Abstract
The experimental objective was to examine the role of melatonin and its pathways in the maintenance of pregnancy in lactating dairy cows. Blood samples were collected at days 0, 16 and 32 after timed AI from cows (n = 200) in order to consider plasma melatonin concentrations and to conduct AOPP (advanced oxidation products of proteins) and TBARS (thiobarbituric acid reactive substances) tests. Luminal endometrial cells were collected at day 16 using a Cytobrush in all cows to determine mRNA expressions of melatonin receptor 1 (MT1), mouse double minute 2 (MDM2), MDM2 binding protein (MTBP), BCL2-associated X, apoptosis Regulator (BAX), p53 upregulated modulator of apoptosis (PUMA, gene symbol BBC3), mucin 1 (MUC1) and leukemia inhibitory factor (LIF). Plasma concentrations of melatonin were significantly greater in pregnant cows diagnosed pregnant at day 16 who sustained pregnancy to day 32 compared to nonpregnant cows at day 16, or pregnant at day 16 and who lost embryos by days 32. Concentrations of AOPP and TBARS were greater in nonpregnant cows at day 16 or pregnant at day 16 and who lost embryos by days 32 compared to those diagnosed pregnant at day 16 and who sustained pregnancy to day 32. In pregnant cows, endometrial mRNA expressions of MDM2, MTBP, MTR1 and LIF were higher compared to pregnant–embryo-loss cows (p < 0.05). In contrast, mRNA expressions of BBC3 and MUC1 were greater at day 16 in pregnant–embryo-loss cows compared to pregnant cows (p < 0.05). In conclusion, melatonin status is a modulator of embryo well-being and maintenance of pregnancy in lactating dairy cows.
1. Introduction
Pregnancy is a critical stage of life, during which many physiological processes are modified. Although 85% of inseminations result in fertilization, less than 40% of cows calve after a first insemination [1]. The causes of embryonic loss and sustained pregnancy are multifaceted through maternal and embryonic factors. A considerable number of embryonic losses are attributed to maternal factors, such as an inability of the uterus to support conceptus growth and implantation [2,3,4] and increased oxidative stress [5]. Oxidative stress, inflammatory status, and mRNA expression of white blood cells in postpartum lactating cows are associated with reproductive responses after Timed AI [3].
Trophoblastic cells of the placenta produce melatonin and express MT1 and MT2 receptors [6]. Melatonin acts in an autocrine, intracrine and paracrine manner in the placenta [6,7]. Melatonin is known as a cell-protective molecule with different properties allowing it to exert strong antioxidant potential [8]. It is more effective in reducing free radicals than other well-known natural or chemically synthesized antioxidants [9,10,11]. Kivela et al. (1991) [12] demonstrated an increase in melatonin concentrations in the maternal blood during pregnancy, and Lanoix et al. (2008) [6] suggested that this increase is due to placental production of melatonin. Melatonin improves embryo quality through increasing expression of antioxidant genes that reduce mitochondrial damage and apoptosis [13].
Reactive oxygen species (ROS) may be responsible for causing increased embryo fragmentation, resulting from increased apoptosis [14]. The TP53 gene codes for Tumor Protein P53 which works as a tumor suppressor and regulator of apoptotic processes [15]. The TP 53 protein acts transcriptionally to regulate expression of apoptotic proteins including BAX, also known as bcl-2-like protein 4 (BAX), p53 upregulated Modulator of Apoptosis (PUMA; gene symbol, also known as Bcl-2-binding component 3 BBC3), E3 ubiquitin–protein ligase Mdm2 protein known as Anti-Apoptotic Mouse Double Minute 2 protein (MDM2, gene symbol MDM2), cell cycle proteins such as p21 (product of the CDKN1A gene), and proteins associated with DNA repair such as the Growth Arrest and DNA-Damage-inducible 45 (GADD45) family [16,17]. Post-transcriptional actions of TP53, in which TP53 protein interacts directly with BAX and/or BCL2 proteins, also have been described. [18].
Cows with early embryonic loss were hypothesized to have both a higher oxidative status and lower concentrations of melatonin [6,12]. Melatonin is considered an essential protector of pregnancy that reduces oxidative stress, contributing to maintenance of pregnancy. This experiment investigated melatonin and its potential network of effects in intrauterine luminal cells that differ between lactating cows experiencing early embryonic loss compared to pregnant cows.
2. Material and Methods
2.1. Lactating Cows and Nutrition
This experiment was carried out during the summer at a commercial dairy farm of 3500 milking cows in the northern part of Iran (longitude and latitude, 36.33° N and 53.06° E). The Temperature Humidity index (THI) during the study was between 67 and 73. The photoperiodic conditions were 15 h light: 9 h dark (Sunrise: 6.00 a.m. and Sunset 9.00 p.m.). The study was conducted in accordance with the guidelines of the Iranian Council of Animal Care (1995) and approved by the ethics committee of Sari Agricultural Sciences and Natural Resources University (protocol #1998). All animal procedures were approved by the Iranian Ministry of Agriculture (Permission no. 2018.06.01).
Cyclic Holstein cows (n = 200, average milk production of 34.5 ± 0.6 kg/d and 3.1 ± 0.5 lactations) were enrolled in the study. All cows were evaluated and assigned a body condition score (BCS) on a scale of 1 to 5. An expert technician determined the BCS of cows. The mean BCS ± SEM of cows was 3.1 ± 0.1. Cows were fed a total mixed ration (TMR) formulated to meet the 2001 Nutrient Research Council (NRC 2001 [19] requirements for a lactating dairy cow of 700 kg body weight (BW) and producing 40 kg milk per day.
