Distributions of α- and δ-TOCopherol in Intact Olive and Soybean Oil-in-Water Emulsions at Various Acidities: A Test of the Sensitivity of the Pseudophase Kinetic Model
Abstract
:1. Introduction
1.1. The Problem of Determining Antioxidant Distributions in Intact Emulsions
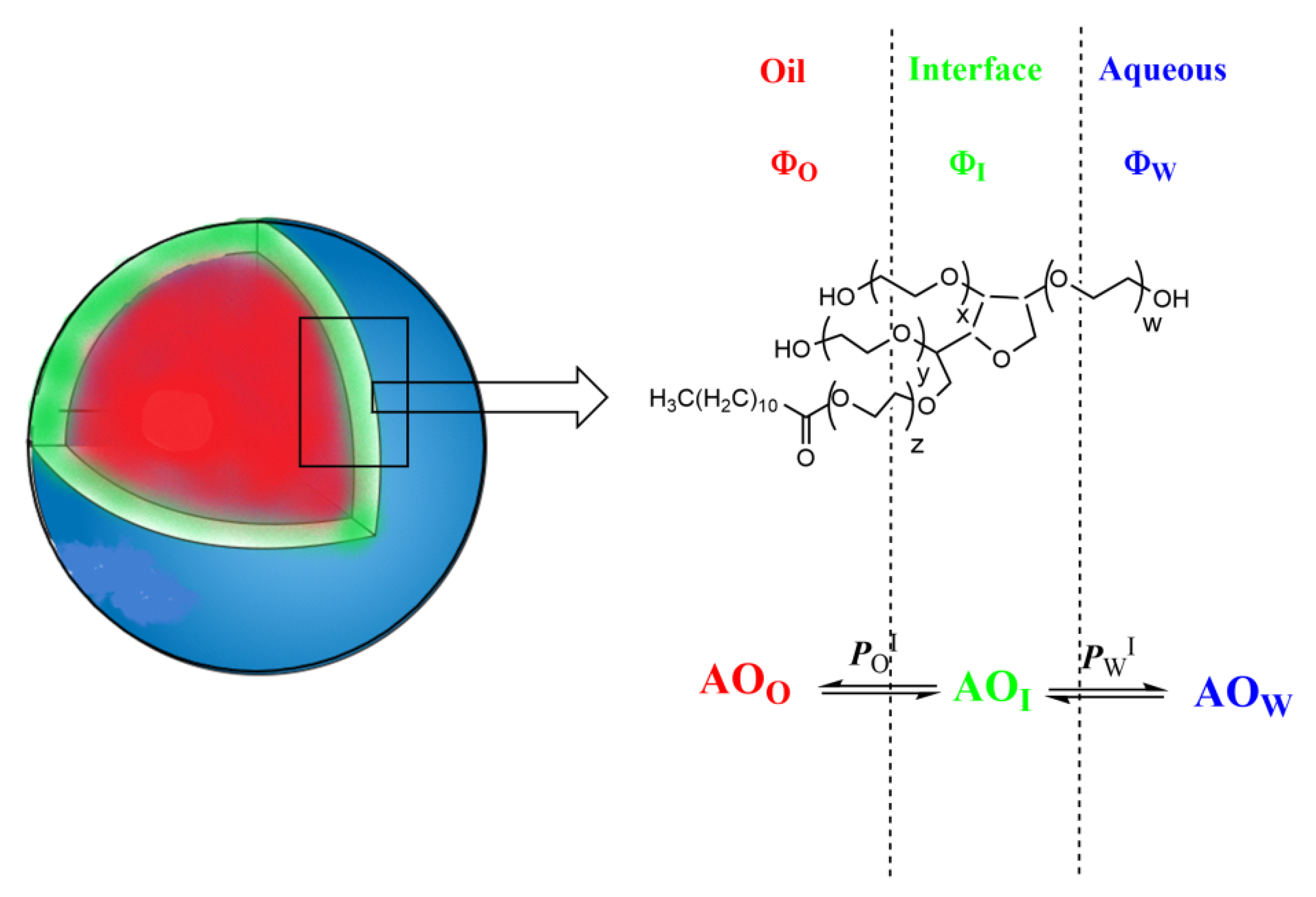
1.2. Pseudophase Kinetic Approach: Determining Antioxidant Distributions in Intact Emulsions
1.3. Separating Medium from Concentration Effects on the Rates of Reactions in Emulsions: Is the Pseudophase Kinetic Model a Trustable Method?
1.4. Applicability and Importance of the Study
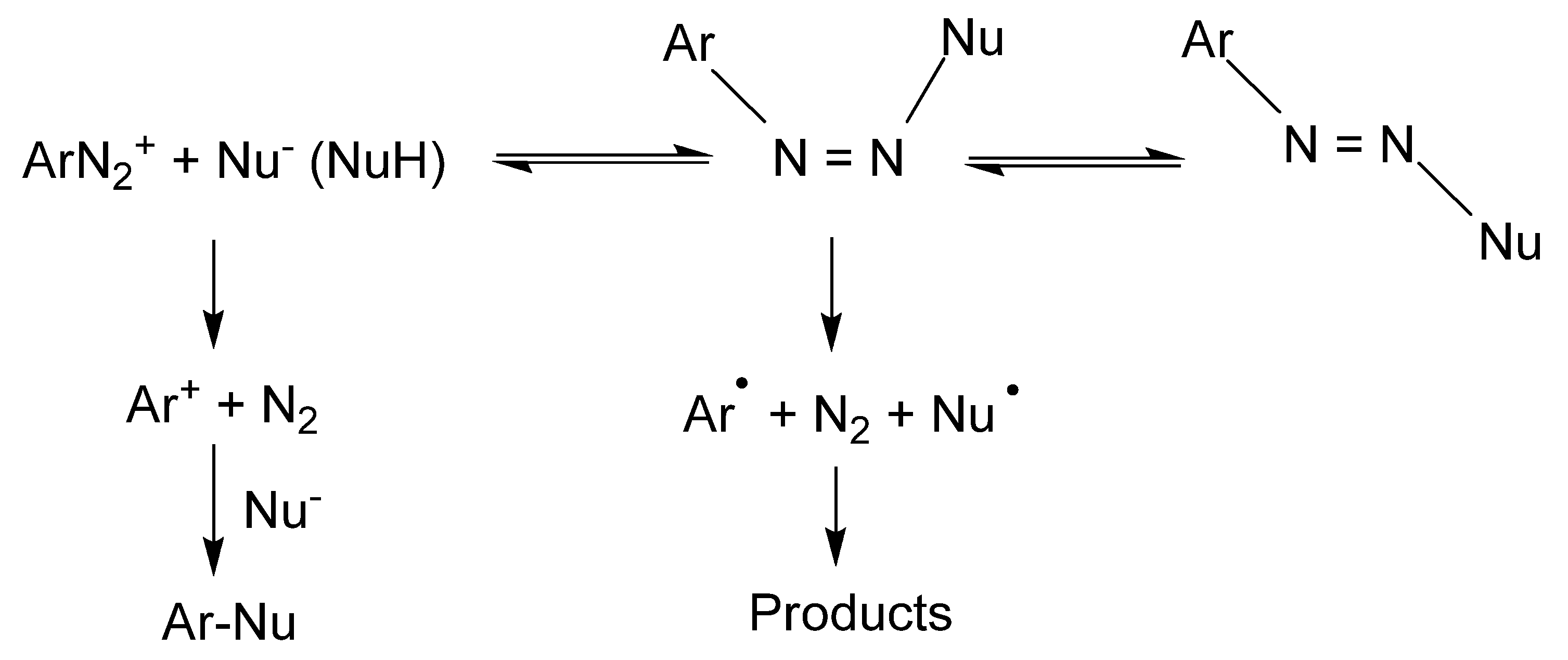
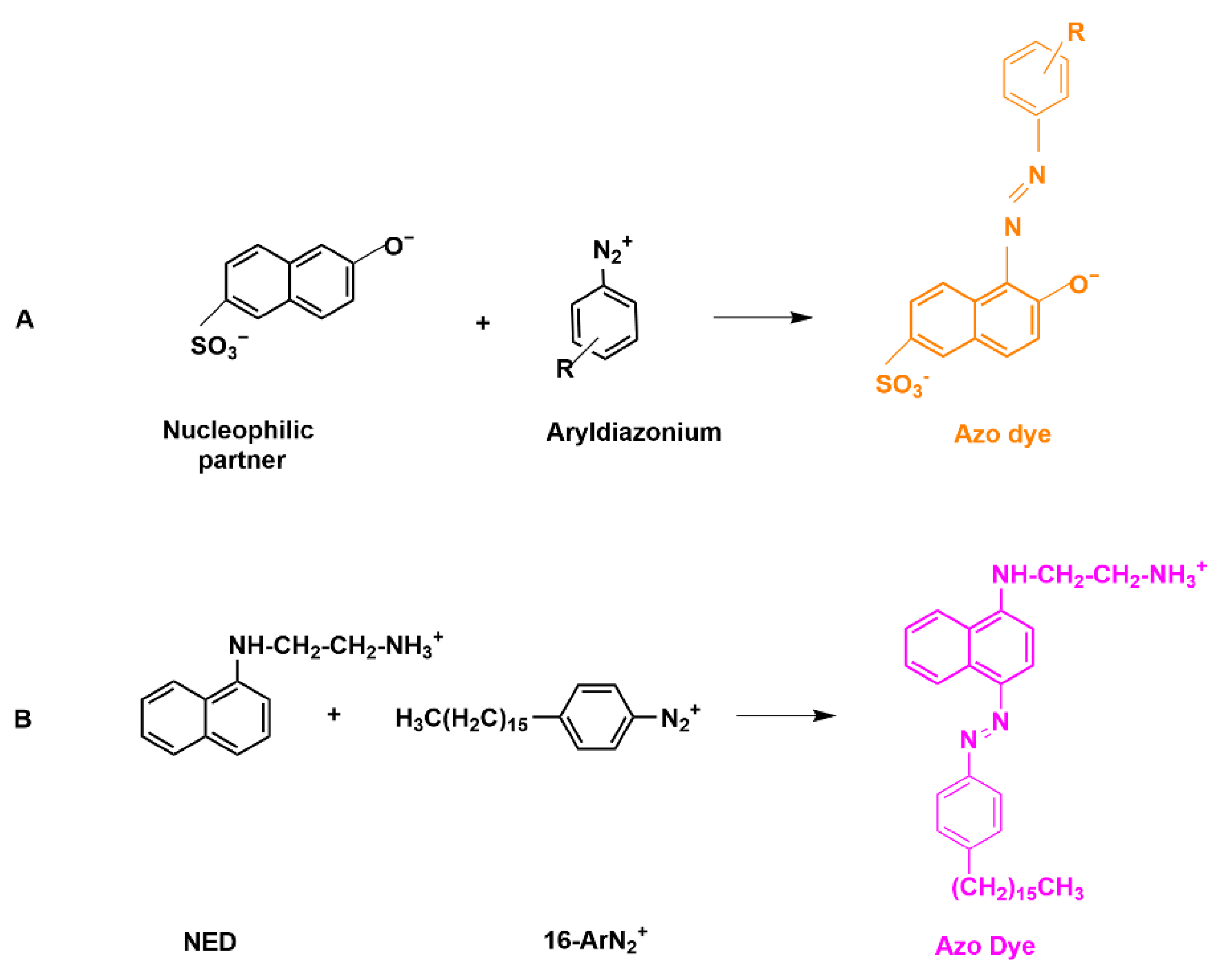
2. A Brief Insight into the Chemistry of Aryldiazonium Ions: C- and O-Coupling Reactions
3. Materials and Methods
3.1. Materials
3.2. Preparation of Emulsions
3.3. Methods
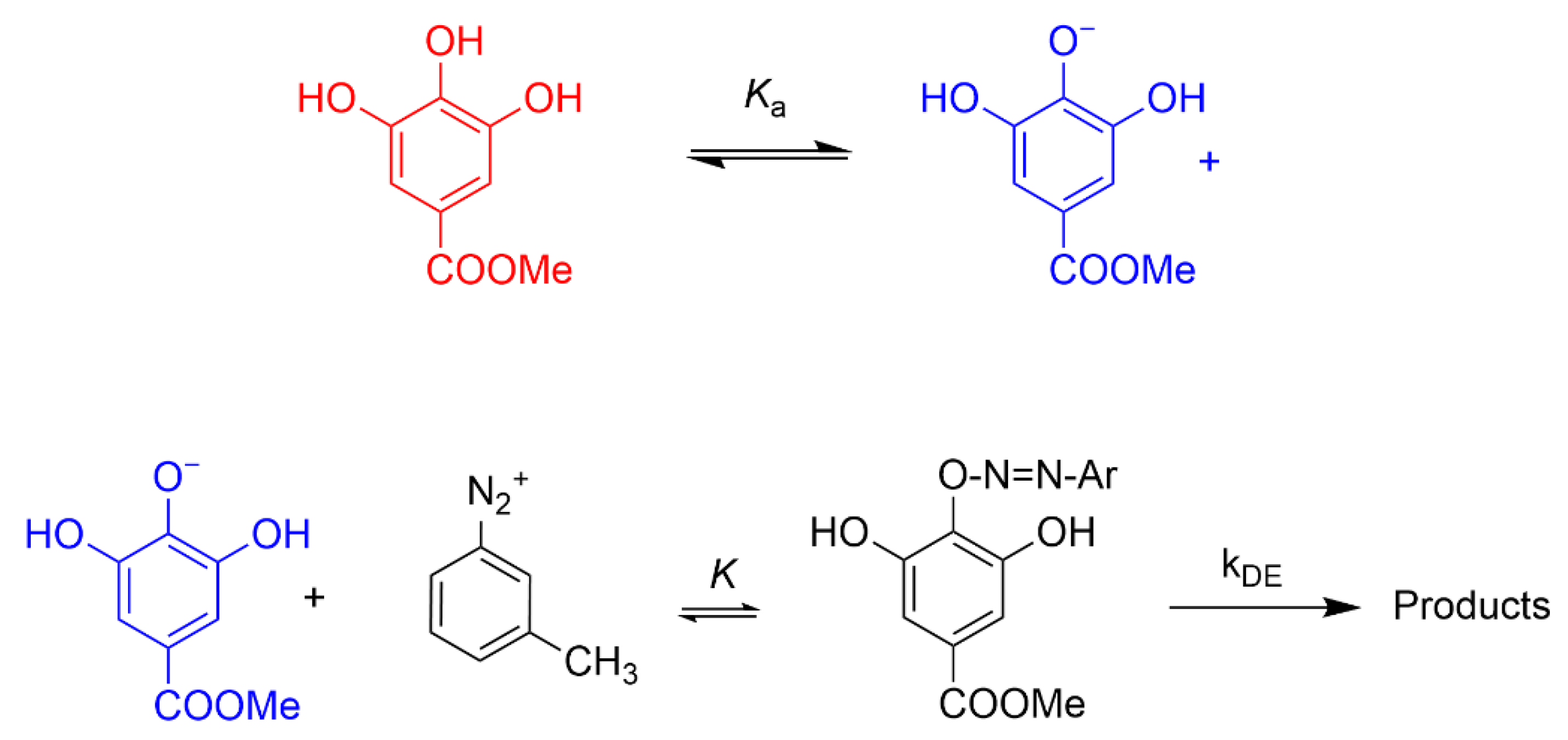
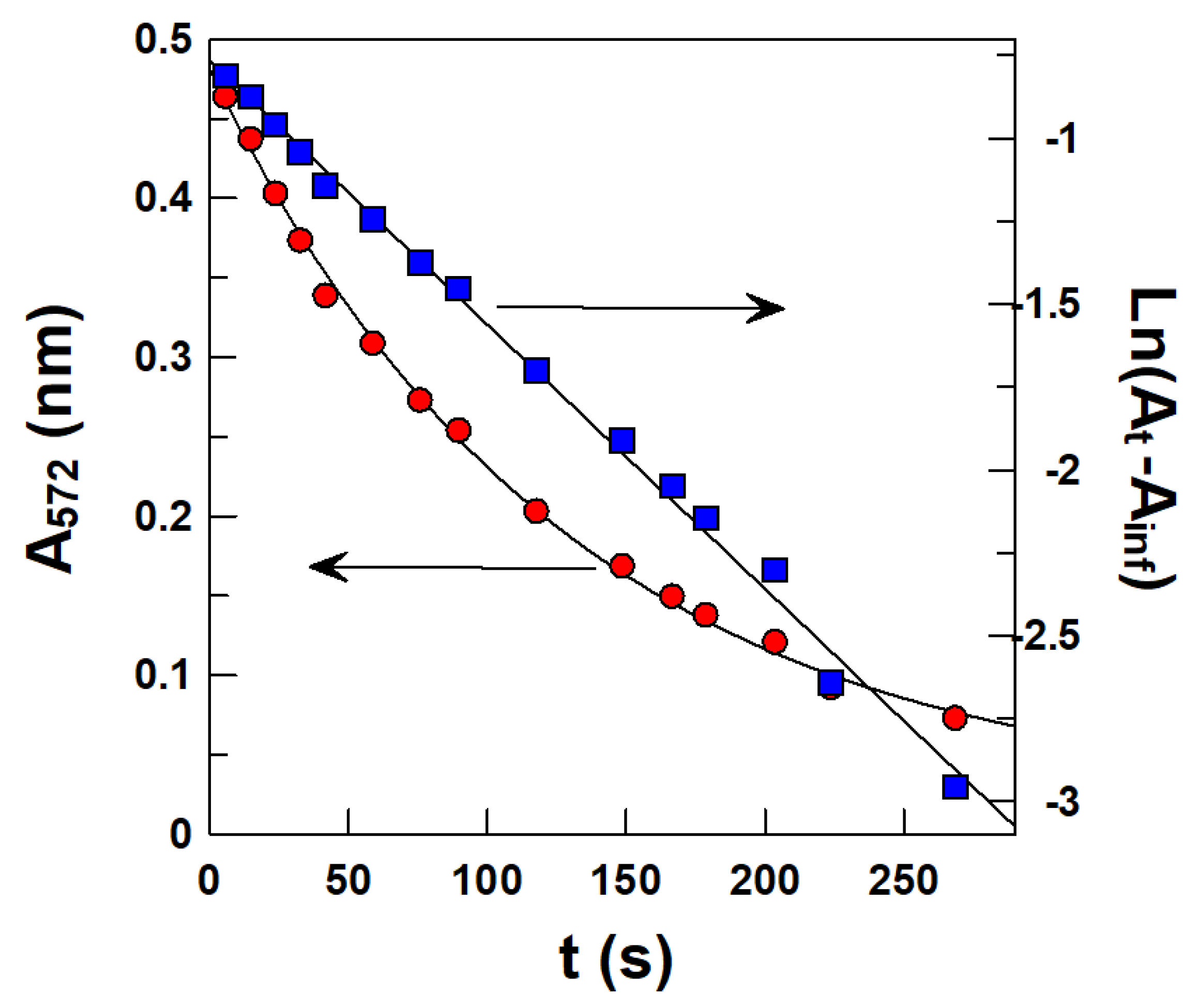
Determining Observed Rate Constant (kobs) Values for the Reaction between 16-ArN2+ and the AOs in Intact Emulsions
3.4. Statistical Analysis
4. Results and Discussion
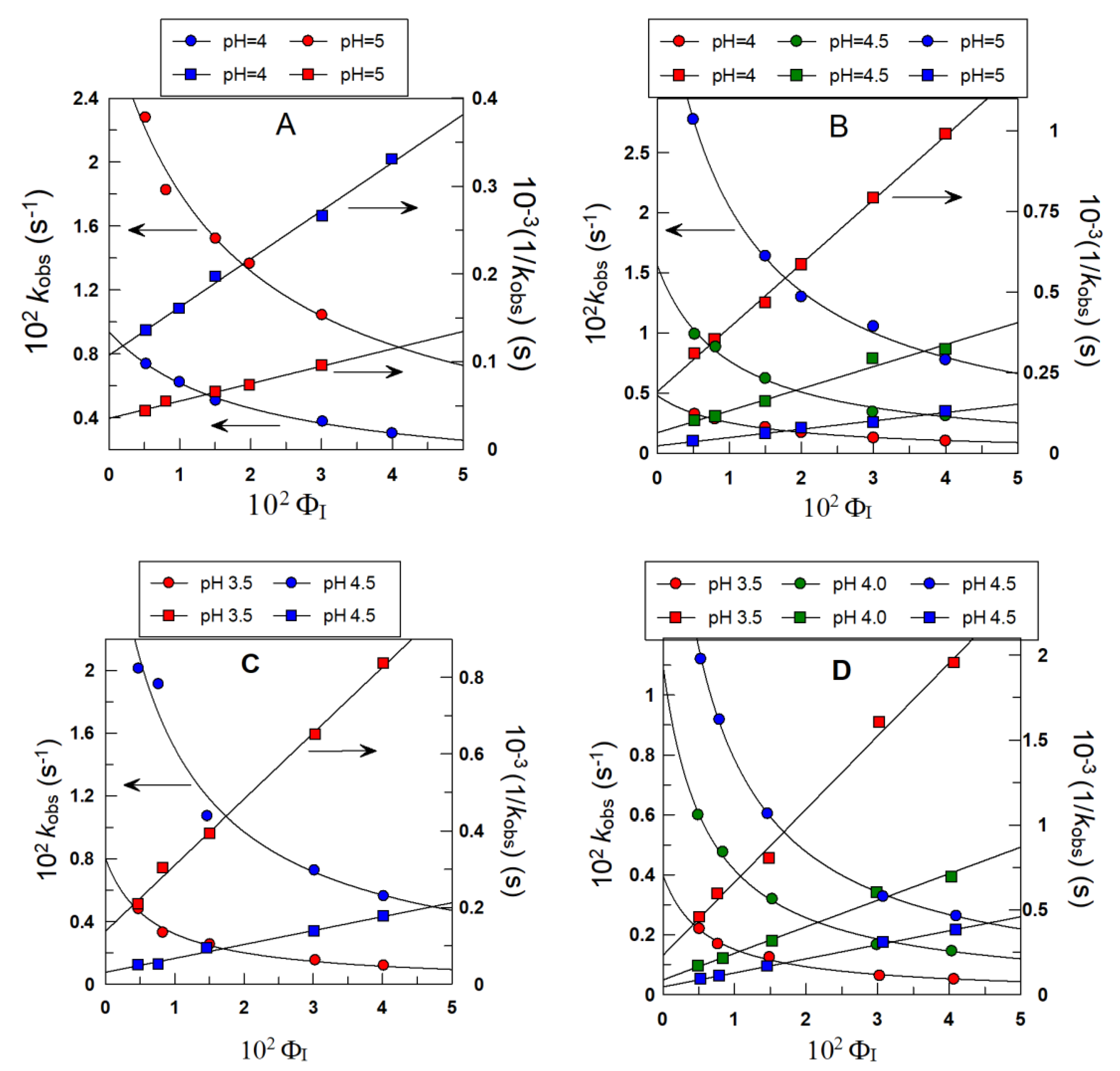
4.1. Partition Constants PoI and Interfacial Rate Constants kI of α- and δ-TOC in Intact Emulsions
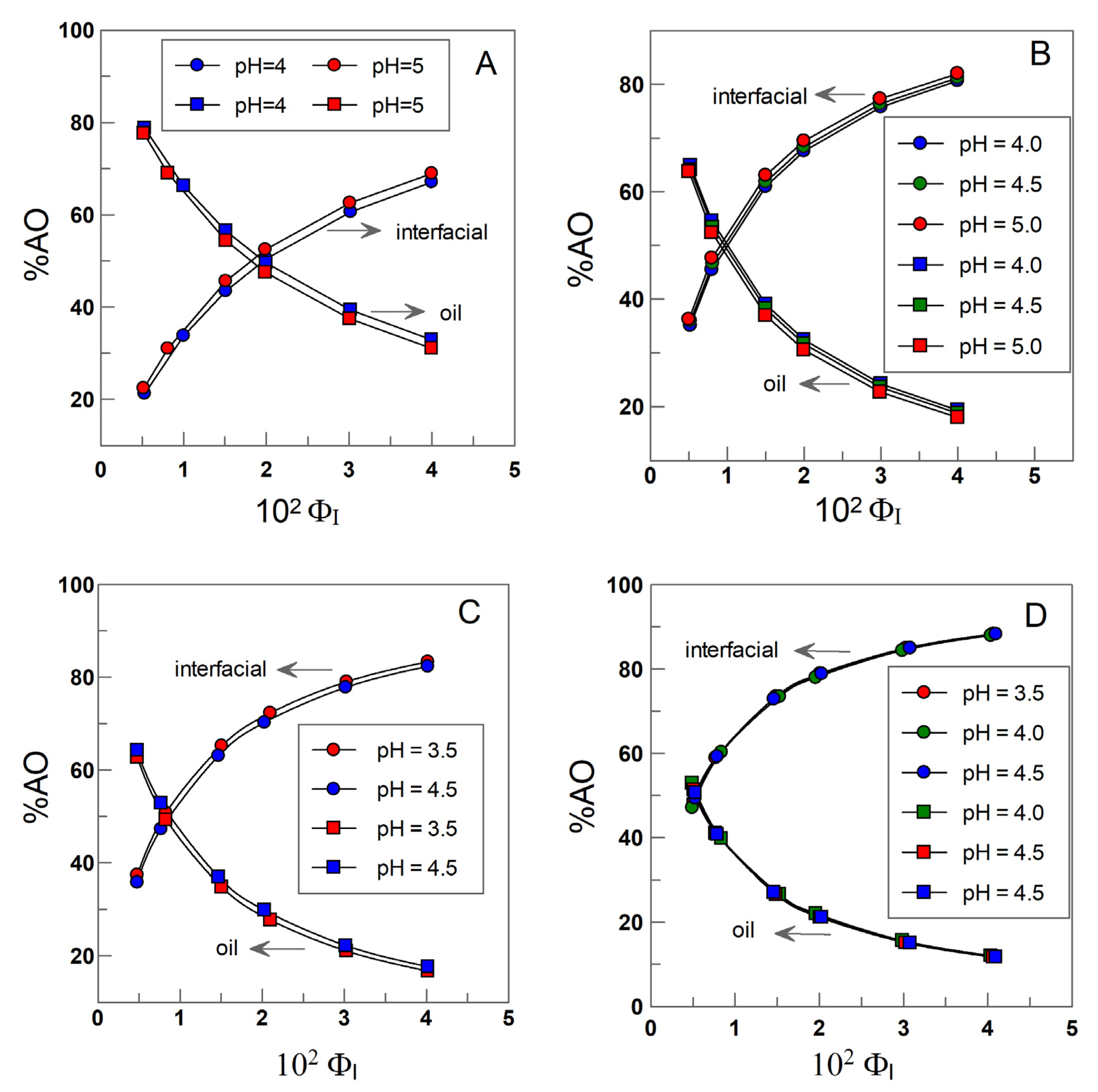
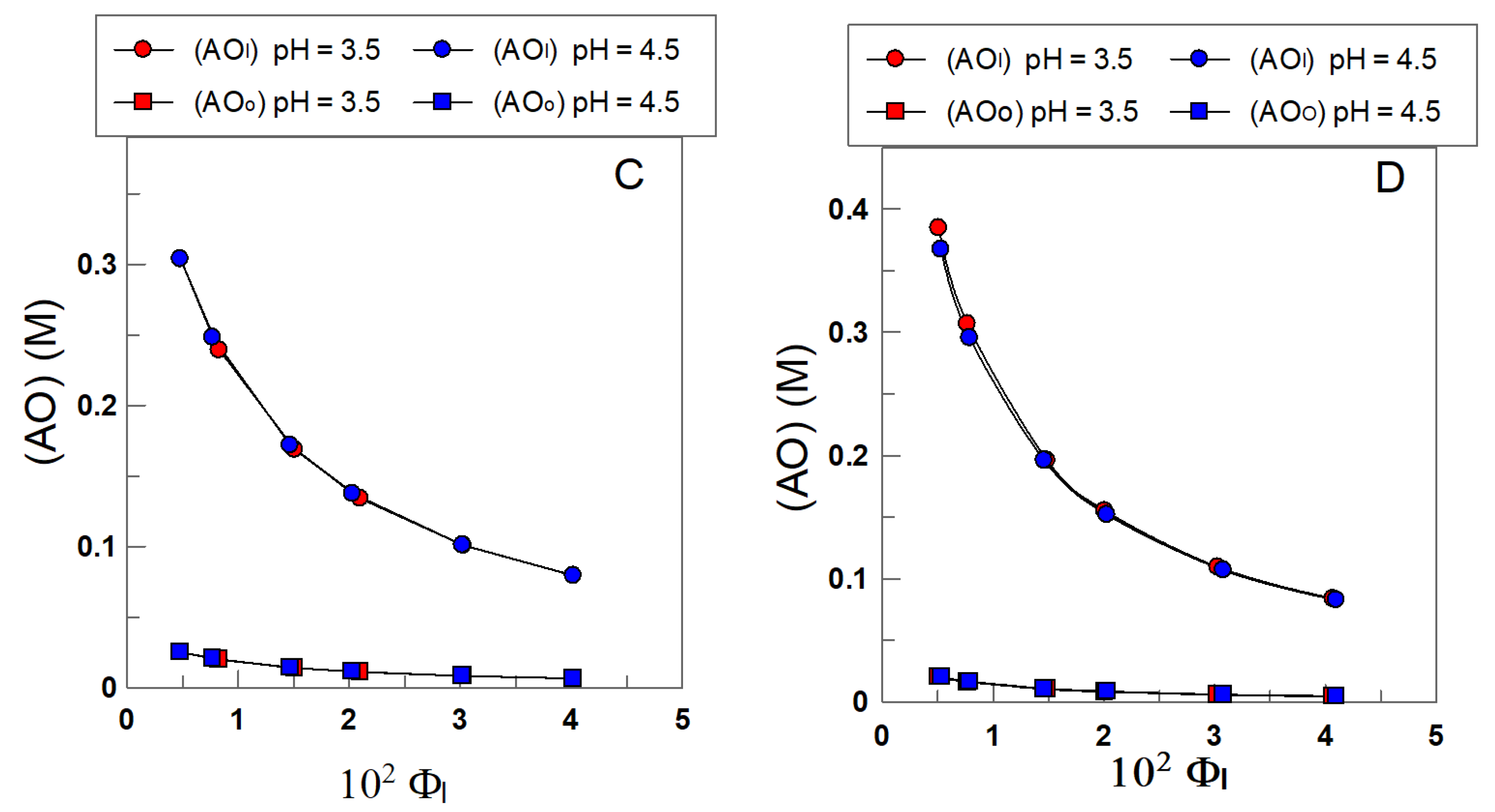
4.2. Distribution and Effective Concentrations of α- and δ-TOC in Soybean and Olive Oil Emulsions
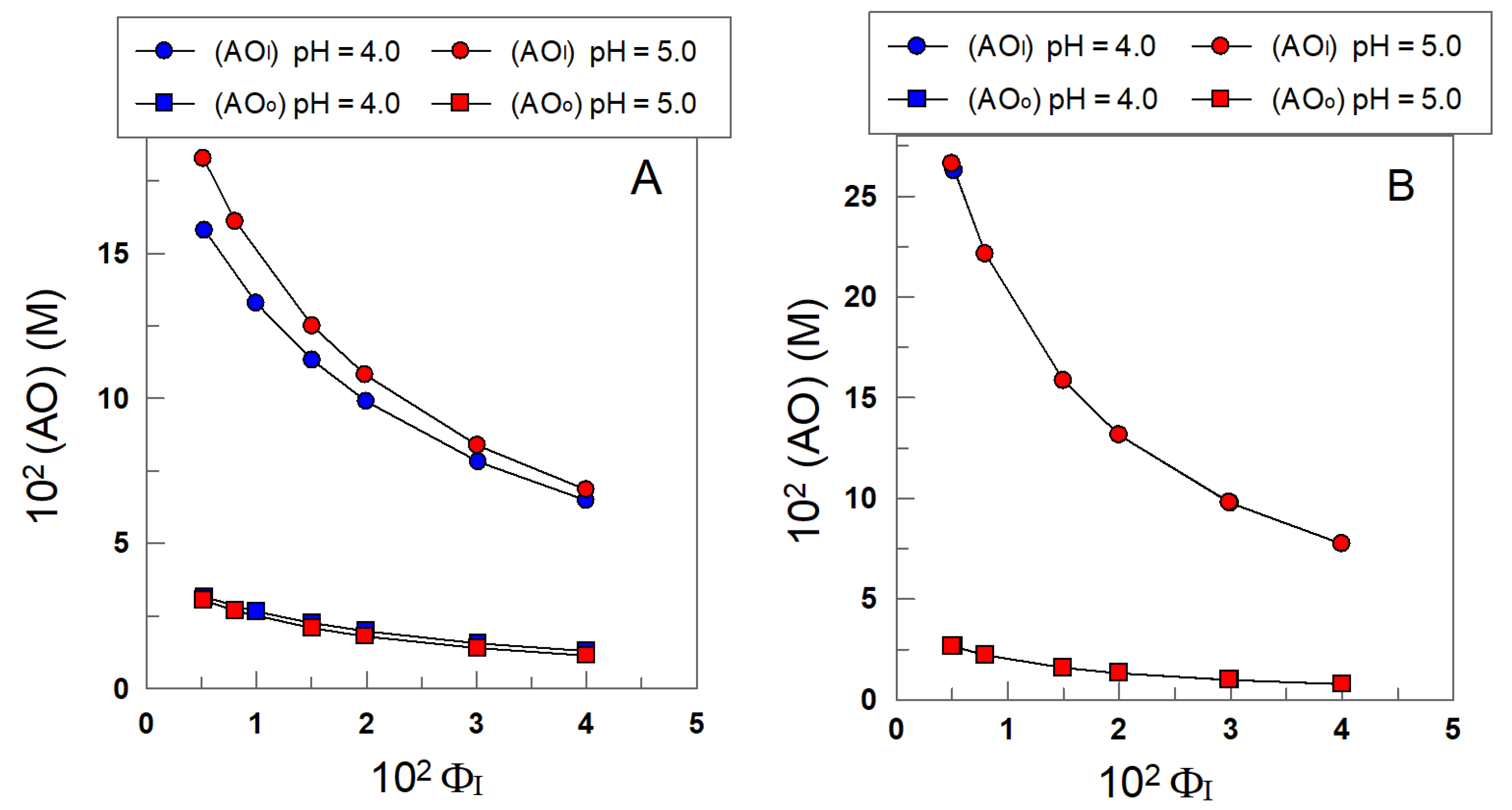
4.3. Effective Concentrations of α-TOC and δ-TOC in the Interfacial and Oil Regions of the Emulsions
5. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaich, K.M. Lipid Antioxidants: More than Just Lipid Radical Quenchers. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 151–184. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Estévez, M. Lipid Oxidation in Meat Systems: Updated Means of Detection and Innovative Antioxidant Strategies. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 93–111. [Google Scholar] [CrossRef]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M. Lipid Oxidation: New Perspectives on an Old Reaction. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 1–72. [Google Scholar] [CrossRef]
- Frankel, E. Lipid Oxidation; The Oily Press, PJ Barnes & Associates: Bridgwater, UK, 2005. [Google Scholar]
- Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Losada-Barreiro, S. Control of Lipid Oxidation in Oil-In Water Emulsions: Effects of Antioxidant Partitioning and Surfactant Concentration. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 201–216. [Google Scholar] [CrossRef]
