
- Labat-Robert, J.; Robert, L. Longevity and aging. Role of free radicals and xanthine oxidase. A review. Pathol. Biol. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 1999; pp. 617–783. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signaling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Ramsay, R.R. Electron carriers and energy conservation in mitochondrial respiration. ChemTexts 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Akbarirad, H.; Gohari Ardabili, A.; Kazemeini, S.M.; Mousavi Khaneghah, A. An overview of some of the important sources of natural antioxidants. Int. Food Res. J. 2016, 23, 928–933. [Google Scholar]
- Suleman, M.; Khan, A.; Baqi, A.; Kakar, M.S.; Samiullah; Ayub, M. Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure Appl. Biol. 2018, 7, 380–388. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khan, A.; Ikram, M.; Rehman, S.; Shah, M.; Nabi, H.H.; Achuchaogu, A.A. In Vitro Antioxidant Properties of Novel Schiff Base Complexes. Asian J. Chem. Sci. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Mohamed, G.G. Synthesis, characterization and biological activity of bis(phenylimine) Schiff base ligands and their metal complexes. Spectrochim. Acta 2006, 64, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Alotaibi, N.H.; Al-Farraj, E.S.; Qasem, H.A.; Alzahrani, S.; Mahfouz, M.K.; Abdou, A. Fabrication, Structural Elucidation, Theoretical, TD-DFT, Vibrational Calculation, and Molecular Docking Studies of Some Novel Adenine Imine Chelates for Biomedical Applications. J. Mol. Liq. 2022, 365, 119961. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Shehata, M.R.; Abdel-Mawgoud, A.A.H. Novel azomethine Pd (II)-and VO (II)-based metallo-pharmaceuticals as anticancer, antimicrobial, and antioxidant agents: Design, structural inspection, DFT investigation, and DNA interaction. J. Phys. Org. Chem. 2019, 32, e4009. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdel-Mawgoud, A.A.H. A robust in vitro anticancer, antioxidant and antimicrobial agents based on new metal-azomethine chelates incorporating Ag (I), Pd (II) and VO (II) cations: Probing the aspects of DNA interaction. Appl. Organomet. Chem. 2020, 34, e5373. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Omran, O.A.; Ahmed, E.A.; Al-Farraj, E.S.; Elkady, E.F.; Alharbi, A.; El-Metwaly, N.M.; Barnawi, I.O.; Abu-Dief, A.M. Design, structural inspection of new bis (1H-benzo [d] imidazol-2-yl) methanone complexes: Biomedical applications and theoretical implementations via DFT and docking approaches. Inorg. Chem. Comm. 2023, 148, 110331. [Google Scholar] [CrossRef]
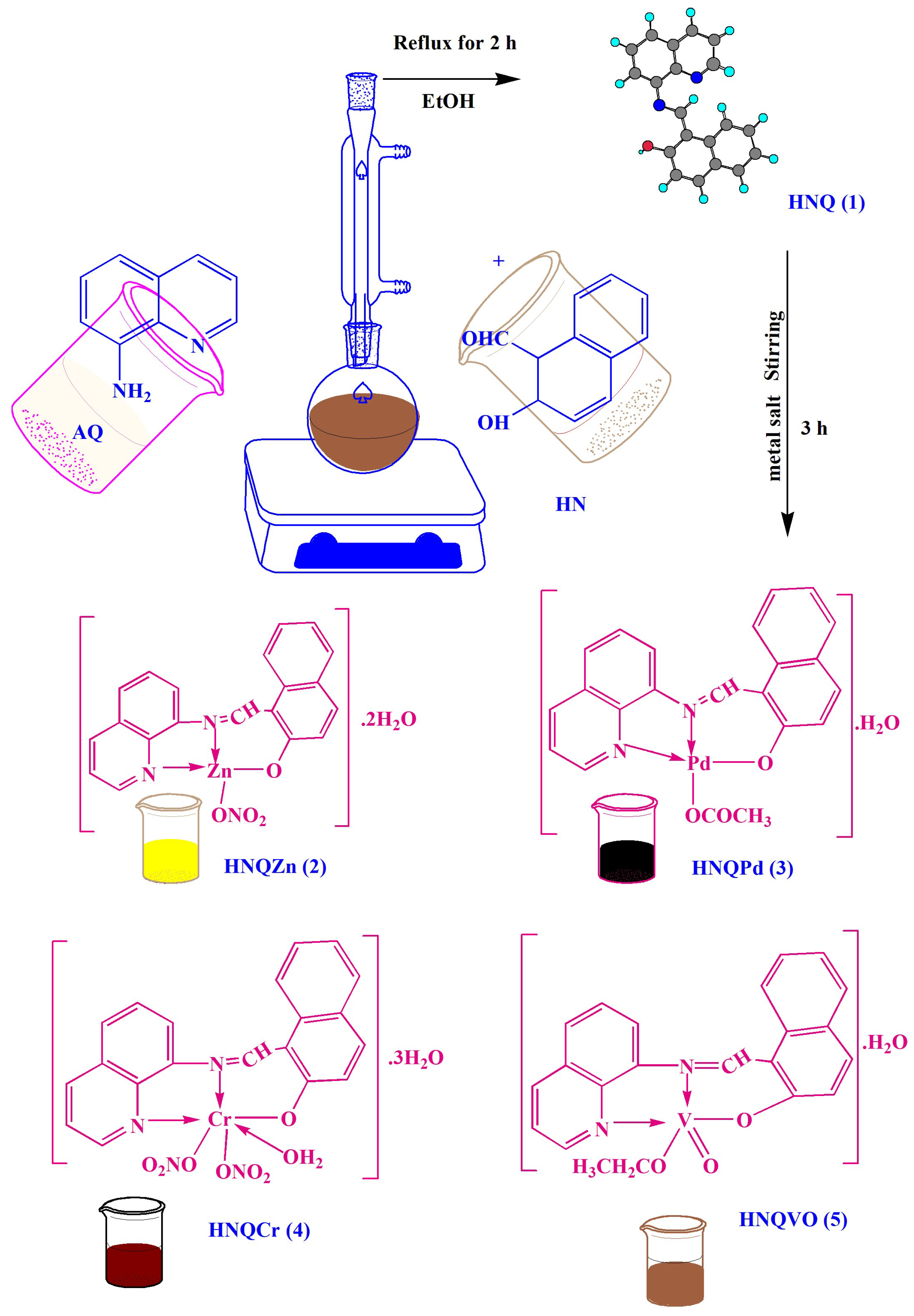
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Alzahrani, S.O.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J. Mol. Struct. 2021, 1242, 130693. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Khalaf, M.M.; Shehata, M.R.; Abu-Dief, A.M. Fabrication, DFT Calculation, and Molecular Docking of Two Fe(III) Imine Chelates as Anti-COVID-19 and Pharmaceutical Drug Candidate. Int. J. Mol. Sci. 2022, 23, 3994. [Google Scholar] [CrossRef]
- Omar, M.M.; Mohamed, G.G.; Hindy, A.M.M. Transition metal complexes of heterocyclic Schiff base Biological activity, spectroscopic and thermal characterization. J. Therm. Anal. Calorim. 2006, 86, 315–325. [Google Scholar] [CrossRef]
- Olive, G.H.; Olive, S. The Chemistry of the Catalyzed Hydrogenation of Carbon Monoxide; Springer Science & Business Media: Berlin, Germany, 1984; Volume 152, pp. 301–315. [Google Scholar]
- Li, Y.; Yang, Z.Y.; Wu, J.C. Synthesis, crystal structures, biological activities and fluorescence studies of transition metal complexes with 3-carbaldehyde chromone thiosemicarbazone. Eur. J. Med. Chem. 2010, 45, 5692–5701. [Google Scholar] [CrossRef]