2.2. TAI Protocol
Ovaries were scanned at 30 ± 2 d postpartum using transrectal ultrasonography (Easi-Scan version 3, BCF Technology Ltd.; Livingston, Scotland, UK), and only cows that had a corpus luteum (CL) were enrolled in the study. All cows were synchronized by a MG6GP protocol: cows received PGF2α (500 µg CLOPROSTENOL a synthetic analogue of prostaglandin F2α, Parnell Technologies, Australia), 4 days later GnRH (100 µg GONADORELIN ACETATE, Parnell Technologies), followed 6 days later by an Ovsynch56 TAI program, as described by Heidari et al. (2017) [20]. An experienced technician with commercially available frozen–thawed semen performed all inseminations.
2.3. Pregnancy Diagnosis
Pregnancy status at day 16 after TAI was predicted via blood cell ISG15 mRNA gene expression. Prediction of pregnancy was based on blood cellular ISG15 expression greater than −7.0 at day 16 after TAI [3,21]. Pregnancy at day 32 after TAI was diagnosed via transrectal ultrasonography. All cows were considered healthy (n = 200) and partitioned into nonpregnant at day 16 after TAI (n = 80), pregnant at day 16 (n = 120), pregnant at day 32 (n = 86), and cows experiencing embryonic losses between days 16–32 after TAI (n = 34)”.
2.4. Melatonin Concentrations
Blood samples were collected at 0, 16 and 32 days after timed (AI) (at 4.00 a.m., almost 2 h before sunrise) from all cows to determine melatonin concentrations. The enzyme-linked immunosorbent assay (ELISA) kit (IBL, Hamburg, Germany, CAT Number RE54021) was used to measure melatonin in plasma. IBL International-Tecan market a serum/plasma ELISA kit (RE54021) which uses a C18 reverse phase extraction procedure for the samples. The samples are applied to the columns and washed sequentially with water and 10% methanol, the melatonin eluted with methanol, the solvent evaporated, and the residue reconstituted for assay. The IBL melatonin antibody being used in the RIA and ELISA kits is reported to have a 1.2% cross reaction with N-acetyl serotonin and 2.5% for 5-methoxy tryptamine and 0.02% for serotonin, but presumably this is decreased following the extraction.
The resultant optical density was read at 405 nm (using 620 nm filter as a reference) on a Multiskan® FC photometer (Thermo Fisher Scientific, Vantaa, Finland). The assay’s working principle is a basic competitive ELISA procedure in which the sample melatonin competes with a biotinylated melatonin for its antibody-binding sites (polyclonal; rabbit). The amount of biotinylated melatonin bound to the antibodies is inversely related to the amount of melatonin in the sample. Finally, the amount of melatonin in the sample is quantified by comparison with a standard melatonin dose–response curve (standard concentration 3.6, 10, 30, 100 and 300 pg/mL provided in the kit). Sensitivity of the melatonin assay was 1.6 pg/mL, and intra-assay and interassay coefficients of variation were 2–5% and 4–9%, respectively.
2.5. Measurements of Oxidative Status
Concentrations of AOPP (Advanced Oxidation Products of Proteins) were measured according to Witko-Sarsat et al. (1996) [22]. The intra-assay and interassay coefficients of variation were 1.02% and 2.27%, respectively. The AOPP values are expressed per unit volume (µmol/L), as well as per g of total protein (µmol/g). For this purpose, total protein concentration in plasma was determined with the Bradford method, (1976) [23] using BSA as standard. The intra- and inter-assay coefficients of variation were 1.42% and 4.16%, respectively. The TBARS (Thiobarbituric Acid Reactive Substances) test [24] was used for measuring oxidized lipids in plasma with a mean intra-assay CV of 7.01% and a mean interassay CV of 8.26%.
2.6. Endometrial Cells
Endometrial samples were collected from all cows at d 16 after AI using a Cytobrush Plus GT (Disposable cytology sampling brush 8”; Viamed Ltd., West Yorkshire, UK, [25]). Briefly, after caudal epidural anesthesia (induced with 5–10 mL lidocaine), the Cytobrush was passed through the cervix, and the tip of Cytobrush was placed in the uterine horn ipsilateral to the CL and against the antimetrial uterine wall. Endometrial cells were collected by rotating the Cytobrush three times clockwise while in contact with the uterine wall. Approximately 80% of cells sampled from the luminal endometrium are epithelial cells (Binelli M. and Co-workers personal communication; manuscript under review 2021). The Cytobrush is retrieved from the AI gun and the tip placed into a 2 mL cryo-tube containing 1 mL of trizol to release cells. The tube is frozen in liquid nitrogen for further storage at −80 °C.
2.6.1. RNA Extraction and cDNA Synthesis
Total RNA was extracted from endometrial cells and used for cDNA synthesis using established protocols in our laboratory [26]. Briefly, RNA was isolated using a commercial kit (NucleoSpin RNA Blood, Cat No. 40200, Macherey-Nagel GmBH&Co. KG, Büren, Germany).
All RNA samples were quantified by spectrophotometry (#ND-1000, Nanodrop Technology Inc.; Wilmington, DE, USA), and the purification of RNA with A260/A280 ratio was between 1.8 and 2.0. Complementary DNA (cDNA) was synthesized from 150 ng RNA using a QuantiTect Reverse Transcription kit (Qiagen, Hilden, Germany, Cat No. 205314), as described by Dirandeh et al. (2021) [3].
2.6.2. Real-Time PCR
Real-time PCR was performed using a 15 mL reaction volume containing 1 mL single-strand cDNA, 7.5 mL of 1 × SYBR Green master mix (Qiagen, GmbH, Germany, Cat. No. 204052), 1 mL of each forward and reverse primers and 4.5 mL of distilled H2O in a Rotor-Gene 6000 Real-Time PCR software (Corbett Research, Sydney, Australia) with specific bovine primers: melatonin receptor 1 (MT1, [27]), mouse double minute 2 (MDM2), MDM2 binding protein (MTBP), BCL2-associated X Apoptosis Regulator (BAX), p53 upregulated modulator of apoptosis (PUMA, gene symbol BBC3, [17]), Mucin 1 (MUC1, [28]) and leukemia inhibitory factor (LIF, [29]), in accordance with MIQE guidelines [30] and using following temperature program: 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C. The internal controls were GAPDH, RPS9, and UXT [3]. The geometric mean of the internal control genes was used to normalize the expression data. The relative levels of mRNA were analyzed by the 2−ΔΔCt method [31].