- Bravo-Díaz, C. Advances in the control of lipid peroxidation in oil-in-water emulsions: Kinetic approaches. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C. Chapter 8—Oxidative Stability and Shelf Life of Food Emulsions. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; AOCS Press: Urbana, IL, USA, 2016; pp. 287–312. [Google Scholar] [CrossRef] [Green Version]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil–water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef]
- Meireles, M.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Monteiro, L.S. Control of antioxidant efficiency of chlorogenates in emulsions: Modulation of antioxidant interfacial concentrations. J. Sci. Food Agric. 2019, 99, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Mitrus, O.; Żuraw, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Paiva-Martins, F. Targeting Antioxidants to Interfaces: Control of the Oxidative Stability of Lipid-Based Emulsions. J. Agric. Food Chem. 2019, 67, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K. Metals and lipid oxidation. Contemporary issues. Lipids 1992, 27, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zembyla, M.; Murray, B.S.; Sarkar, A. Water-in-oil emulsions stabilized by surfactants, biopolymers and/or particles: A review. Trends Food Sci. Technol. 2020, 104, 49–59. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, M.; Mao, Z.; Xiao, J.; Huang, Q.; Lin, X.; Cao, Y. Modulation of interfacial phenolic antioxidant distribution in Pickering emulsions via interactions between zein nanoparticles and gallic acid. Int. J. Biol. Macromol. 2020, 152, 223–233. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, W.; Xiao, J.; Chen, Y.; Cao, Y. Interfacial Engineering of Pickering Emulsion Co-Stabilized by Zein Nanoparticles and Tween 20: Effects of the Particle Size on the Interfacial Concentration of Gallic Acid and the Oxidative Stability. Nanomaterials 2020, 10, 1068. [Google Scholar] [CrossRef]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Frankel, E.N.; Finley, J.W. How To Standardize the Multiplicity of Methods To Evaluate Natural Antioxidants. J. Agric. Food Chem. 2008, 56, 4901–4908. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Sørensen, A.-D.M.; Bayrasy, C.; Lecomte, J.; Jacobsen, C.; Decker, E.A.; Villeneuve, P. CHAPTER 8—Role of Hydrophobicity on Antioxidant Activity in Lipid Dispersions: From the Polar Paradox to the Cut-Off Theory. In Lipid Oxidation; Logan, A., Nienaber, U., Pan, X., Eds.; AOCS Press: Urbana, IL, USA, 2013; pp. 261–296. [Google Scholar] [CrossRef]