- Hranjec, M.; Starčević, K.; Pavelić, S.K.; Lučin, P.; Pavelić, K.; Zamola, G.K. Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles. Eur. J. Med. Chem. 2011, 46, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Díaz-Torres, R.; Sañudo, E.C.; Abdel-Rahman, L.H.; Aliaga-Alcalde, N. Novel sandwich triple-decker dinuclear NdIII-(bis-N, N′-p-bromo-salicylideneamine-1, 2-diaminobenzene) complex. Polyhedron 2013, 64, 203–208. [Google Scholar] [CrossRef]
- Rao, N.V.; Choudhury, T.D.; Deb, R.; Paul, M.K.; Rao, T.R.; Francis, T.; Smalyukh, I.I. Fluorescent lanthanide complexes of Schiff base ligands possessing N-aryl moiety: Influence of chain length on crossover (calamitic to discotic) phase behavior. Liq. Crys. 2010, 37, 1393–1410. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Wajid, A.; Mahmood, K.; Maah, M.J.; Yusoff, I. Spectral investigation of the activities of amino substituted bases. Int. J. Chem. Eng. Appl. 2011, 2, 252–255. [Google Scholar]
- Ashassi-Sorkhabi, H.; Shabani, B.; Aligholipour, B.; Seifzadeh, D. The effect of some Schiff bases on the corrosion of aluminum in hydrochloric acid solution. Appl. Surf. Sci. 2006, 252, 4039–4047. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Bella, S.D. Aggregation properties of bis(salicylaldiminato) zinc(II) Schiff-base complexes and their Lewis acidic character. Dalton. Trans. 2012, 41, 387–395. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Abu-Dief, A.M. Design, synthesis, structural inspection of Pd2+, VO2+, Mn2+ and Zn2+ chelates incorporating ferrocenyl thiophenol ligand: DNA interaction and pharmaceutical studies. Appl. Organomet. Chem. 2021, 35, e6169. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Ibrahim, M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. J. Bas. Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Alorabi, Q.A.; Abdelbaset, M.; Zabin, A.S. Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl) benzene. Chemosensors 2020, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Abu-Dief, A.M.; El-Sagher, H.M.; Shehata, M.R. Fabrication, spectroscopic characterization, calf thymus DNA binding investigation, antioxidant and anticancer activities of some antibiotic azomethine Cu (II), Pd (II), Zn (II) and Cr (III) complexes. Appl. Organomet. Chem. 2019, 33, e4943. [Google Scholar] [CrossRef]
- Al-Abdulkarim, H.A.; El-Khatib, R.M.; Aljohani, F.S.; Mahran, A.; Alharbi, A.; Mersal, G.A.M.; El-Metwaly, N.M.; Abu-Dief, A.M. Optimization for synthesized quinoline-based Cr3+, VO2+, Zn2+, and Pd2+ complexes: DNA interaction, bio-logical assay and in-silico treatments for verification. J. Mol. Liq. 2021, 339, 116797. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdel-Mawgoud, A.A.H. Novel di- and tri-azomethine compounds as chemosensors for the detection of various metal ions. Int. J. Nanomater. Chem. 2019, 5, 1–17. [Google Scholar]
- Al-Saeedi, S.I.; Abdel-Rahman, L.H.; Abu-Dief, A.M.; Abdel-Fatah, S.M.; Alotaibi, T.M.; Alsalme, A.M.; Nafady, A. Catalytic Oxidation of Benzyl Alcohol Using Nanosized Cu/Ni Schiff-Base Complexes and Their Metal Oxide Nanoparticles. Catalysts 2018, 8, 452. [Google Scholar] [CrossRef] [Green Version]
- Kuddushi, M.M.Y.; Malek, M.A.H.; Patidar, V.L.; Patel, M.S.; Patel, R.K.; Dave, R.H. Synthesis and Characterization Of Schiff Base Aniline With 5- Bromo -2- Hydroxyl Benzaldehyde And Their Metal Complexes. Int. J. Recent Sci. Res. 2018, 9, 26026–26030. [Google Scholar]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Bawazeer, A.M.; Shaaban, S.; Adam, M.S.S. Targeting CtDNA Binding and Elaborated In-Vitro Assessments Concerning Novel Schiff Base Complexes: Synthesis, Characterization, DFT and Detailed in-Silico Confirmation. J. Mol. Liq. 2021, 322, 114977. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Abu-Dief, A.M.; Makhlouf, M.M.; Shaaban, S.; Alzahrani, S.O.; Alkhatib, F.; Masaret, G.S.; Mohamed, M.A.; Alsehli, M.; El-Metwaly, N.M.; et al. Tailoring, structural inspection of novel oxy and non-oxy metal-imine chelates for DNA interaction, pharmaceutical and molecular docking studies. Polyhedron 2021, 201, 115167. [Google Scholar] [CrossRef]