2.7. Statistical Analysis
All data were analyzed using SAS (Windows; SAS Institute, Cary, NC, USA). Melatonin concentrations, total protein, AOPP and TBARS were analyzed using PROC MIXED of SAS (2001) with the following model:
where µ is the population mean, αi is the pregnancy group effect, c(αj) is the cows (group) effect, which is the interaction for testing the pregnancy group effect, τk is the effect of sampling day after TAI, ατ*ik is the interaction effect of pregnancy group and sampling day after TAI, and eijk is the residual error for testing day and day * sampling effects. Pregnancy groups for the time analyses were nonpregnant (n = 114), pregnant (n = 80) and pregnant–embryo loss (n = 6).
Yijk = µ + αi + c(αj) + τk + ατ*ik + eijk
Gene expression data for day 16 are presented as fold changes relative to one of the pregnancy groups. These were calculated using the method described by Yuan et al. (2006) [32].
Statistical analyses were performed on ΔCt values as described by Livak et al. (2001) [32]. Statistical differences were declared significant at p ≤ 0.05 and tendencies at p ≤ 0.10.
The simple correlations between mRNA expressions of uterine intraluminal cells and partial correlations adjusted for ISG15 expression of peripheral blood cells were analyzed using Pearson correlation analyses in SPSS 21 statistical software.
3. Results
Milk yields (kg/d) were similar between groups (pregnant = 45.3 ± 0.5, pregnant–embryo loss = 44.9 ± 0.7 and nonpregnant = 45.6 ± 0.8). Likewise, body condition scores were similar between groups (pregnant = 3.0 ± 0.25, pregnant–embryo loses = 3.25 ± 0.15 and nonpregnant = 2.9 ± 0.25).
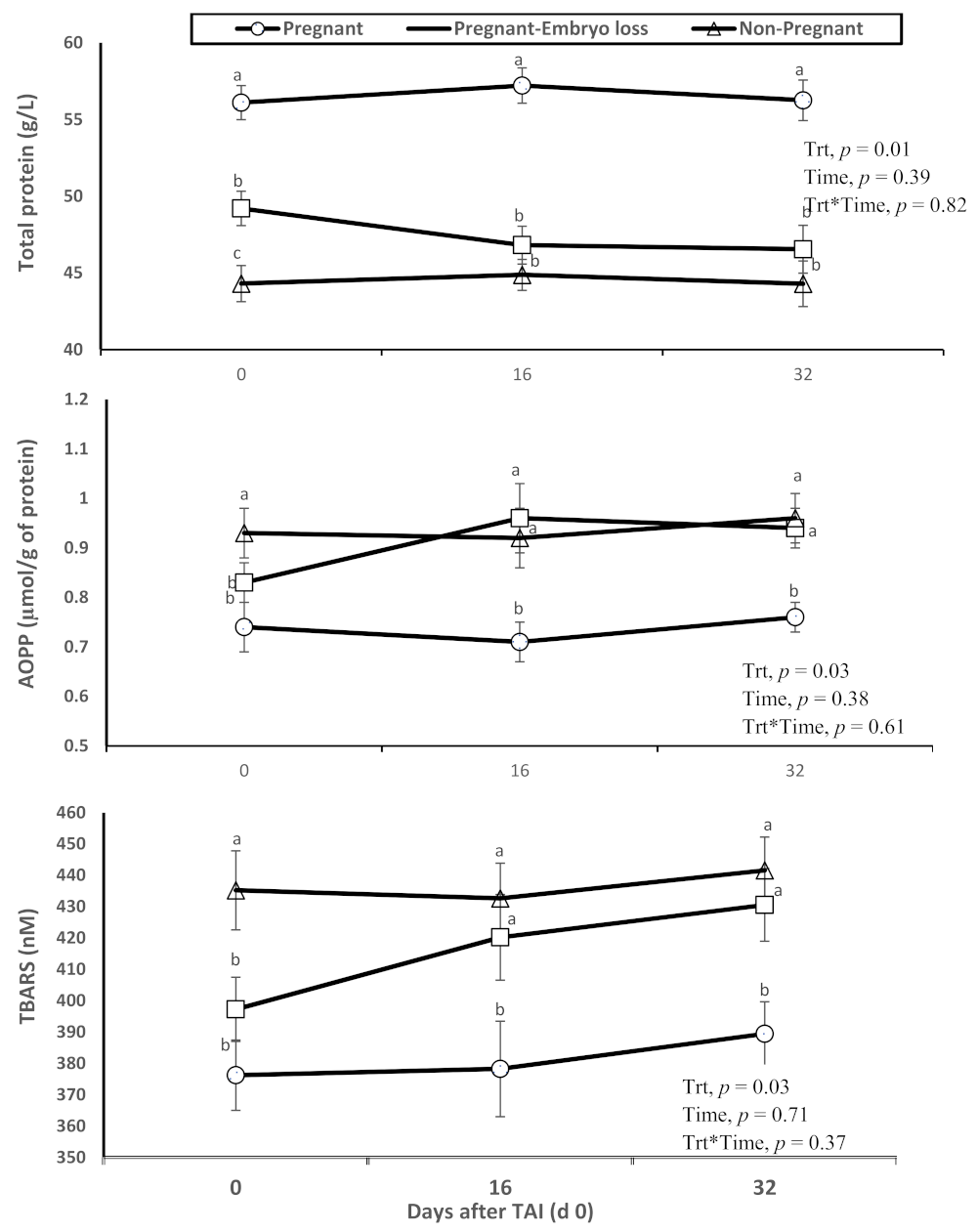
The pregnant lactating dairy cows that had lower metabolic oxidation products (AOPP and TBARS) had higher overall total protein concentrations in plasma. Furthermore, relative differences were evident at TAI on day 0. Advanced oxidation protein products (AOPP) and TBARS (i.e., measurement of oxidized lipids due to lipid peroxidation) were greater in nonpregnant cows at day 16 or pregnant cows at day 16 who lost embryos by days 32, when compared to lactating cows diagnosed pregnant at day 16 who sustained pregnancy to day 32 (Figure 1).