- Laguerre, M.; Bily, A.; Birtić, S. Chapter 7—Lipid oxidation in food. In Lipids and Edible Oils; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 243–287. [Google Scholar] [CrossRef]
- Dar, A.A.; Bravo-Diaz, C.; Nazir, N.; Romsted, L.S. Chemical kinetic and chemical trapping methods: Unique approaches for determining respectively the antioxidant distributions and interfacial molarities of water, counter-anions, and other weakly basic nucleophiles in association colloids. Curr. Opin. Colloid Interface Sci. 2017, 32, 84–93. [Google Scholar] [CrossRef]
- Bravo-Díaz, C.; Romsted, L.S.; Liu, C.; Losada-Barreiro, S.; Pastoriza-Gallego, M.J.; Gao, X.; Gu, Q.; Krishnan, G.; Sánchez-Paz, V.; Zhang, Y.; et al. To Model Chemical Reactivity in Heterogeneous Emulsions, Think Homogeneous Microemulsions. Langmuir 2015, 31, 8961–8979. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Paiva-Martins, F.; Losada-Barreiro, S.; Bravo-Díaz, C. Modeling Chemical Reactivity at the Interfaces of Emulsions: Effects of Partitioning and Temperature. Molecules 2021, 26, 4703. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; McClements, D.J.; Bourlieu-Lacanal, C.; Durand, E.; Figueroa-Espinoza, M.C.; Lecomte, J.; Villeneuve, P. Hurdles in Predicting Antioxidant Efficacy in Oil-in-water emulsions. Trends Food Sci. Technol. 2017, 67, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What Makes Good Antioxidants in Lipid-Based Systems? The Next Theories Beyond the Polar Paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Sagis, L.; Schroën, K. Formation, Structure, and Functionality of Interfacial Layers in Food Emulsions. Annu. Rev. Food Sci. Technol. 2018, 9, 551–587. [Google Scholar] [CrossRef]
- Stöckman, H.; Schwarz, K. Partitioning of Low Molecular Weight Compounds in Oil-in-Water Emulsions. Langmuir 1999, 15, 6142–6149. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Stöckman, H.; Schwarz, K.; Heinoen, I.M.; Hopia, A.I. Antioxidant Activity and Partitioning of Phenolic Acids in Bulk and Emulsified Methyl Linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef]
- Jacobsen, C.; Schwarz, K.; Stöckmann, H.; Meyer, A.S.; Adler-Nissen, J. Partitioning of Selected Antioxidants in Mayonnaise. J. Agric. Food. Chem. 1999, 47, 3601–3610. [Google Scholar] [CrossRef]
- Schwarz, K.; Frankel, E.N.; German, J.B. Partition behaviour of antioxidative phenolic compounds in heterophasic systems. Lipid/Fett 1996, 98, 115–121. [Google Scholar] [CrossRef]