- Nikic, P.; Dragicevic, D.; Jerotic, D.; Savic, S.; Djukic, T.; Stankovic, B.; Matic, M. Polymorphisms of Antioxidant Enzymes SOD2 (rs4880) and GPX1 (rs1050450) Are Associated with Bladder Cancer Risk or Its Aggressiveness. Medicina 2023, 59, 131. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. Antioxidants 2019, 10, 1–29. [Google Scholar]
- Halliwell, B. Antioxidant characterization: Methodology and mechanism. Biochem. Pharmacol 1995, 49, 1341–1348. [Google Scholar] [CrossRef]
- De La Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Karihtala, P.; Soini, Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis 2007, 115, 81–103. [Google Scholar] [CrossRef]
- Bouamrane, O.L.; Hellal, A.; Hachama, K.; Touafri, L.; Haddadi, I.; Layaida, H.; Bensouici, C. Effect of the bifunctionalization of aminomethylphosphonic acid on the structural, electronic, vibrational, thermodynamic and antioxidant activity: Microwave-assisted synthesis, Density Functional Theory studies and DPPH radical scavenging activity. J. Mol. Struct 2022, 1250, 131714. [Google Scholar] [CrossRef]
- Knight, J.A. Free radicals: Their history and current status in aging and disease. Ann. Clin. Lab. Sci. 1998, 28, 331–346. [Google Scholar] [PubMed]
- Jacob, R.A. Three eras of vitamin C discovery. Subcell. Biochem. 1996, 25, 1–16. [Google Scholar] [PubMed]
- Moreau, D. Comptes Rendus des Séances et Mémoires de la Société de Biologie; Au Bureau de la Gazette médicale: Paris, France, 1922; Volume 86, p. 321. [Google Scholar]
- Mattill, H.A. Antioxidants. Annu. Rev. Biochem. 1947, 16, 177–192. [Google Scholar] [CrossRef]
- German, J.B. Food processing and lipid oxidation. Adv. Exp. Med. Biol. 1999, 459, 23–50. [Google Scholar]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Niki, E. Comparative study on dynamics of antioxidative action of alpha-tocopheryl hydroquinone, ubiquinol, and alpha-tocopherol against lipid peroxidation. Free Radic. Biol. Med. 1999, 27, 334–346. [Google Scholar] [CrossRef]
- Yang, M.-H.; Lin, H.-J.; Choong, Y.-M. A rapid gas chromatographic method for direct determination of BHA, BHT and TBHQ in edible oils and fats. Food Res. Int. 2002, 35, 627–633. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Madhavi, D.L.; Deshpande, S.S.; Salunkhe, D.K. Introduction. In Food Antioxidants: Technological, Toxicological, and Health Perspectives; Madhavi, D.L., Deshpande, S.S., Salunkhe, D.K., Eds.; Dekker: New York, NY, USA, 1996; pp. 1–4. [Google Scholar]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. Adv. Food Nutr. Res. 2019, 90, 1–81. [Google Scholar] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [PubMed]
- Biesalski, H.K.; Grune, T.; Tinz, J.; Zöllner, I.; Blumberg, J.B. Reexamination of a meta-analysis of the effect of antioxidant supplementation on mortality and health in randomized trials. Nutrients 2010, 2, 929–949. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Angeloni, C. New Mechanisms of Action of Natural Antioxidants in Health and Disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar]
- Murphy, M.P. Antioxidants as therapies: Can we improve on nature. Free Radic. Biol. Med 2014, 66, 20–23. [Google Scholar] [CrossRef]
- Florido, J.; Rodriguez-Santana, C.; Martinez-Ruiz, L.; López-Rodríguez, A.; Acuña-Castroviejo, D.; Rusanova, I.; Escames, G. Understanding the mechanism of action of melatonin, which induces ROS production in cancer cells. Antioxidants 2022, 11, 1621. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Schramm, M. Reactive oxygen species: Not omnipresent but important in many locations. Front. Cell Dev. Biol. 2021, 9, 716406. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Scaloni, A.; Giustarini, D.; Cavarra, E.; Tell, G.; Lungarella, G.; Milzani, A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: The contribution of redox proteomics. Mass Spectrom. Rev 2005, 24, 55–99. [Google Scholar] [CrossRef]