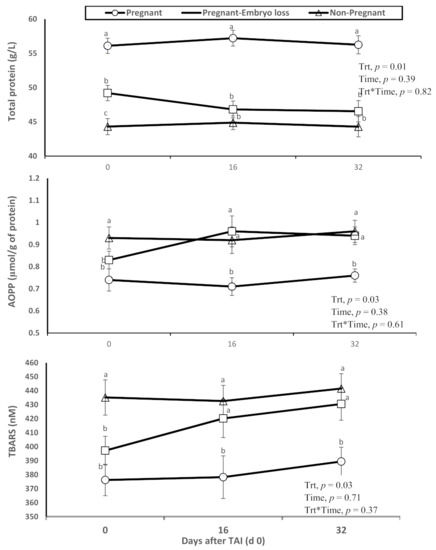
Figure 1.
Changes in the systemic oxidative status (total protein content in plasma, advanced oxidation products of proteins (AOPP) and thiobarbituric acid reactive substances (TBARS)), of nonpregnant, pregnant and pregnant dairy cows with embryo loss between d 16 to 32 days after TAI (d 0). Values are means ± standard error of means. Different letters indicate a significant statistical difference in the specific day between the highlighted black symbols (p < 0.05).
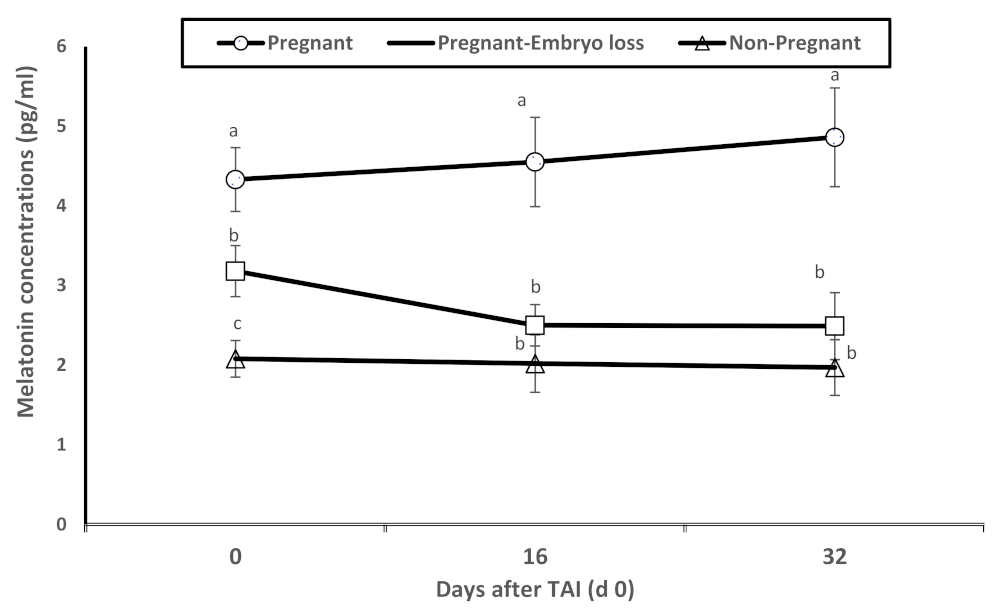
Dynamic changes in melatonin plasma concentrations were associated with pregnancy statuses. Melatonin concentrations were significantly greater in pregnant lactating cows diagnosed pregnant at day 16 with sustained pregnancy to day 32 compared to nonpregnant lactating cows at day 16 or pregnant at day 16 with lost embryos by days 32 (Figure 2). As observed in Figure 1, these differences were evident on day 0 at TAI.
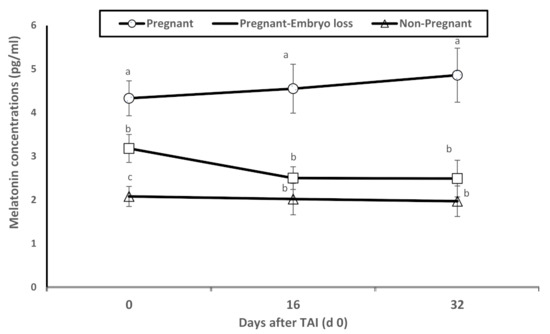
Figure 2.
Mean plasma levels of melatonin (pg/mL) of nonpregnant, pregnant and pregnant dairy cows with embryo loss between d 16 to 32 days after TAI (d 0). Values are means ± standard error of means. Different letters indicate a significant statistical difference in the specific day between the highlighted black symbols (p < 0.05).