- Romsted, L.S.; Bravo-Díaz, C. Modelling chemical reactivity in emulsions. Curr. Opin. Colloid Interface Sci. 2013, 18, 3–14. [Google Scholar] [CrossRef]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Aliaga, C.; Bravo-Díaz, C. Interfacial kinetics in olive oil-in-water nanoemulsions: Relationships between rates of initiation of lipid peroxidation, induction times and effective interfacial antioxidant concentrations. J. Colloid Interface Sci. 2021, 604, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Differential Partitioning of Bioantioxidants in Edible Oil–Water and Octanol–Water Systems: Linear Free Energy Relationships. J. Chem. Eng. Data 2018, 63, 2999–3007. [Google Scholar] [CrossRef]
- Amézqueta, S.; Subirats, X.; Fuguet, E.; Rosés, M.; Ràfols, C. Chapter 6—Octanol-Water Partition Constant. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–208. [Google Scholar] [CrossRef]
- Loureiro, D.R.P.; Soares, J.X.; Lopes, D.; Macedo, T.; Yordanova, D.; Jakobtorweihen, S.; Nunes, C.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Accessing lipophilicity of drugs with biomimetic models: A comparative study using liposomes and micelles. Eur. J. Pharm. Sci. 2018, 115, 369–380. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Polyphenolic Antioxidants in Lipid Emulsions: Partitioning Effects and Interfacial Phenomena. Foods 2021, 10, 539. [Google Scholar] [CrossRef]
- Oehlke, K.; Garamus, V.; Heins, A.; Stöckman, H.; Schwarz, K. The partitioning of emulsifiers in o/w emulsions: A comparative study of SANS, ultrafiltration and dialysis. J. Colloid Interface Sci. 2008, 322, 294–303. [Google Scholar] [CrossRef]
- Heins, A.; Sokolowski, T.; Stöckmann, T.; Schwarz, K. Investigating the Location of Propyl Gallate at Surfaces and Its Chemical Microenvironment by 1H NMR. Lipids 2007, 42, 561–572. [Google Scholar] [CrossRef]
- Oehlke, K.; Heins, A.; Stöckmann, H.; Schwarz, K. Impact of emulsifier microenvironments on acid-base equilibrium and activity of antioxidants. Food Chem. 2010, 118, 48–55. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Villeneuve, P.; Jacobsen, C. Alkyl caffeates as antioxidants in O/W emulsions: Impact of emulsifier type and endogenous tocopherols. Eur. J. Lipid Sci. Technol. 2017, 119, 1600276. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Paiva-Martins, F. Effects of Emulsion Droplet Size on the Distribution and Efficiency of Antioxidants. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 217–235. [Google Scholar] [CrossRef]