- Sproll, C.; Ruge, W.; Andlauer, C.; Godelmann, R.; Lachenmeier, D.W. HPLC analysis and safety assessment of coumarin in foods. Food Chem. 2008, 109, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.H. The mechanism of antioxidant action in vitro. EAFSS 1990, 1–18. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide AND Peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, N.; Kitts, D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Fd. Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.K.; Jang, M.; Song, M.J.; Kim, D.; Kim, Y.; Jang, H.H. Redox-mediated mechanism of chemoresistance in cancer cells. Antioxidants 2019, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Štarha, P.; Trávníček, Z. Non-platinum complexes containing releasable biologically active ligands. Coord. Chem. Rev. 2019, 395, 130–145. [Google Scholar] [CrossRef]
- de Fátima, Â.; Pereira, C.d.P.; Gonçalves Olímpio, C.R.S.D.; de Freitas Oliveira, B.G.; Franco, L.L.; da Silva, P.H.C. Schiff bases and their metal complexes as urease inhibitors—A brief review. J. Adv. Res. 2018, 13, 113–126. [Google Scholar] [CrossRef]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Development of Metal Complexes for Treatment of Coronaviruses. Int. J. Mol. Sci. 2022, 23, 6418. [Google Scholar] [CrossRef] [PubMed]
- Milaeva, E.R. Metal-Based Antioxidants—Potential Therapeutic Candidates for Prevention the Oxidative Stress—Related Carcinogenesis: Mini-Review. Curr. Top. Med. Chem. 2011, 11, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F.; Sánchez, S.; Iradi, M.; Azman, N.; Almajano, M. Avocado Seeds: Extraction Optimization and Possible Use as Antioxidant in Food. Antioxidants 2014, 3, 439–454. [Google Scholar] [CrossRef] [Green Version]
- Sabri, A.C.; Nassima, M.-S.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M.; Chamani, J. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [Green Version]
- Anwar, H.; Hussain, G.; Mustafa, I. Antioxidants from Natural Sources. In Antioxidants in Foods and Its Applications; InTech: Vienna, Austria, 2018; pp. 3–28. [Google Scholar] [CrossRef] [Green Version]
- Kozsup, M.; Zhou, X.; Farkas, E.; Bényei, A.C. Sylvestre Bonnet, Tamás Patonay, Krisztina Kónya, Péter Buglyó, Synthesis, characterization and cytotoxicity studies of Co(III)-flavonolato complexes. J. Inorg. Biochem. 2021, 217, 111382. [Google Scholar] [CrossRef]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Transition metal complexes of symmetrical and asymmetrical Schiff bases as antibacterial, antifungal, antioxidant, and anticancer agents: Progress and prospects. Rev. Inorg. Chem. 2015, 35, 191–224. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Alkhatib, F.; Alzahrani, S.O.; Abu-Dief, A.M. Development and structure elucidation of new VO2+, Mn2+, Zn2+, and Pd2+ complexes based on azomethine ferrocenyl ligand: DNA interaction, antimicrobial, antioxidant, anticancer activities, and molecular docking. Appl. Organomet. Chem. 2021, 35, e6154. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Khatib, R.M.; Salah, M.E.; Alzahrani, S.; Alkhatib, F.; El-Sarrag, G.; Ismael, M. Tailoring, Structural elucidation, DFT calculation, DNA interaction and pharmaceutical applications of some aryl hydrazone Mn (II), Cu (II) and Fe (III) complexes. J. Mol. Struct. 2021, 1244, 131017. [Google Scholar] [CrossRef]
- Yıldırım, N.; Bilgiçli, A.T.; Alici, E.H.; Arabacı, G.; Yarasir, M.N. Formation, characterization, aggregation, fluorescence and antioxidant properties of novel tetrasubstituted metal-free and metallophthalocyanines bearing (4- (methylthio)phenoxy) moieties. J. Mol. Struct. 2017, 1144, 66–79. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study. Int. J. Mol. Sci. 2022, 23, 9360. [Google Scholar] [CrossRef] [PubMed]