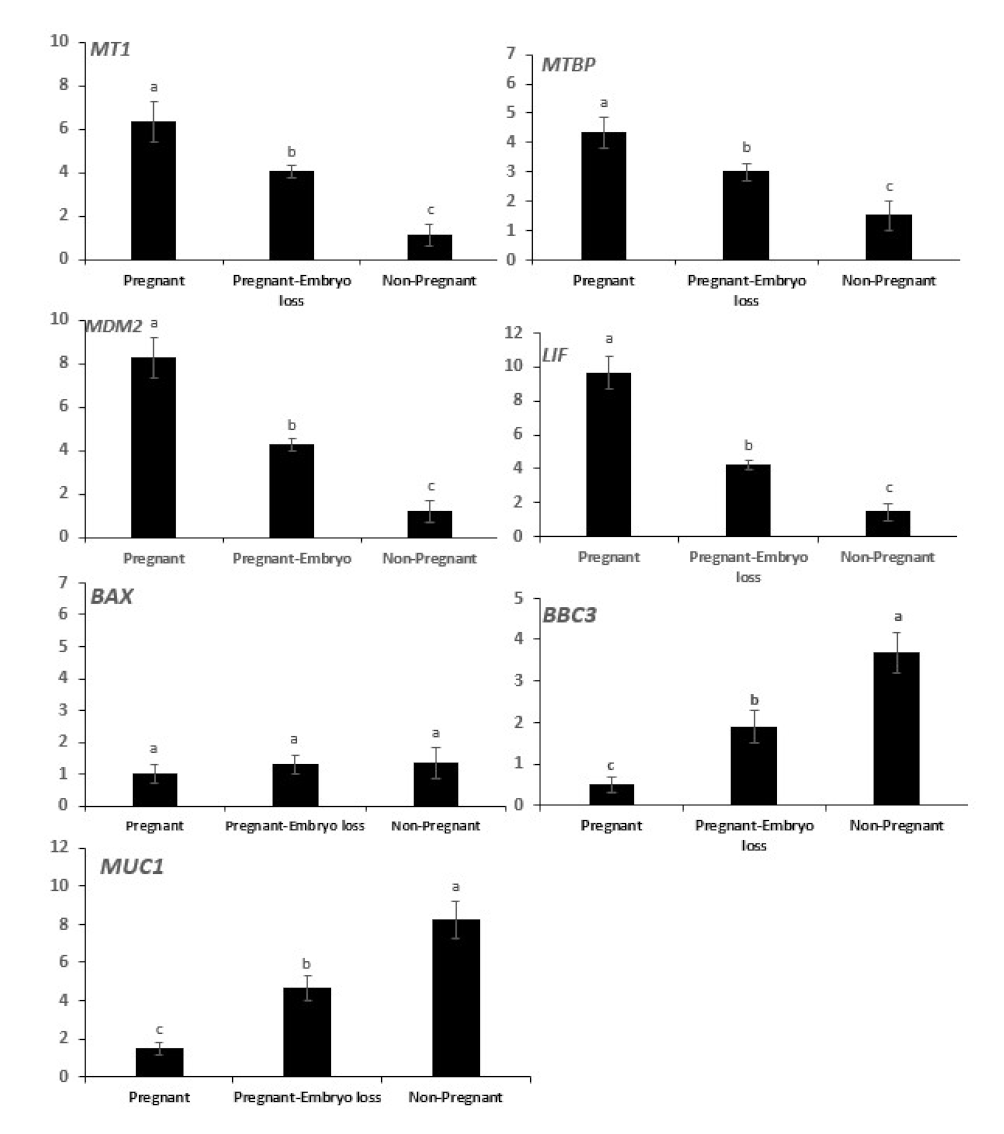
The mRNA expressions of MT1, MTBP, MDM2, LIF were greater in pregnant cows at day 16 and declined sequentially in pregnant cows that lost embryos, and they were lowest in nonpregnant cows at day 16 (Figure 3). Conversely, mRNA expressions of BBC3 and MUC1 were lowest in pregnant cows at day 16, they progressively increased in pregnant cows that lost embryos and were highest in nonpregnant cows. The expression of BAX mRNA was low and similar among the three groups (Figure 3).
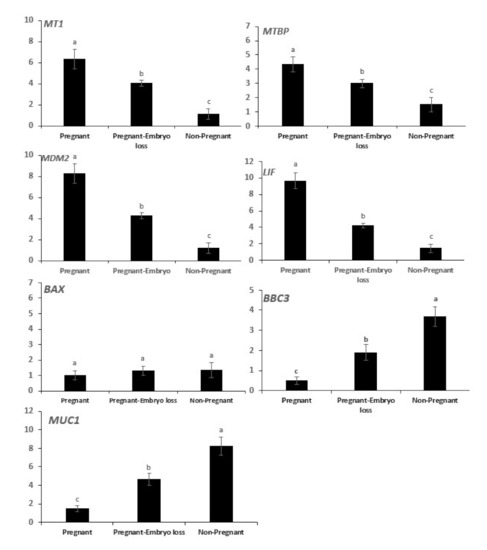
Figure 3.
Relative mRNA expression of melatonin receptor 1 (MT1), mouse double minute 2 (MDM2), MDM2 binding protein (MTBP), DNA-damage-inducible 45 (GADD45), BCL2-associated X, apoptosis regulator (BAX), p53 upregulated modulator of apoptosis (PUMA, gene symbol BBC3), Mucin 1 (MUC1) and leukemia inhibitory factor (LIF) in day 16 endometrial samples of pregnant cows diagnosed pregnant at day 16 with sustained pregnancy to day 60 compared to nonpregnant cows at day 16 or cows pregnant at day 16 who lost embryos by days 32 or 60. Error bars represent the 95% CI of the adjusted fold change. Different letters indicate differences between groups (p < 0.05).
Following the classification of pregnancy statuses, gene expression of ISG15 in peripheral blood cells was used to distinguish pregnancy statuses of the three experimental groups. The array of gene expressions of intraluminal cells differed among the three pregnancy statuses (Figure 3). Associations of mRNA gene expressions among intraluminal endometrial cells and potential associated expressions with ISG15 in blood cells among the 200 cows were evaluated. It was considered appropriate to report both simple (Table 1) and standard partial correlations between genes in luminal uterine endometrial cells adjusted for IGF15 mRNA expression in blood cells (Table 2).

Table 1.
Simple correlations of genes expressed in uterine luminal cells collected at day 16 after insemination from pregnant, pregnant with embryo loss, and nonpregnant lactating dairy cows.

Table 2.
Standard partial correlations 1 of genes expressed in uterine luminal cells collected at day 16 after insemination from pregnant, pregnant with embryo loss, and nonpregnant lactating dairy cows.