- Romsted, L.S. Introduction to Surfactant Self-Assembly. In Supramolecular Chemistry: From Molecules to Nanomaterials; Gale, P.A., Steed, J.W., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2012; pp. 181–203. [Google Scholar]
- Gu, Q.; Bravo-Díaz, C.; Romsted, L.S. Using the pseudophase kinetic model to interpret chemical reactivity in ionic emulsions: Determining antioxidant partition constants and interfacial rate constants. J. Colloid Interface Sci. 2012, 400, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Quina, F.H.; Lissi, E.A. Photoprocesses in Microaggregates. Acc. Chem. Res. 2004, 37, 703–710. [Google Scholar] [CrossRef] [PubMed]
- López-Cornejo, P.; Pérez, P.; García, F.; de la Vega, R.; Sánchez, F. Use of the Pseudophase Model in the Interpretation of Reactivity under Restricted Geometry Conditions. An Application to the Study of the [Ru(NH3)5pz]2+ + S2O82− Electron-Transfer Reaction in Different Microheterogeneous Systems. J. Am. Chem. Soc. 2002, 124, 5154–5164. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, L.; Leis, J.R.; Mejuto, J.C.; Perez-Lorenzo, M. Microemulsions as microreactors in physical organic chemistry. Pure Appl. Chem. 2007, 79, 1111–1123. [Google Scholar] [CrossRef] [Green Version]
- Bunton, C.A.; Nome, F.; Quina, F.H.; Romsted, L.S. Ion Binding and Reactivity at Charged Aqueous Interfaces. Acc. Chem. Res. 1991, 24, 357–364. [Google Scholar] [CrossRef]
- Yi, B.; Kim, M.-J.; Lee, J. Oxidative stability of oil-in-water emulsions with α-TOCopherol, charged emulsifier, and different oxidative stress. Food Sci. Biotechnol. 2018, 27, 1571–1578. [Google Scholar] [CrossRef]
- Kiralan, S.S.; Doğu-Baykut, E.; Kittipongpittaya, K.; McClements, D.J.; Decker, E.A. Increased Antioxidant Efficacy of Tocopherols by Surfactant Solubilization in Oil-In-Water Emulsions. J. Agric. Food Chem. 2014, 62, 10561–10566. [Google Scholar] [CrossRef]
- Yamauchi, R. Oxidation Products of Vitamin E in the Peroxidation of Liposomal and Biological Systems. J. Clin. Biochem. Nutr. 2004, 34, 111–120. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [Green Version]
- Azzi, A. Molecular mechanism of a-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [PubMed]
- Burton, G.W.; Ingold, K.U. Vitamin E: Application of the Principles of Physical Organic Chemistry to the Exploration of Its Structure and Function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar]