- Amaral, G.P.; Puntel, G.O.; Corte, C.L.D.; Dobrachinski, F.; Barcelos, R.P.; Bastos, L.L.; Ávila, D.S.; Rocha, J.B.T.; da Silva, E.O.; Puntel, R.L.; et al. The antioxidant properties of different phthalocyanines. Toxicol. Vitr. 2012, 26, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Et Biophys. Acta (BBA) 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Xu, J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol. Appl. Biochem. 2019, 66, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative stress, nutritional antioxidants and beyond. Sci. China Life Sci. 2019, 63, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Willems, P.H.G.M.; Rossignol, R.; Dieteren, C.E.J.; Murphy, M.P.; Koopman, W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef]
- Lv, D.; Xiong, X.; Yang, H.; Wang, M.; He, Y.; Liu, Y.; Yin, Y. Effect of dietary soy oil, glucose, and glutamine on growth performance, amino acid profile, blood profile, immunity, and antioxidant capacity in weaned piglets. Sci. China Life Sci. 2018, 61, 1233–1242. [Google Scholar] [CrossRef]
- Maulucci, G.; Daniel, B.; Cohen, O.; Avrahami, Y.; Sasson, S. Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol. Asp. Med. 2016, 49, 49–77. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Mazzoli, A.; Giacco, A.; Liverini, G.; Iossa, S. A possible link between hepatic mitochondrial dysfunction and diet-induced insulin resistance. Eur. J. Nutr. 2015, 55, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauck, A.K.; Bernlohr, D.A. Oxidative stress, and lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; He, Y.; Sen, B.; Wang, G. Reactive oxygen species and their applications toward enhanced lipid accumulation in oleaginous microorganisms. Bioresour. Res. Technol. 2020, 307, 123234. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cuevas, I.; Kuligowski, J.; Cárcel, M.; Cháfer-Pericás, C.; Asensi, M.; Solberg, R.; Cubells, E.; Nuñez, A.; Saugstad, O.D.; Vento, M.; et al. Protein-bound tyrosine oxidation, nitration and chlorination by-products assessed by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2016, 913, 104–110. [Google Scholar] [CrossRef]
- Saladino, J.; Liu, M.; Live, D.; Sharp, J.S. Aliphatic peptidyl hydroperoxides as a source of secondary oxidation in hydroxyl radical protein footprinting. J. Am. Soc. Mass Spectrom. 2009, 20, 1123–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J. Protein and Peptide Alkoxyl Radicals Can Give Rise to C-Terminal Decarboxylation and Backbone Cleavage. Arch. Biochem. Biophys. 1996, 336, 163–172. [Google Scholar] [CrossRef]
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Estévez, M.; Luna, C. Dietary protein oxidation: A silent threat to human health? Crit. Rev. Food Sci. Nutr. 2017, 57, 3781–3793. [Google Scholar] [CrossRef]
- Ren, J.; Bi, Y.; Sowers, J.R.; Hetz, C.; Zhang, Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 2021, 18, 499–521. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Salaheldeen, M.; El-Dabea, T. Recent advances in the development of gold nanoparticles for drug delivery systems. J. Mod. Nanotechnol. 2021. [Google Scholar] [CrossRef]
- Ohshima, H. Genetic and epigenetic damage induced by reactive nitrogen species: Implications in carcinogenesis. Toxicol. Lett. 2003, 140–141, 99–104. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Kowalska, M.; Piekut, T.; Prendecki, M.; Sodel, A.; Kozubski, W.; Dorszewska, J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Wu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Nickel carcinogenesis mechanism: DNA damage. Int. J. Mol. Sci. 2019, 20, 4690. [Google Scholar] [CrossRef] [Green Version]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of the antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Ionita, P. The Chemistry of DPPH Free Radical and Congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of Free Radical Scavenging Activity of Plant Extracts Through DPPH Assay: An EPR and UV-Vis Study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Luís, M.M.; Marcela, A.S.; Salette, R.; José, L.F.C.