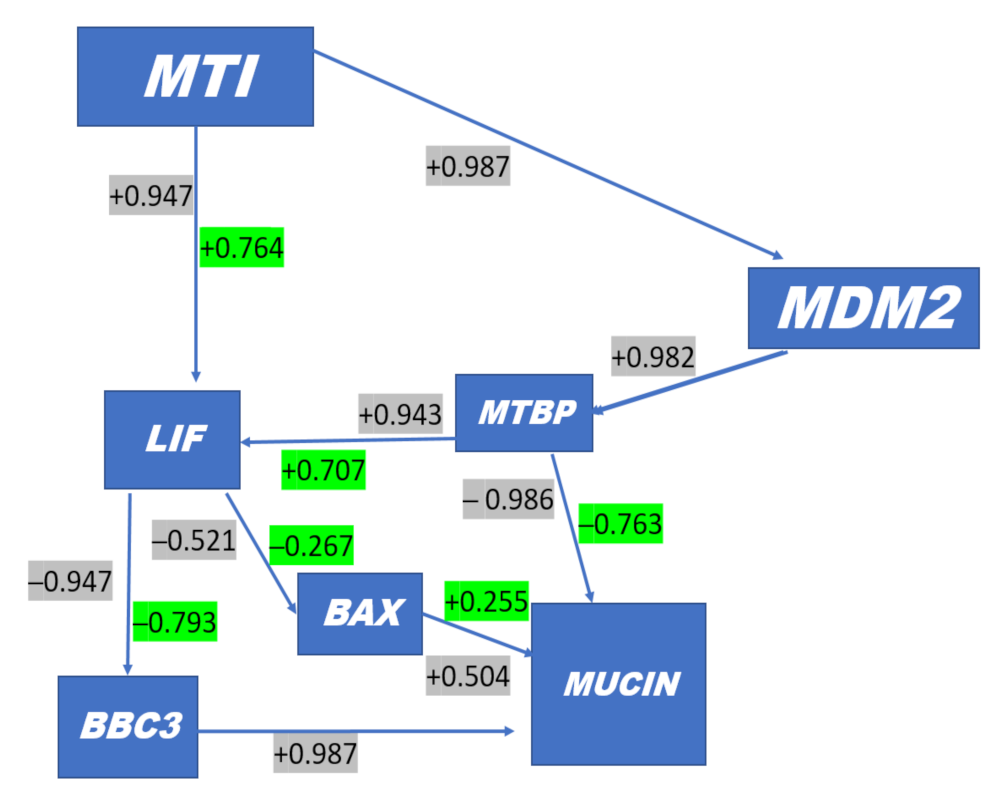
Among intraluminal endometrial cells, significant (p < 0.01) simple correlations (+ or -) were detected among all the genes (Table 1 Simple correlations). For example, positive correlations of MDM2 expressions with MTBP (r = 0.982), LIF (r = 0.979) and MT1 (r = 0.987) were detected (Table 1), as opposed to negative correlations of MDM2 expressions with BAX (r = −0.574), BBC3 (r = −0.983) and Mucin (r = −0.990). The cross section of gene associations with BAX were consistently lower. Of interest was that the ISG15 gene expressions in peripheral blood cells at day 16 were correlated strongly with the full array of gene expressions of intraluminal endometrial cells (i.e., r = 0.89 to 0.973, or −0.972 to −0.977; Table 2). An examination of Standard Partial Correlations (Table 2) detected 6 of 21 correlations (29%) in which partial correlations adjusted for ISG15 (i.e., MTBP/MUC1, MTBP/LIF, BAX/MUC1, BAX/LIF, BBC3/LIF, LIF/MT1) were lower than the respective simple correlations (Table 1). This is indicative that ISG15 gene expression in blood cells was associated with differential expression of specific genes in specific intraluminal endometrial cells.
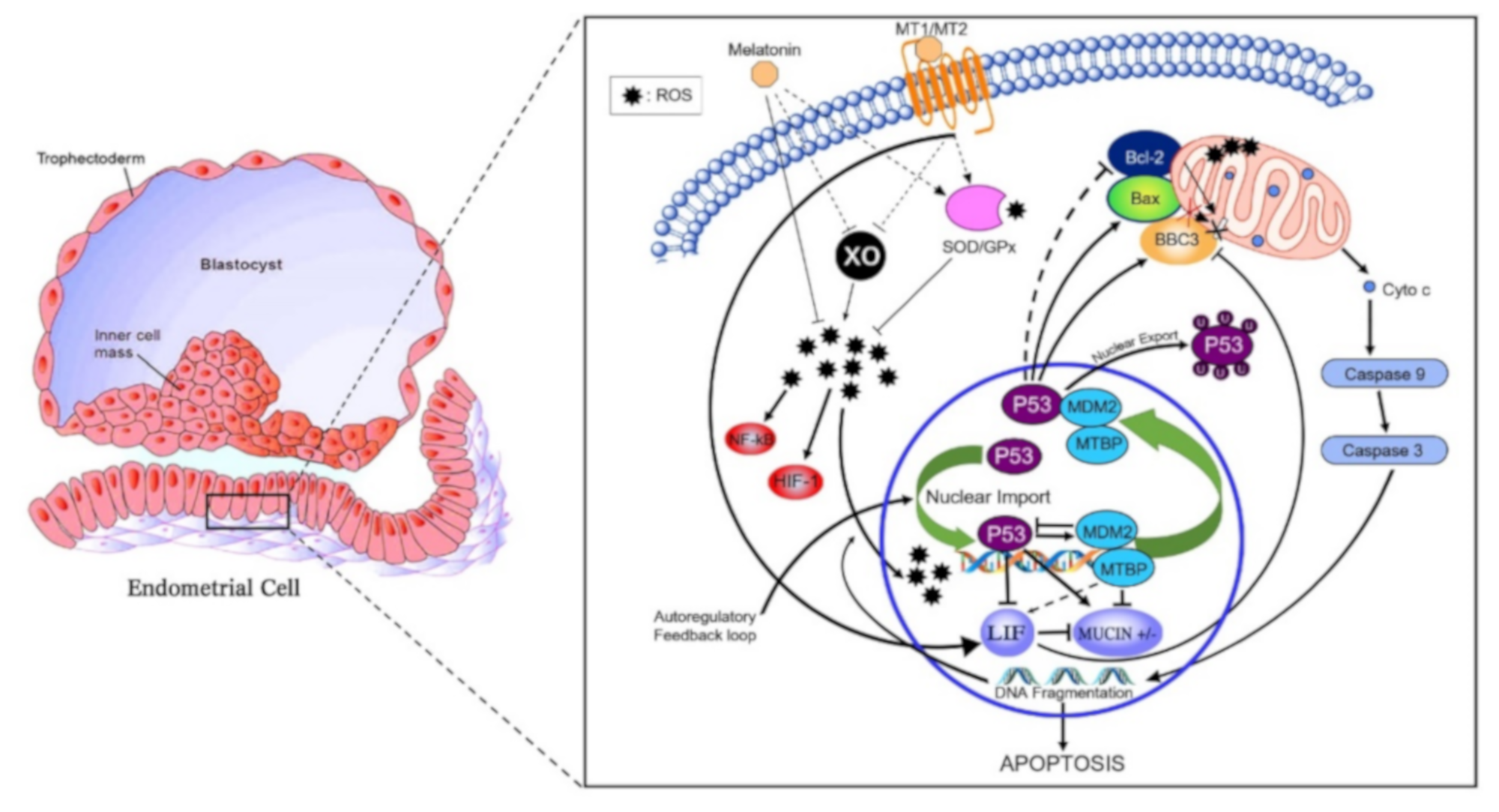
An overall proposed schematic representation depicting the direct protective and antiapoptotic pathways of melatonin within the bovine uterine endometrial cell is presented in Figure 4. The potential integrated associations of gene expression supporting the schematic are depicted in Figure 4. Increased plasma concentrations of melatonin at insemination and early pregnancy acting via increased MT1 receptor expression enhances MDM2 gene expression at day 16 (Figure 3). Gene expressions of LIF and MDM2 appear to be central intracellular focal points leading to an ultimate decrease in gene expression of mucin. This appears to be mediated by a MDM2-induced increase in MTBP expression acting in concert with ISG15 expression leading to a decrease in Mucin expression. Concurrently, increased MT1 expression acting in concert with ISG15 expression enhances expression of LIF that targeted a moderate decrease in BAX gene expression. The decrease in BAX expression would reduce the positive effect of BAX on Mucin expression. An additional critical