- Bravo Díaz, C. Diazohydroxides, diazoethers and related species. In The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids; Rappoport, Z., Liebman, J.F., Eds.; John Wiley & Sons: Chichester, UK, 2011; Volume 2, p. 853. [Google Scholar]
- Zollinger, H. Color Chemistry; VCH: Weinheim, Germany, 1991. [Google Scholar]
- Zollinger, H. Diazo Chemistry II. Aliphatic, Inorganic and Organometallic Compounds; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Zollinger, H. Diazo Chemistry I: Aromatic and Heteroaromatic Compounds; Wiley-VCH: Weinheim, Germany, 1994; Volume 107. [Google Scholar]
- Lu, L.-L.; Lu, X.-Y. Solubilities of gallic acid and its esters in water. J. Chem. Eng. Data 2007, 52, 37–39. [Google Scholar]
- Szele, I.; Zollinger, H. Azo Coupling reactions: Structure and mechanism. In Preparative Organic Chemistry, 1983; Springer: New York, NY, USA, 1986. [Google Scholar]
- Hegarty, A.F. Kinetics and Mechanisms of Reactions Involving Diazonium and Diazo Groups. In The Chemistry of Diazonium and Diazo Compounds; Patai, S., Ed.; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Costas-Costas, U.; Gonzalez-Romero, E.; Bravo-Díaz, C. Effects of Ascorbic Acid on Arenediazonium Reactivity: Kinetics and Mechanism of the Reaction. Helv. Chim. Acta 2001, 84, 632–648. [Google Scholar] [CrossRef]
- Hanson, P.; Jones, J.R.; Taylor, A.B.; Walton, P.H.; Timms, A.W. Sandmeyer reactions. Part 7. An investigation into the reduction steps of Sandmeyer hydroxylation and chlorination reactions. J. Chem. Soc. Perkin Trans. 2002, 2, 1135–1150. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Diaz, C. Reactivity of 3-Methylbenzenediazonium Ions with Gallic Acid. Kinetics and Mechanism of the reaction. Helv. Chim. Acta 2009, 92, 2009–2023. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sánchez-Paz, V.; Bravo-Díaz, C. Kinetics and Mechanism of the Reaction Between an Arenediazonium ion and Methyl Gallate in aqueous solution. Evidence for Diazo Ether Formation through an O-Coupling Reaction. Helv. Chim. Acta 2007, 90, 1559–1573. [Google Scholar] [CrossRef]
- Saunders, K.H.; Allen, R.L.M. Aromatic Diazo Compounds, 3rd ed.; Edward Arnold: Baltimore, MD, USA, 1985. [Google Scholar]
- Machackova, O.; Sterba, V.; Valter, K. Kinetics and mechanism of diazo coupling. XXIV. Coupling kinetics of benzenediazonium ions with resorcinol and its O-methyl derivatives. Collect. Czechoslov. Chem. Commun. 1972, 37, 1851–1860. [Google Scholar] [CrossRef]