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Satish, B.N.; Dilipkumar, P. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Marchi, R.C.; Campos, I.A.; Santana, V.T.; Carlos, R.M. Chemical implications and considerations on techniques used to assess the in vitro antioxidant activity of coordination compounds. Coord. Chem. Rev. 2022, 451, 214275. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Changjiang, G.; Jijun, Y.; Jingyu, W.; Yunfeng, L.; Jing, X.; Yugang, J. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Özyûrek, M.; Gûçlû, K.; Bekdeser, B.; Bener, M. The CUPRAC Methods of Antioxidant Measurement for Beverages. In Processing and Impact Antioxidants Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 235–244. [Google Scholar] [CrossRef]
- Sumathi, S.; Mahadevi, P. Synthesis, characterization and biological activities of Co (II), Cu (II), Zn (II), and Cd (II) metal complexes of 1, 10 phenanthroline based Schiff base. Res. Sq. 2022, 1–16. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Arciszewska, Ż.; Gama, S.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Gołębiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic Acid/Eu (III) complexes: Solution equilibrium studies, structure characterization and biological activity. Int. J. Mol. Sci. 2022, 23, 888. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, F.S.; Abu-Dief, A.M.; El-Khatib, R.M.; Al-Abdulkarim, H.A.; Alharbi, A.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Structural inspection for Novel Pd (II), VO (II), Zn (II) and Cr (III)-Azomethine metal chelates: DNA interaction, Biological Screening and theoretical treatments. J. Mol. Struct. 2021, 1246, 131139. [Google Scholar] [CrossRef]
- Nongpiur, C.G.L.; Tripathi, D.K.; Poluri, K.M.; Rawat, H.; Kollipara, M.R. Ruthenium, rhodium and iridium complexes containing diazafluorene derivative ligands: Synthesis and biological studies. J. Chem. Sci. 2022, 134, 1–14. [Google Scholar] [CrossRef]
- Priya, J.; Madheswari, D. Biomolecular docking interactions, cytotoxicity and antioxidant property evaluations with novel Mn (II), Ni (II), Cd (II) and Pb (II) Schiff base ligand complexes: Synthesis and characterization. J. Biosci. 2022, 47, 1–13. [Google Scholar] [CrossRef]
- Elaaraj, I.; Raouan, S.E.; Nakkabi, A.; Es-sounni, B.; Koraichi, I.; Fahim, M. Synthesis, characterization and antioxidant, antibacterial activity Zn2+, Cu2+, Ni2+ and Co2+, complexes of ligand [2-(thiophen-2-yl)-1-(thiophen-2-ylmethyl)-1H-benzo [d] imidazole]. J. Ind. Chem. Soc. 2022, 99, 100404. [Google Scholar] [CrossRef]
- Gur’eva, Y.A.; Zalevskaya, O.A.; Shevchenko, O.G.; Slepukhin, P.A.; Makarov, V.A.; Kuchin, A.V. Copper (ii) complexes with terpene derivatives of ethylenediamine: Synthesis, and antibacterial, antifungal and antioxidant activity. RSC Adv. 2022, 12, 8841–8851. [Google Scholar] [CrossRef]
- Devi, J.; Kumar, S.; Kumar, B.; Asija, S.; Kumar, A. Synthesis, structural analysis, in vitro antioxidant, antimicrobial activity and molecular docking studies of transition metal complexes derived from Schiff base ligands of 4-(benzyloxy)-2-hydroxybenzaldehyde. Res. Chem. Intermed. 2022, 48, 1541–1576. [Google Scholar] [CrossRef]
- Ali, S.; Singh, V.; Tripathi, V. Microwave assisted synthesis of heterocyclic metal complexes and evaluation of their in vitro anticancer activity against oral cancer cells, antioxidant and molecular docking study. Ind. J. Chem. (IJC) 2022, 61, 385–391. [Google Scholar]
- Medetalibeyoglu, H. Synthesis, antioxidant activity, spectroscopic, electronic, nonlinear optical (NLO) and thermodynamic properties of 2-ethoxy-4-[(5-oxo-3-phenyl-1, 5-dihydro-1, 2, 4-triazol-4-ylimino)-methyl]-phenyl-4-methoxybenzoate: A theoretical and experimental study. J. Iran Chem. Soc. 2022, 19, 1015–1038. [Google Scholar] [CrossRef]