factor intracellularly is the degree of mitochondrial regulation of apoptosis. High expression of LIF in pregnancy at day 16 is associated with low gene expression of BBC3 in the two nonpregnant statuses (Figure 3). Expression of BBC3 is a major upstream regulator of the apoptotic pathway in mitochondria that ultimately influences both nuclear and mitochondrial pathways leading to cellular apoptosis. The high LIF expression associated with a negative decrease in BBC3 expression (Figure 3 and Figure 4) exerts an antiapoptotic effect, partly associated with ISG15 expression in blood cells.
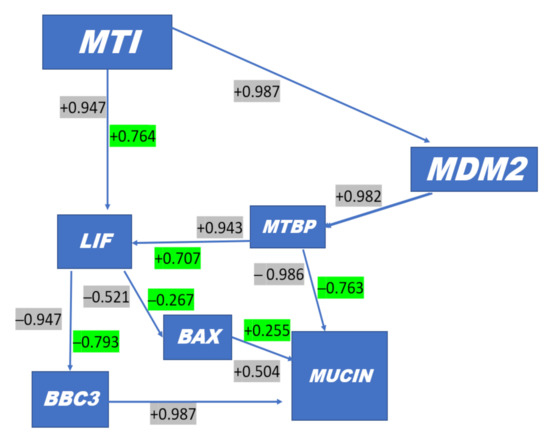
Figure 4.
Simple (Gray) and Standard Partial correlations (Green) in uterine luminal endometrial cells responsive to ISG 15 expression in blood cells. Based on magnitude of decrease in correlation between simple and partial correlations, the systems in cellular target components are modulated by ISG15 e.g., MTBP with Mucin is highly correlated negatively but when adjusted to average ISG15 expression the relationship is reduced, which indicates that ISG15 targets MTBP. In contrast, MDM2 is highly negatively correlated with MUC1; however, when MDM2 is negatively correlated with MUC1 and adjusted for ISG15, the correlation is not reduced but it stays the same. + MT1 Melatonin Receptor is found in peripheral tissues; + MDM2 gene encodes the MDM2 protein that binds and ubiquitinates p53 (i.e., inhibits p53) and stimulates degradation; + MTBP gene inhibits cell migration in vitro and suppresses the invasive behavior of tumor cells (by similarity). This may play a role in MDM2-dependent p53/TP53 homeostasis by binding of MDM2 to MTBP; -BAX, a proapoptotic activator, effects mitochondria membrane voltage-dependent anion channels (VDACs) involved in P54-mediated apoptosis; -BBC3 gene encodes Bcl-2-binding component 3 protein; BBC3 also known as p53 upregulated modulator of apoptosis (PUMA); + MUCIN provides instructions for making a protein called mucin 1. This protein is one of several mucin proteins that make up mucus; + LIF gene encodes interleukin 6 family cytokine, involved in induction of hematopoietic differentiation, neuronal cell differentiation, and kidney development. This plays a role in immune tolerance at the maternal–fetal interface.