- González-Romero, E.; Malvido-Hermelo, B.; Bravo-Díaz, C. Effects of b-cyclodextrin on the electrochemical behavior of a model arenediazonium ion. Kinetics and mechanism of the reaction. Langmuir 2002, 18, 46–55. [Google Scholar] [CrossRef]
- Pazo-Llorente, R.; Bravo-Díaz, C.; González-Romero, E. pH Effects on Ethanolysis of Some Arenediazonium Ions: Evidence for Homolytic Dediazoniation Proceeding through Formation of Transient Diazo Ethers. Eur. J. Org. Chem. 2004, 2004, 3221–3226. [Google Scholar] [CrossRef]
- Pazo-Llorente, R.; Maskill, H.; Bravo-Díaz, C.; González-Romero, E. Dediazoniation of 4-nitrobencenediazonium ions in acidic MeOH/H2O mixtures: Role of acidity and MeOH concentration on the formation of transient diazo ethers that initiate homolytic dediazoniation. Eur. J. Org. Chem. 2006, 2006, 2201–2209. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sánchez-Paz, V.; Pastoriza-Gallego, M.J.; Bravo-Diaz, C. Micellar Effects on the Reaction between an Arenediazonium Ion and the Antioxidants Gallic Acid and Octyl Gallate. Helv. Chim. Acta 2008, 91, 21–34. [Google Scholar] [CrossRef]
- Lisete-Torres, P.; Losada-Barreiro, S.; Albuquerque, H.; Sánchez-Paz, V.; Paiva-Martins, F.; Bravo-Díaz, C. Distribution of hydroxytyrosol and hydroxytyrosol acetate in olive oil emulsions and their antioxidant efficiency. J. Agric. Food Chem. 2012, 60, 7318–7325. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Sánchez Paz, V.; Bravo-Díaz, C. Effects of emulsifier hydrophile–lipophile balance and emulsifier concentration the distributions of gallic acid, propyl gallate, and a-tocopherol in corn oil emulsions. J. Colloid Interface Sci. 2013, 389, 1–9. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sánchez Paz, V.; Bravo Díaz, C.; Paiva Martins, F.; Romsted, L.S. Temperature and emulsifier concentration effects on gallic acid distribution in a model food emulsion. J. Colloid Interface Sci. 2012, 370, 73–79. [Google Scholar] [CrossRef]
- Romsted, L.S.; Bravo-Díaz, C. Determining Antioxidant Distributions in Intact Emulsions by Kinetic Methods: Application of Pseudophase Models. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 31–48. [Google Scholar] [CrossRef]
- Thomas, A.; Matthäus, B.; Fiebig, H.-J. Fats and Fatty Oils. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–84. [Google Scholar] [CrossRef]
- Aliaga, C.; Rezende, M.C. Location, Orientation and Buoyance Effects of Radical Probes as Studied by EPR. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 133–150. [Google Scholar] [CrossRef]