- Damena, T.; Zeleke, D.; Desalegn, T.; Demissie, T.B.; Eswaramoorthy, R. Synthesis, Characterization, and Biological Activities of Novel Vanadium (IV) and Cobalt (II) Complexes. ACS Omega 2022, 7, 4389–4404. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Al-Abdulkarim, H.A.; Alzahrani, S.; El-Sarrag, G.; Ismael, M. Synthesis, structuralelucidation, DFT calculation, biological studies and DNA inter-action of some aryl hydrazone Cr3+, Fe3+, and Cu2+ chelates. Comput. Biol. Chem. 2022, 97, 107643. [Google Scholar] [CrossRef] [PubMed]
- Qasem, H.A.; Aouad, M.R.; Al-Abdulkarim, H.A.; Al-Farraj, E.S.; Attar, R.M.S.; El-Metwaly, N.M.; Abu-Dief, A.M. Tailoring of some novel bis-hydrazone metal chelates, spectral based characterization and DFT calculations for pharmaceutical applications and in-silico treatments for verification. J. Mol. Struct. 2022, 1264, 133263. [Google Scholar] [CrossRef]
- Sen, S.; Chowdhury, N.; Kim, T.W.; Paul, M.; Debnath, D.; Jeon, S.; Bagchi, A.; Im, J.; Biswas, G. Anticancer, Antibacterial, Antioxidant, and DNA-Binding Study of Metal-Phenalenyl Complexes. Bioinorg. Chem. Appl. 2022. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the antioxidant and anti-inflammatory activities of selected plant compounds and their metal ions complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Turan, N.; Buldurun, K.; Türkan, F.; Aras, A.; Çolak, N.; Murahari, M.; Bursal, E.; Mantarcı, A. Some metal chelates with Schiff base ligand: Synthesis, structure elucidation, thermal behavior, XRD evaluation, antioxidant activity, enzyme inhibition, and molecular docking studies. Mol. Div. 2022, 26, 2459–2472. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Alkhatib, F.; Abumelha, H.M.; El-Dabea, T.; Ali El-Remaily, M.A.E.A.A. Structural, conformational and therapeutic studies on new thiazole complexes: Drug-likeness and MOE-simulation assessments. Res. Chem. Intermed. 2021, 47, 1979–2002. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Alkhatib, F.; Abualnaja, M.M.; El-Dabea, T.; Ali, M.A.E.A.A. Synthesis and characterization of Fe (III), Pd (II) and Cu (II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J. Mol. Liq. 2021, 326, 115277. [Google Scholar] [CrossRef]
- Alzahrani, S.O.; Abu-Dief, A.M.; Alkhamis, K.; Alkhatib, F.; El-Dabea, T.; El-Remaily, M.A.E.A.A.; El-Metwaly, N.M. Synthesis and structural elucidation for new pyrano thiazole complexes: Biological screening and effects on DNA through in-vitro and in-silico approaches. J. Mol. Liq. 2021, 332, 115844. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Seyada, A.A.; Shadia, A.E.; Ahmed, K.A.E.; Ahmed, M.E. New transition metal complexes of 2,4-dihydroxybenzaldehyde benzoylhydrazone Schiff base (H2dhbh): Synthesis, spectroscopic characterization, DNA binding/cleavage and antioxidant activity. J. Mol. Struct. 2018, 1158, 39–50. [Google Scholar]


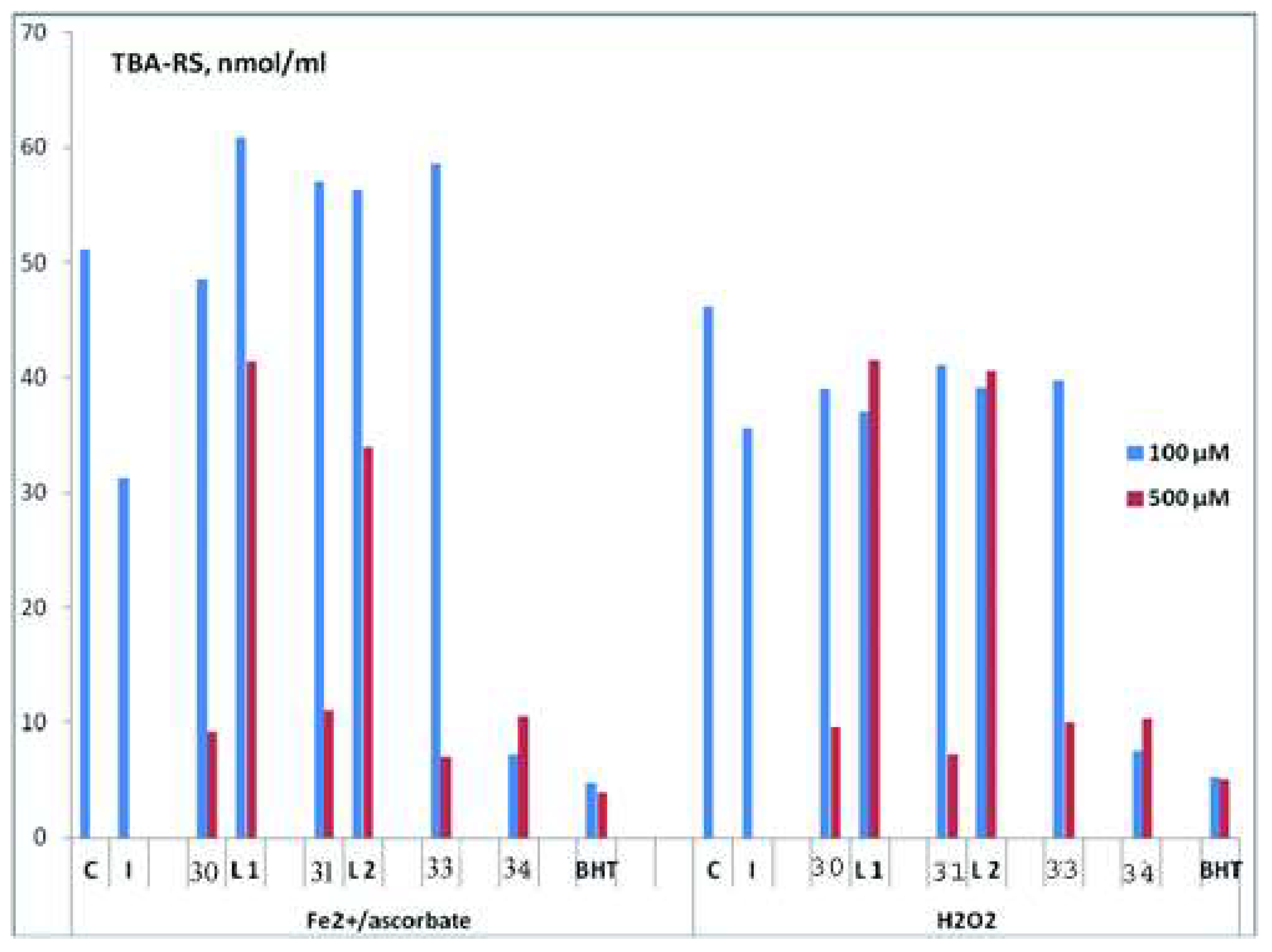
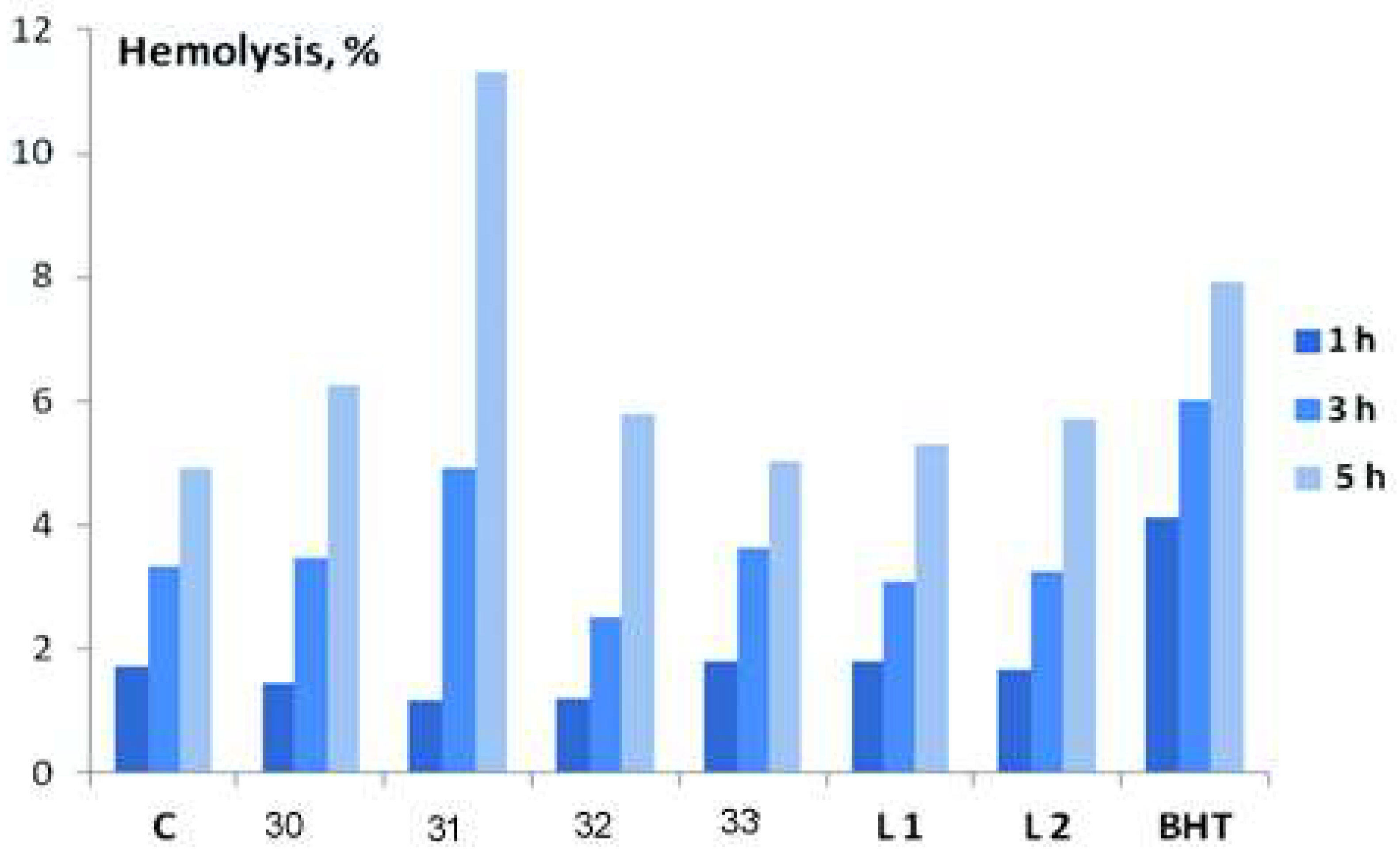

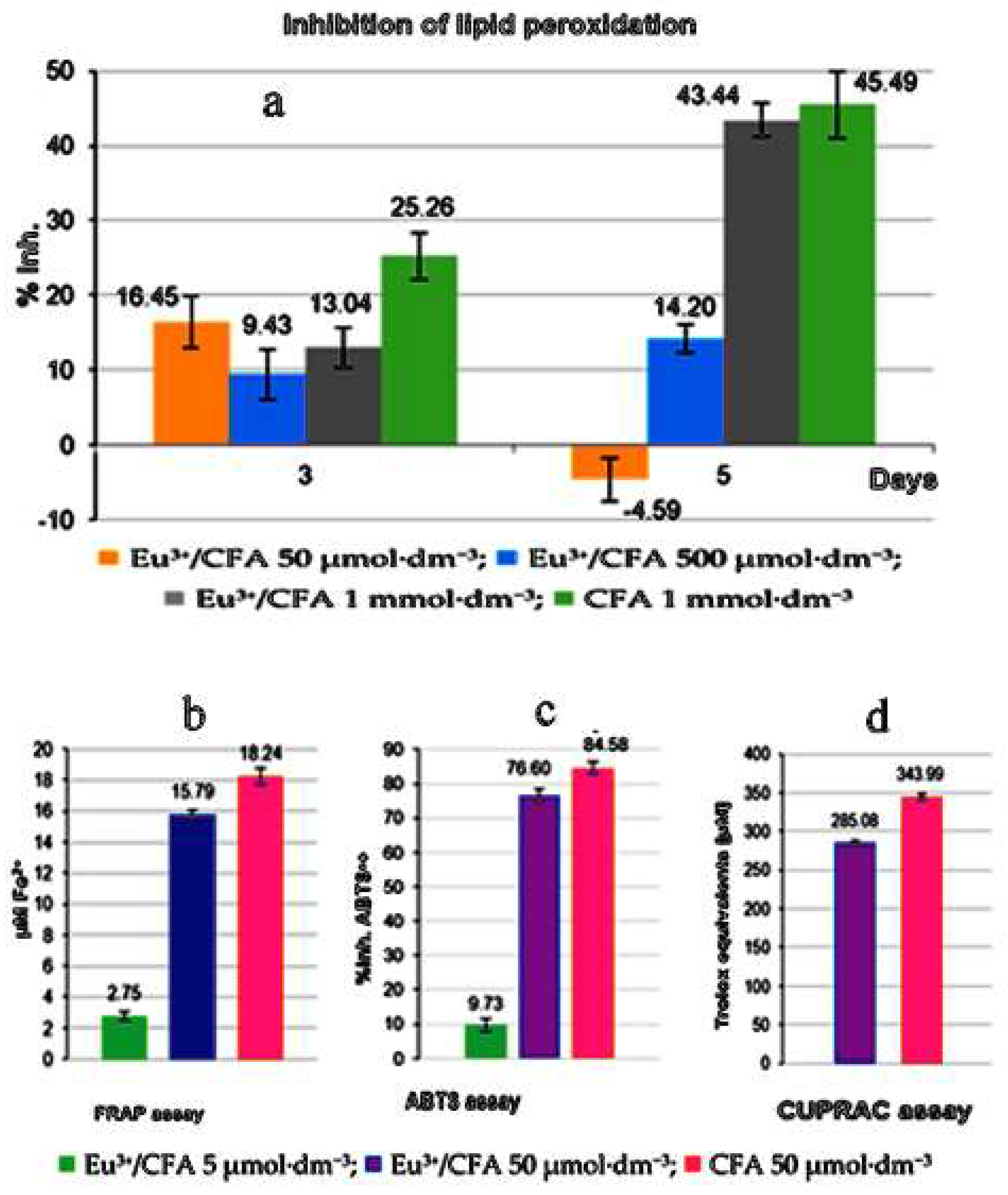
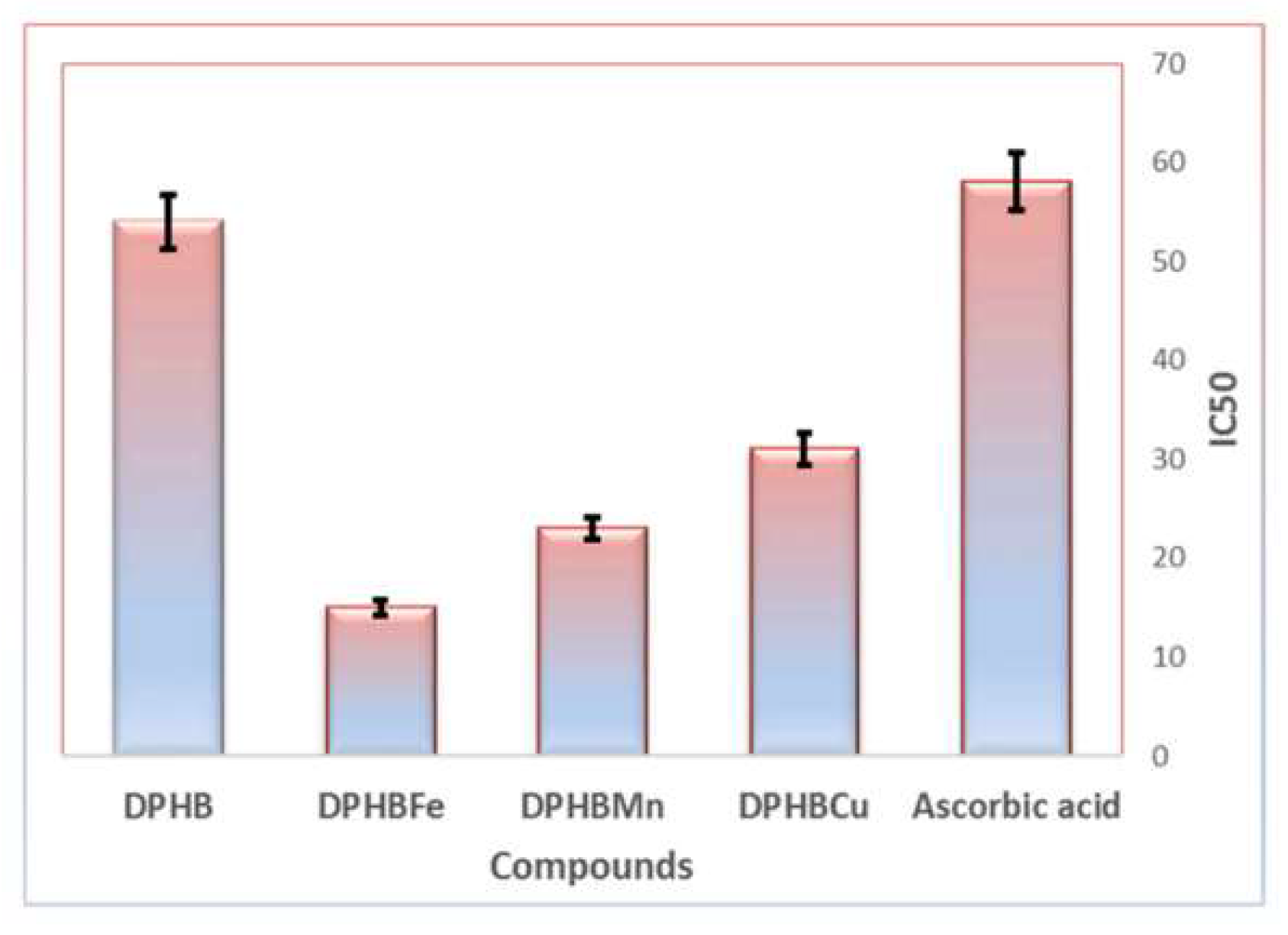
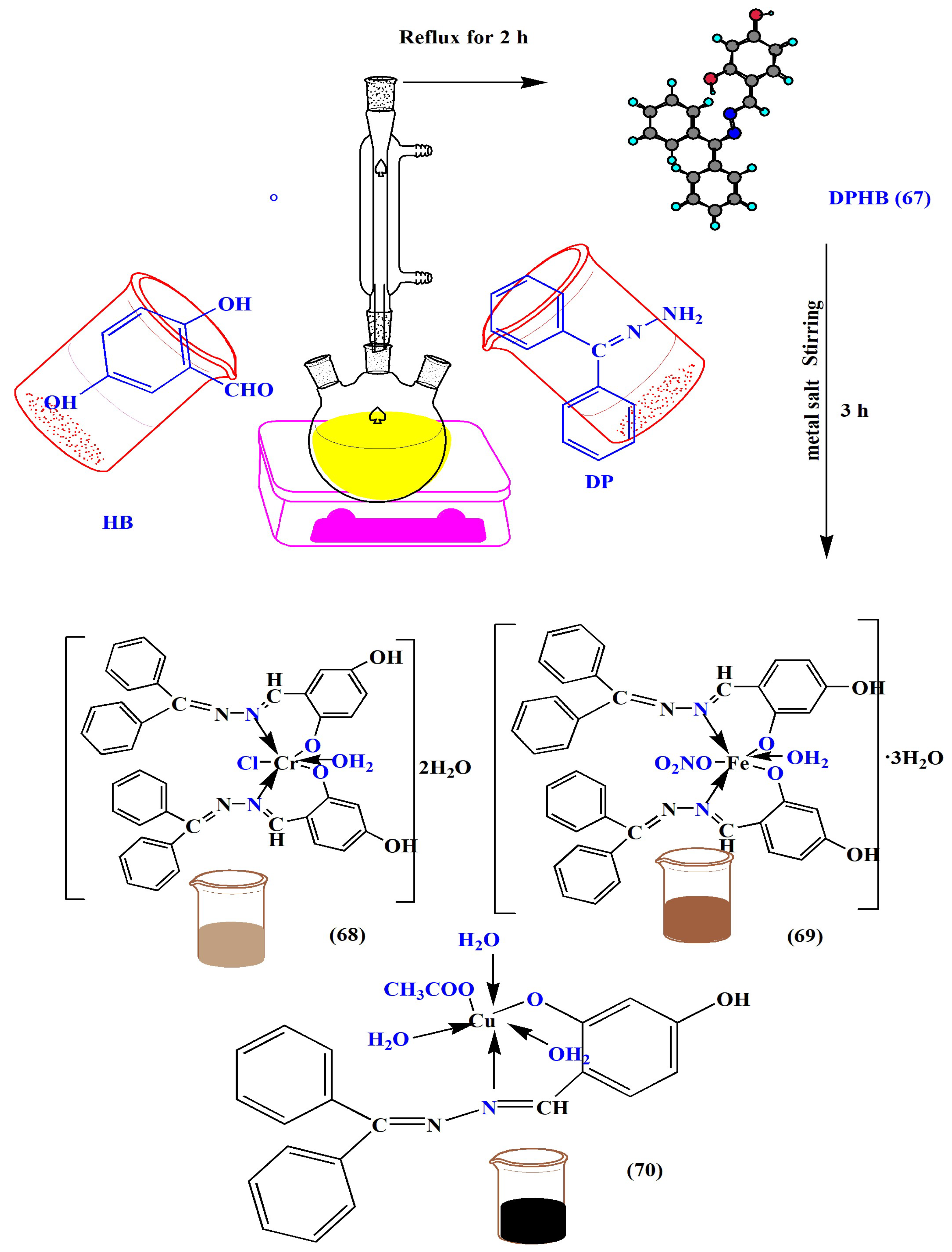


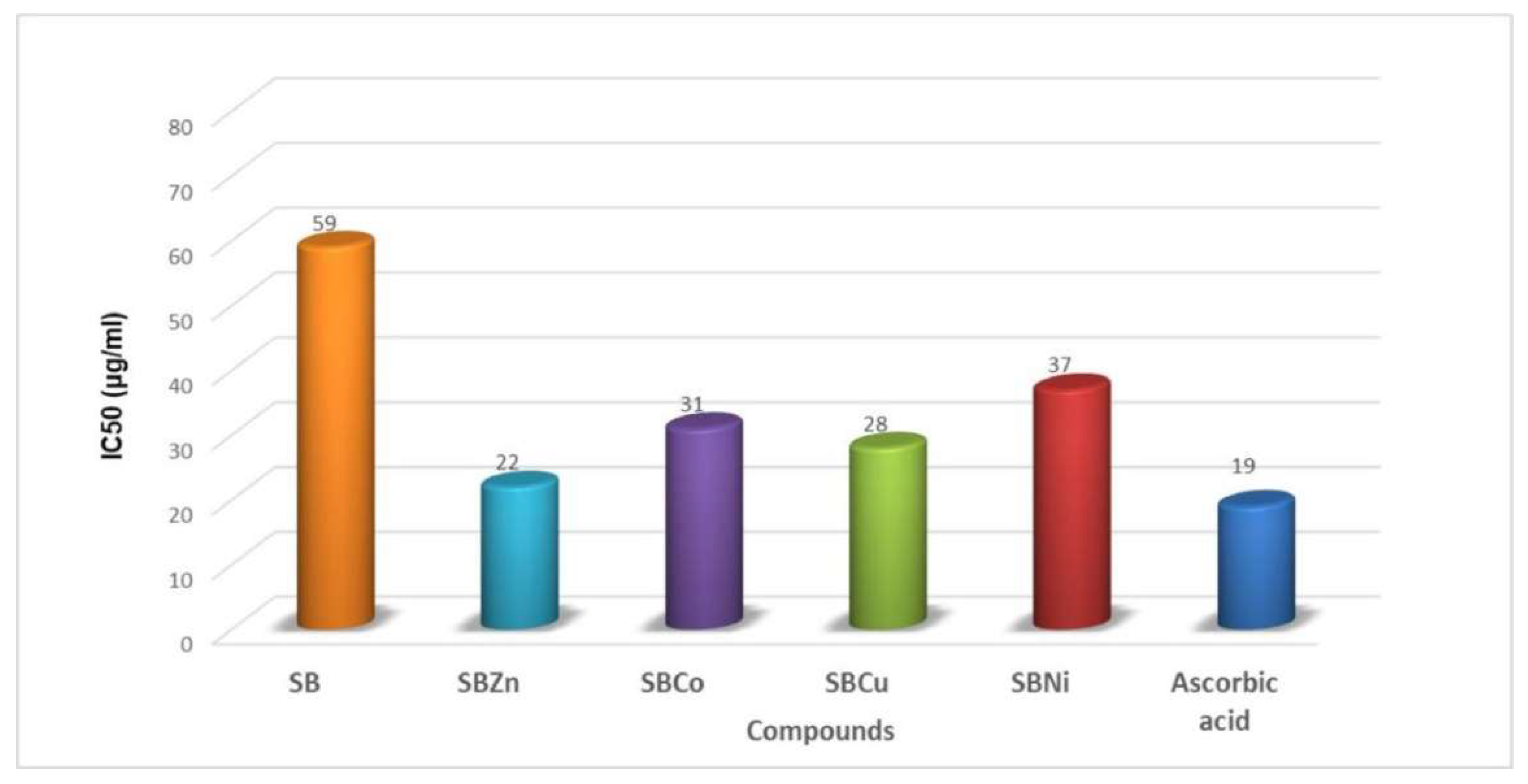
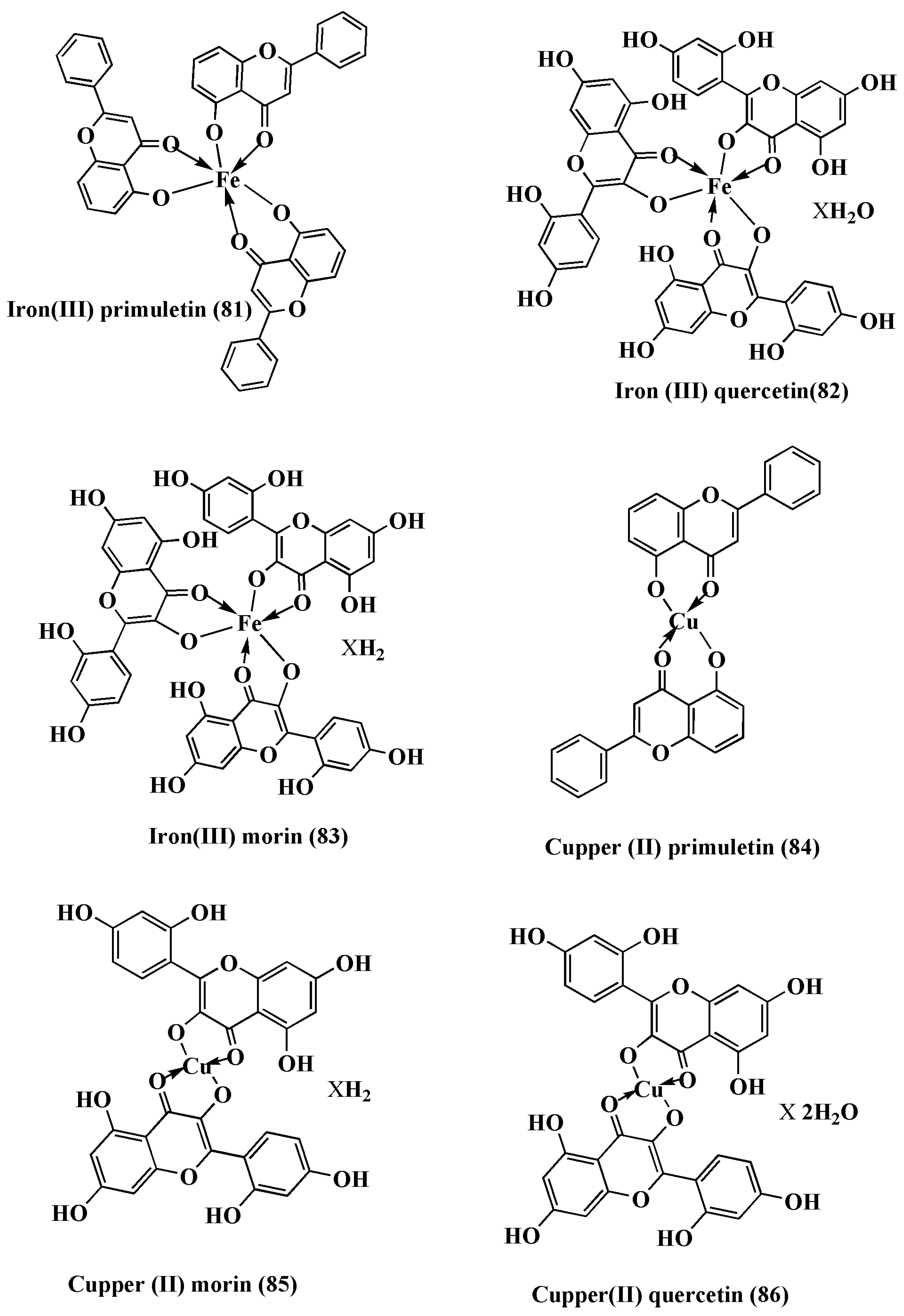

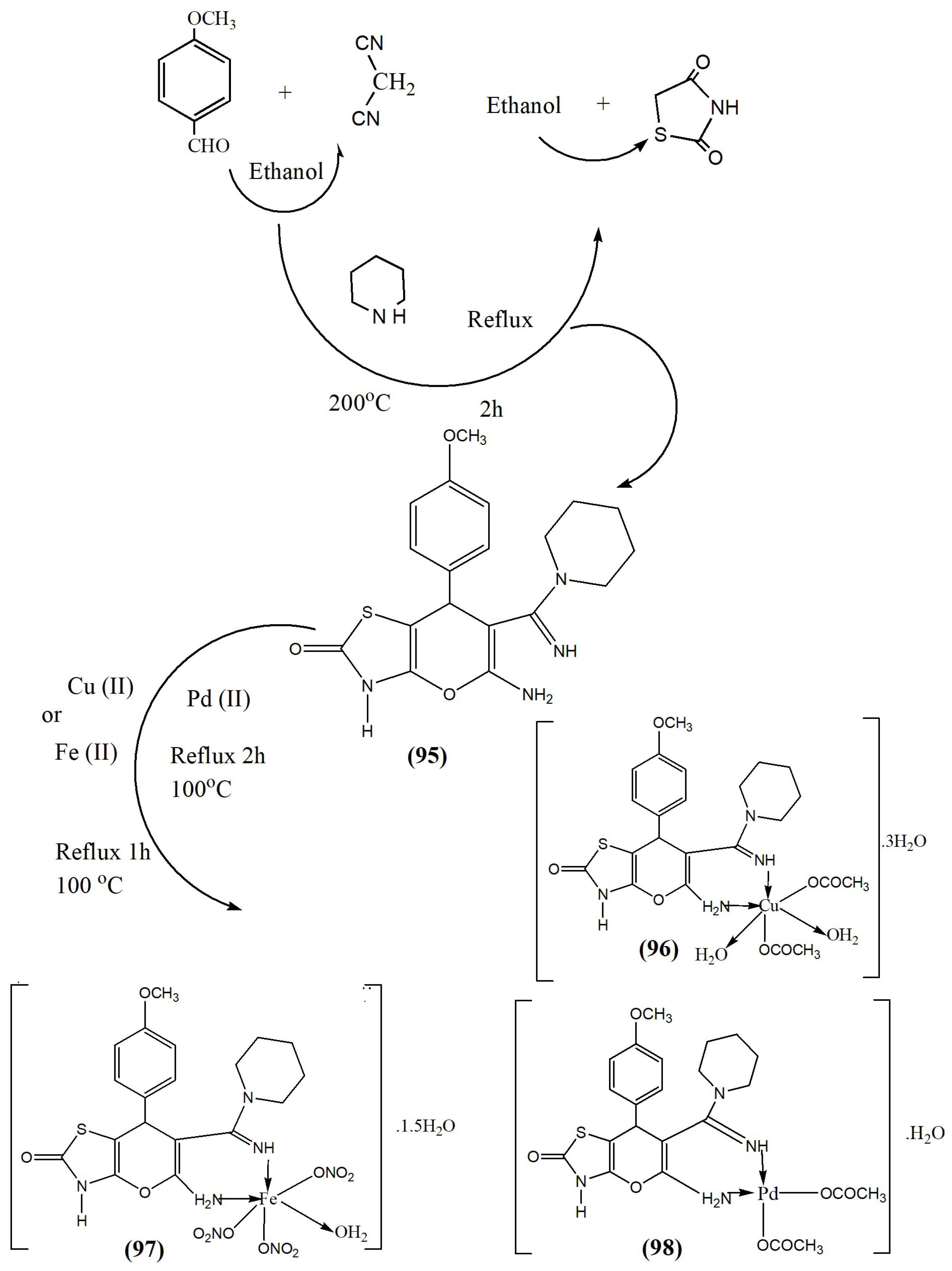
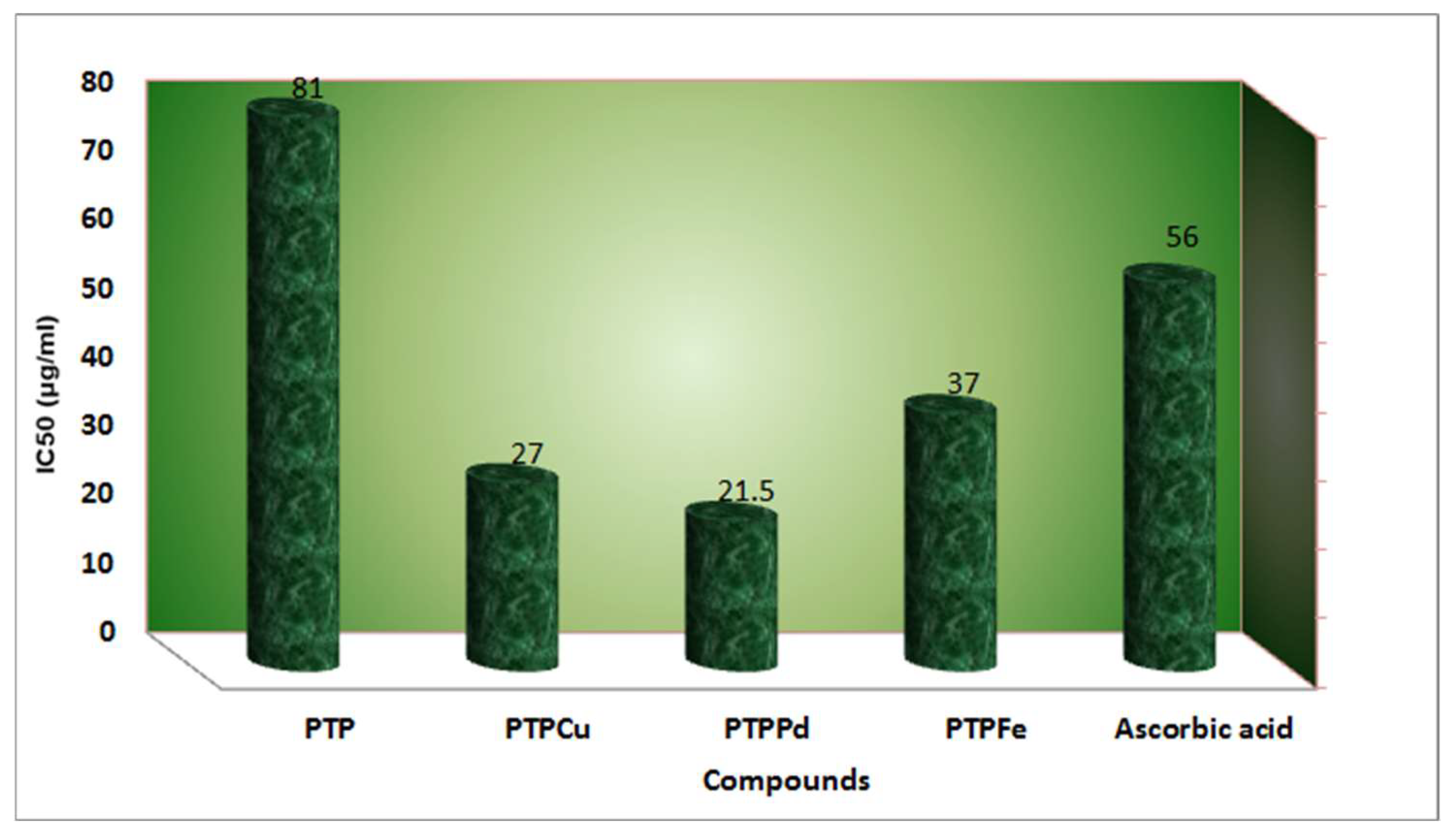
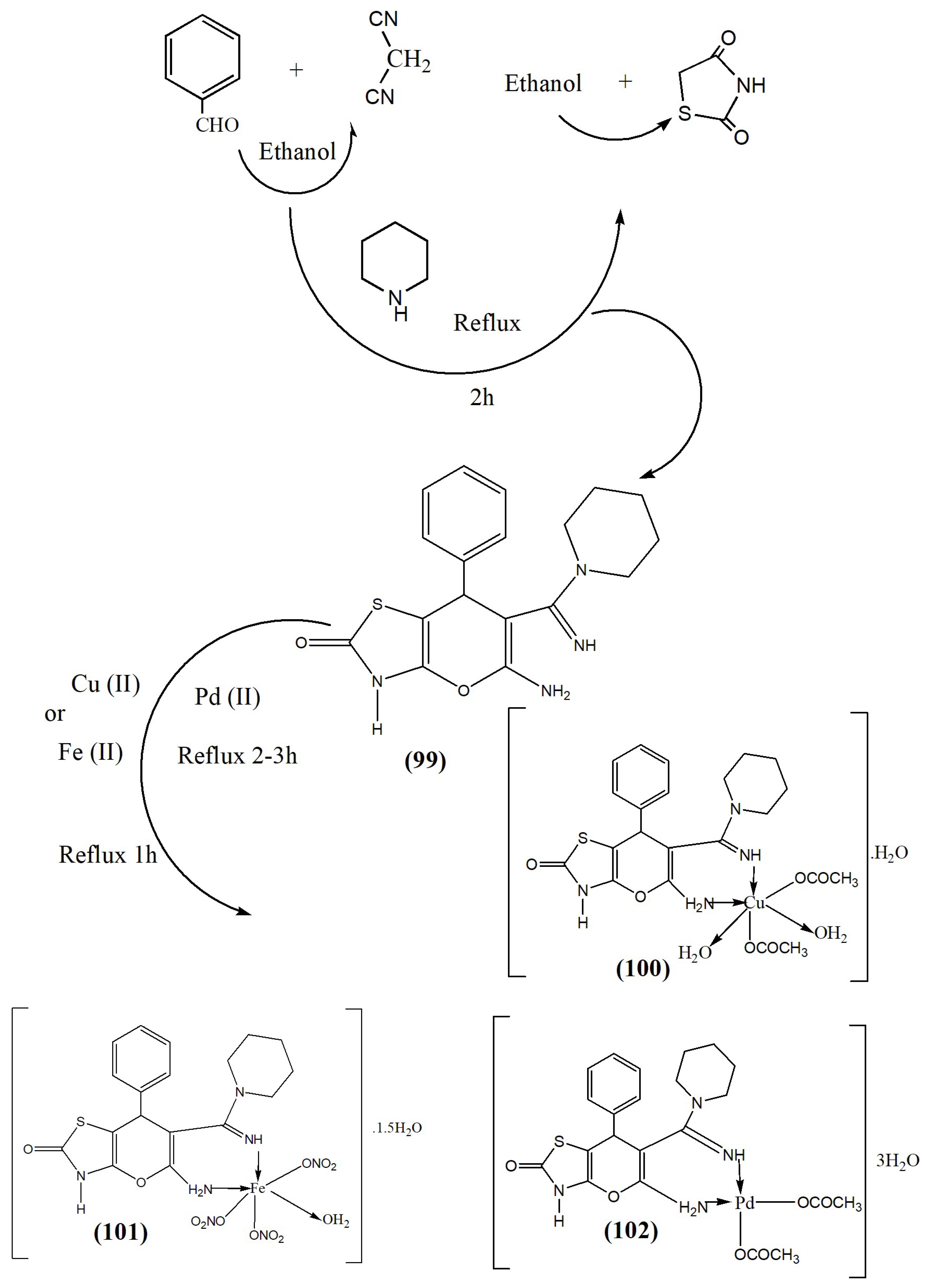

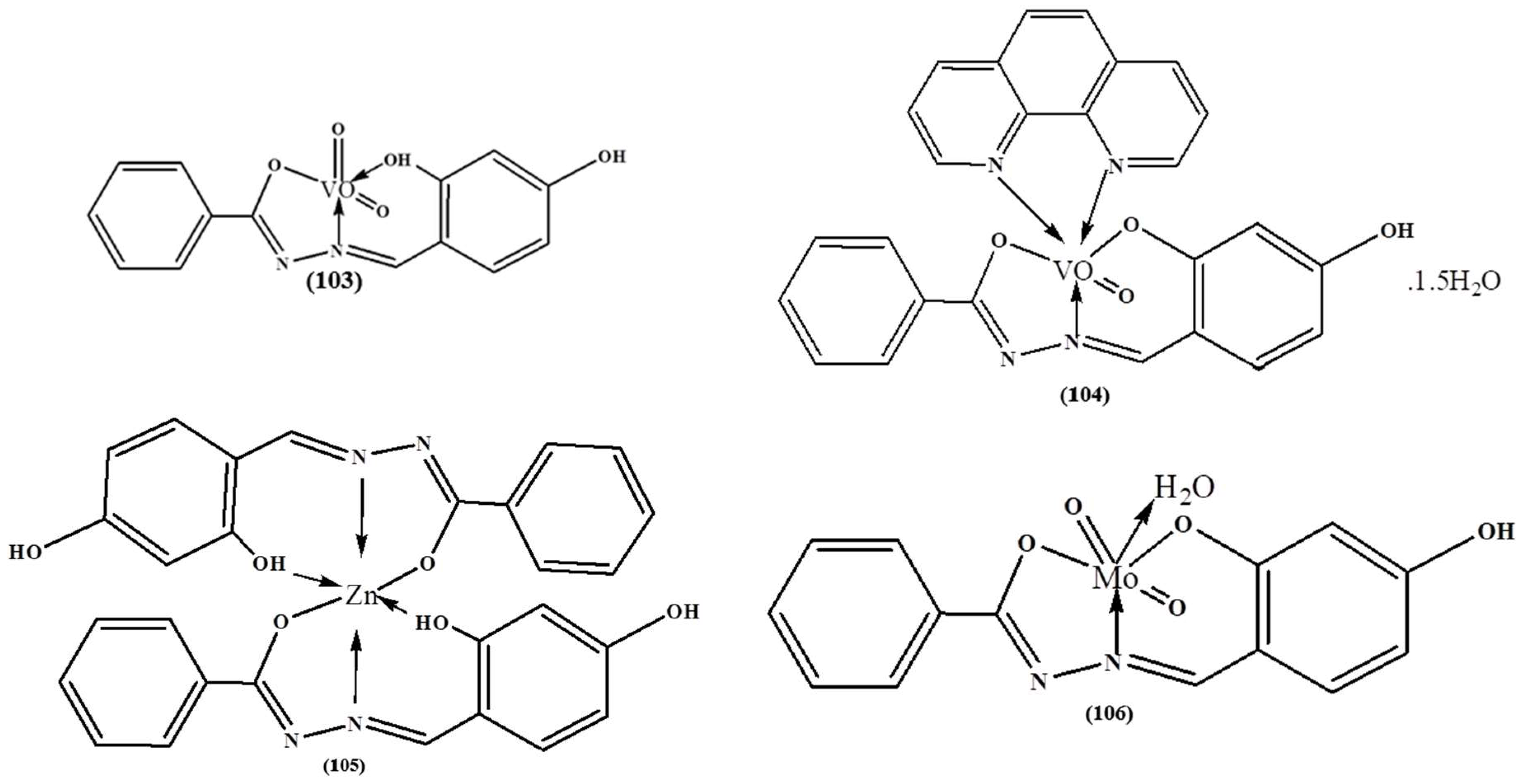
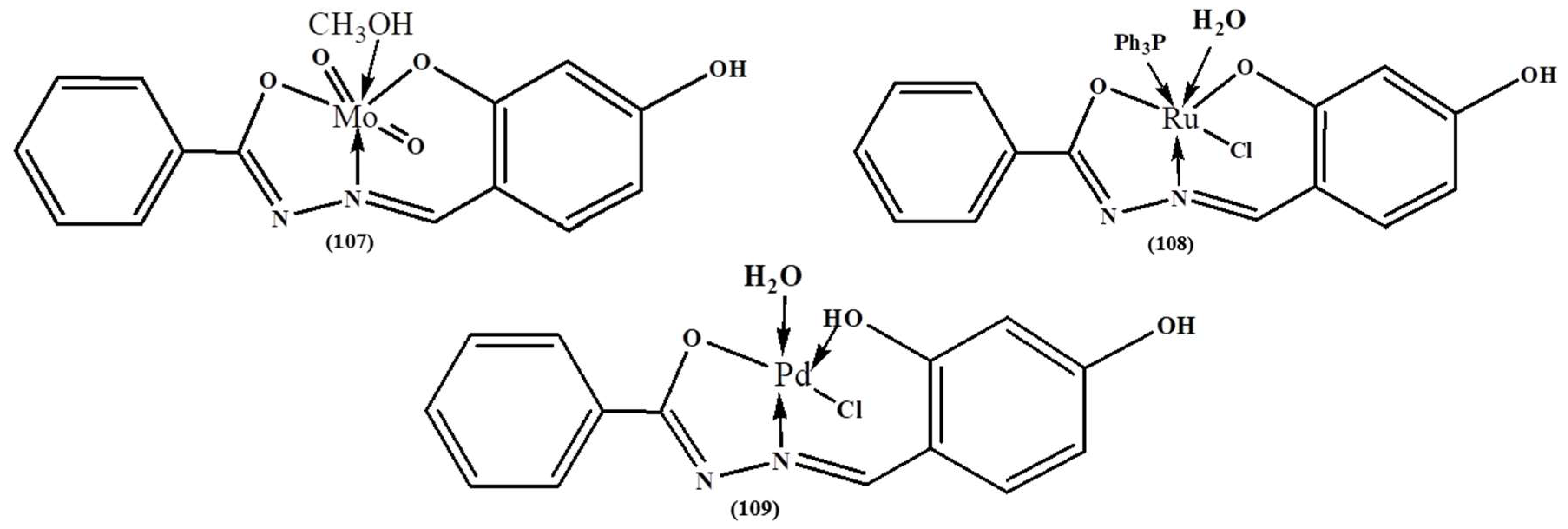
| Complex | % of Antioxidant Activity | |
|---|---|---|
| DPPH | H2O2 | |
| Cd complex (7) | 86.1 | 80.6 |
| Cu complex (8) | 78.1 | 71.4 |
| Co complex (9) | 74.2 | 72.2 |
| Zn complex (10) | 81.6 | 74.1 |
| α-tocopherol | 89.5 | 83.7 |
| Tested Compound | % DRSA ± Std. Error |
|---|---|
| L1(11) | 1.60 ± 0.12 |
| L2(12) | 78.45 ± 0.11 |
| L3(13) | 95.20 ± 0.14 |
| [(benzene)Ru(L1)Cl]PF6 (14) | 6.30 ± 0.25 |
| [(p-cymene)Ru(L2)Cl]PF6 (15) | 7.60 ± 0.23 |
| [(benzene)Ru(L2)Cl]PF6 (16) | 8.0 ± 0.06 |
| [(p-cymene)Ru(L3)Cl]PF6 (17) | 77.0 ± 0.02 |
| [(benzene)Ru(L3)Cl]PF6 (18) | 77.0 ± 0.08 |
| [Cp*Rh(L1)Cl]PF6 (19) | 3.70 ± 0.44 |
| [Cp*Rh(L2)Cl]PF6 (20) | 62.50 ± 0.12 |
| [Cp*Rh(L3)Cl]PF6 (21) | 81.20 ± 0.27 |
| [Cp*Ir(L2)Cl]PF6 (22) | 40.60 ± 0.35 |
| [Cp*Ir(L1)Cl]PF6 (23) | 11.0 ± 0.08 |
| [Cp*Ir(L3)Cl]PF6 (24) | 80.30 ± 0.11 |
| AA | 100 |
| Complex | Ligand (25) | Mn(II) Complex (26) | Ni(II) Complex (27) | Cd(II) Complex (28) | Pb(II) Complex (29) |
|---|---|---|---|---|---|
| IC50 values (mg/mL) | 0.53 ± 0.05 | 0.36 ± 0.13 | 0.20 ± 0.11 | 0.43 ± 0.05 | 0.35 ± 0.07 |
| C. No | Tested Compounds | IC50 (μM) |
|---|---|---|
| 36 | 4-((4-(benzyloxy)-2-hydroxybenzylidene)amino)-[1,1′-biphenyl]-3-ol (BHAP) | 6.13 ± 0.06 |
| 37 | 6-((4-(benzyloxy)-2-hydroxybenzylidene)amino)-4,6-dichloro -3-methylphenol (BHACM) | 5.76 ± 0.06 |
| 38 | 2-((4-(benzyloxy)-2-hydroxybenzylidene)amino)-6-chloro -4-nitrophenol (BHACN) | 4.98 ± 0.12 |
| 39 | 5-(benzyloxy)-2-(((2-hydroxyphenyl)imino)methyl)phenol (BHIMP) | 7.09 ± 0.07 |
| 40 | [Cupper(BHAP) (acetate)(H2O)2] | 3.81 ± 0.04 |
| 41 | [Cobalt(BHAP) (acetate)(H2O)2] | 5.20 ± 0.15 |
| 42 | [Zinc(BHAP) (acetate)(H2O)2] | 4.35 ± 0.07 |
| 43 | [Nickel(BHAP) (acetate)(H2O)2] | 4.76 ± 0.05 |
| 44 | [Cupper (BHACM) (acetate)(H2O)2] | 3.23 ± 0.04 |
| 45 | [Cobalt (BHACM)(acetate)(H2O)2] | 5.11 ± 0.12 |
| 46 | [Zinc (BHACM)(acetate)(H2O)2] | 4.35 ± 0.13 |
| 47 | [Nickel (BHACM) (acetate)(H2O)2] | 4.66 ± 0.08 |
| 48 | [Cupper (BHACN) (acetate)(H2O)2] | 2.98 ± 0.09 |
| 49 | [Cobalt (BHACN) (acetate)(H2O)2] | 4.53 ± 0.08 |
| 50 | [Zinc (BHACN) (acetate)(H2O)2] | 3.76 ± 0.05 |
| 51 | [Nickel (BHACN) (acetate)(H2O)2] | 4.02 ± 0.06 |
| 52 | [Cupper (BHIMP) (acetate)(H2O)2] | 3.89 ± 0.14 |
| 53 | [Cobalt (BHIMP) (acetate)(H2O)2] | 5.38 ± 0.16 |
| 54 | [Zinc (BHIMP) (acetate)(H2O)2] | 4.22 ± 0.12 |
| 55 | [Nickel (BHIMP) (acetate)(H2O)2] | 4.93 ± 0.09 |
| Ascorbic acid | 1.95 ± 0.07 |
| Chelate | IC50 (mg/mL) |
|---|---|
| Ascorbic acid | 0.042 |
| Mn-phenalenyl complex (76) | 0.059 |
| Al-phenalenyl complex (77) | 0.156 |
| Fe-phenalenyl complex (78) | 0.115 |
| Ni-phenalenyl complex (79) | 0.143 |
| Co-phenalenyl complex (80) | 0.086 |
| Ki (μM) | IC50 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AChE | BChE | GST | AChE | r2 | BChE | r2 | GST | r2 | |
| TBHPC (87) | 7.13 ± 0.84 * | 5.75 ± 1.03 * | 11.06 ± 1.26 | 14.63 | 0.88 | 11.42 | 0.94 | 17.77 | 0.96 |
| TBHPCFe (88) | 8.89 ± 0.83 | 8.04 ± 1.44 | 9.37 ± 1.06 * | 16.98 | 0.94 | 15.21 | 0.99 | 14.08 | 0.94 |
| TBHPCNi (89) | 9.27 ± 1.28 | 7.88 ± 1.86 | 10.87 ± 1.33 | 13.61 | 0.836 | 12.95 | 0.98 | 15.24 | 0.98 |
| TBHPCCo (90) | 7.73 ± 0.74 | 10.24 ± 1.66 | 9.98 ± 0.91 | 16.07 | 0.96 | 18.74 | 0.93 | 16.98 | 0.99 |
| Ethacrynic acid ** | - | - | 0.386 ± 0.04 | - | - | - | - | 0.374 | 0.96 |
| Tacrine *** | 0.56 ± 0.06 | 1.32 ± 0.15 | - | 0.85 | 0.99 | 2.49 | 0.90 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Lateef, H.M.A.; El‐Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Recent Overview of Potent Antioxidant Activity of Coordination Compounds. Antioxidants 2023, 12, 213. https://doi.org/10.3390/antiox12020213
El-Lateef HMA, El‐Dabea T, Khalaf MM, Abu-Dief AM. Recent Overview of Potent Antioxidant Activity of Coordination Compounds. Antioxidants. 2023; 12(2):213. https://doi.org/10.3390/antiox12020213
Chicago/Turabian StyleEl-Lateef, Hany M. Abd, Tarek El‐Dabea, Mai M. Khalaf, and Ahmed M. Abu-Dief. 2023. "Recent Overview of Potent Antioxidant Activity of Coordination Compounds" Antioxidants 12, no. 2: 213. https://doi.org/10.3390/antiox12020213
APA StyleEl-Lateef, H. M. A., El‐Dabea, T., Khalaf, M. M., & Abu-Dief, A. M. (2023). Recent Overview of Potent Antioxidant Activity of Coordination Compounds. Antioxidants, 12(2), 213. https://doi.org/10.3390/antiox12020213