- OECD. Test No. 107: Partition Coefficient (n-Octanol/Water): Shake Flask Method; OECD: Paris, France, 1995. [Google Scholar] [CrossRef]
- Menger, F.M.; Portnoy, C.E. Chemistry of reactions proceeding inside molecular aggregates. J. Am. Chem. Soc. 1967, 89, 4698–4703. [Google Scholar] [CrossRef]
- Romsted, L.S.; Bunton, C.; Yao, J. Micellar Catalysis, A useful misnomer. Curr. Opin. Colloid Interface Sci. 1997, 2, 622–628. [Google Scholar] [CrossRef]
- Amado, S.; García-Rio, L.; Leis, J.R.; Ríos, A. Reactivity of Anions with Organic Substrates Bound to Sodium Dodecyl Sulfate Micelles: A Poisson-Boltzmann/Pseudophase Approach. Langmuir 1997, 13, 687–692. [Google Scholar] [CrossRef]




| α-TOC | δ-TOC | ||||
|---|---|---|---|---|---|
| Oil | pH | POI | 102 kI (M−1 s−1) | POI | 103 kI (M−1 s−1) |
| Soybean | 3.5 | 12 ± 2 | 1.5 ± 0.4 | 19 ± 5 | 5.8 ± 0.2 |
| 4.0 | 13 ± 3 | 4.0 ± 0.7 | 18 ± 4 | 15.9 ± 1.1 | |
| 4.5 | 12 ± 3 | 12 ± 2 | 18 ± 2 | 30.0 ± 0.4 | |
| Olive | 4.0 | 5 ± 0 | 4.0 ± 0.2 | 10 ± 1 | 12.6 ± 0.3 |
| 4.5 | 6 ± 1 | 13 ± 1.0 | 11 ± 2 | 36.5 ± 3.1 | |
| 5.0 | 6 ± 1 | 27 ± 3.0 | 11 ± 2 | 96.4 ± 5.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ventoso, L.; Toba-Pérez, A.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Distributions of α- and δ-TOCopherol in Intact Olive and Soybean Oil-in-Water Emulsions at Various Acidities: A Test of the Sensitivity of the Pseudophase Kinetic Model. Antioxidants 2022, 11, 2477. https://doi.org/10.3390/antiox11122477
Fernández-Ventoso L, Toba-Pérez A, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C. Distributions of α- and δ-TOCopherol in Intact Olive and Soybean Oil-in-Water Emulsions at Various Acidities: A Test of the Sensitivity of the Pseudophase Kinetic Model. Antioxidants. 2022; 11(12):2477. https://doi.org/10.3390/antiox11122477
Chicago/Turabian StyleFernández-Ventoso, Lucía, Artai Toba-Pérez, Sonia Losada-Barreiro, Fátima Paiva-Martins, and Carlos Bravo-Díaz. 2022. "Distributions of α- and δ-TOCopherol in Intact Olive and Soybean Oil-in-Water Emulsions at Various Acidities: A Test of the Sensitivity of the Pseudophase Kinetic Model" Antioxidants 11, no. 12: 2477. https://doi.org/10.3390/antiox11122477
APA StyleFernández-Ventoso, L., Toba-Pérez, A., Losada-Barreiro, S., Paiva-Martins, F., & Bravo-Díaz, C. (2022). Distributions of α- and δ-TOCopherol in Intact Olive and Soybean Oil-in-Water Emulsions at Various Acidities: A Test of the Sensitivity of the Pseudophase Kinetic Model. Antioxidants, 11(12), 2477. https://doi.org/10.3390/antiox11122477