4. Discussion
Oxidative stress (OS) is due to an imbalance between oxidants and antioxidants [33]. Due to abrupt and sustained environmental changes (e.g., THI), at the time of insemination and pregnancy, etc., transitioning from hypoxic to hyperoxic status increases reactive oxygen species (ROS) of the maternal–conceptus cells. This transition causes OS and atrophy of cells resulting in death of the localized conceptus (embryo and endometrial interface) [34].
The relatively greater values of TBARS and AOPP in cows with early embryonic loss are indicative of oxidative damage of lipids (TBARS) and proteins (AOPP) (Figure 1). Similar results were described by Dirandeh et al. (2021) [3] reporting decreased total antioxidant capacity (TAC), SOD and GPx in cows with embryonic loss, as well as increased lipid peroxidation. Zhao et al. (2019) [35] reported that GPx abundance was greater in cyclic cows compared to cows with inactive ovaries, suggesting that OS in ovaries may lead to failure in follicular development to form a cumulus structure that promotes oocyte production.
In the present study, plasma concentrations of melatonin were significantly greater in pregnant cows compared to nonpregnant cows at day 16 or cows pregnant at day 16 with lost embryos by day 32 (Figure 2). Melatonin, through direct scavenging and indirect antioxidant actions, limits oxidative stress in all cells and protects DNA and other components from damage [36]. The antioxidative actions of melatonin and its metabolites are extremely vast, including ability to neutralize ROS [10]. Melatonin also directly and indirectly increases antioxidant enzyme expressions of superoxide dismutase (SOD) and glutathione peroxidase (GPx) (Figure 5). These are bifunctional effects of directly increasing enzyme expressions associated with detoxification of ROS and increasing mRNA expressions of antiapoptotic pathways of melatonin via MT1 and MT2 (Figure 5). Use of melatonin in embryo culture has caused reductions in oxidative stress and apoptosis [37]. Moreover, melatonin has increased the number of blastocyst cells, glutathione activity, and reduced both oxidative stress and apoptosis [38]. In rats exposed to oxidative stress induced by sodium fluoride (NaF), melatonin improved levels of catalase (CAT) and superoxide dismutase (SOD), which counteracted ROS activity on embryos [13].
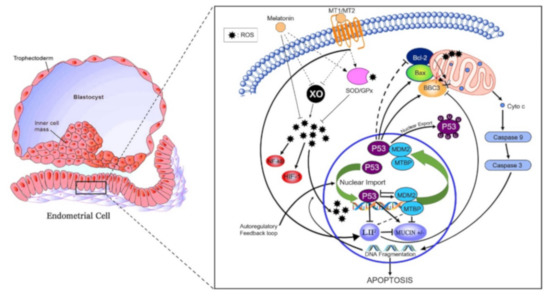
Figure 5.
Proposed antiapoptotic pathway of melatonin in the bovine endometrial cell. Abrupt environmental change at pregnancy from hypoxic to hyperoxic induces xanthine oxidase (XO) expression and activity, thereby generating reactive oxygen species (ROS). ROS-induced DNA fragmentation also activates p53. Regulation of the Bax/Bcl-2 ratio pathway leads to the activation of caspase 9 and caspase 3. Downstream effects of caspase 3 include increasing DNA fragmentation. Melatonin, via its receptors, indirectly increases expression and activity of superoxide dismutase (SOD) 1 and 2 as well as glutathione peroxidase (GPx) antioxidant enzymes. XO expression and activity is indirectly reduced by melatonin and its receptors. Moreover, melatonin signaling creates a positive feedback loop between MDM2/MTBP that regulates p53 by two main mechanisms: inducing p53 ubiquitination that suppresses the transactivation domain of p53, hence preventing transcription of downstream targets (i.e., increase Bcl-2: Bas ratio and BBC3); secondly, melatonin binding to MT1 increases LIF. The increase in LIF decreases BBC3 therefore reducing apoptosis. The decrease in BBC3 expression reduces the positive regulation of MUC1 expression thus allowing cell adhesion.
In the present study, mRNA expression of MTI receptor in uterine luminal epithelial cells was significantly greater in pregnant cows compared to nonpregnant cows at day 16 or cows pregnant at day 16 but who lost embryos by days 32 (Figure 3). In addition to the direct antioxidant activity and inhibition of xanthine oxidase (XO), melatonin-induced functions also are mediated through binding to melatonin membrane receptors MT1 (Figure 5) and MT2 [11]. Following melatonin stimulation, MT1 and MT2 receptors regulating antioxidative and antiapoptotic signaling pathways involved in embryo implantation were identified, e.g., expressions of p53, MUC-1 and LIF (Figure 5). It has been shown that differential expression of MT1 and MT2 receptors in pregnant and nonpregnant human uteri are able to influence the cyclic rhythm of myoendometrial contractility [39]. This is an insightful response because it infers not only melatonin action within the control systems of the brain, but direct melatonin regulation of tissues such as the uterus. Serotonin, the precursor to melatonin, has a dual control system based on differential expression of two isoforms of tryptophan hydroxylase (TPH1 and TPH2) [40]. Indeed, TPH1 is highly expressed throughout a plethora of tissues involved in regulation of metabolism (e.g., small intestine, stomach, ovary, uterus, etc.), However, TPH2 expression was much more restricted to tissues of the central nervous system. Melatonin, as a product of serotonin biosynthesis, has a major metabolic role in the body, and its biosynthesis is not restricted to the pineal but is produced profusely in a diversity of tissues. This is evident by differences detected in plasma melatonin concentrations in highly productive fertile cows compared to cows that did not readily conceive or had embryonic mortality (Figure 1). The high-producing dairy cow is challenged with the metabolic demands and managing oxidative stress. Zhao et al. (2019) [35] characterized an array of differential blood proteins in dairy cows with inactive versus active cyclic ovaries during early lactation and reported that GPx was downregulated in cows with inactive ovaries. Consequently, OS results in lower quality of subsequent ovulatory follicles and consequently lower embryo quality. Distinct differences in antioxidative statuses and differential gene expressions of cells harvested from the luminal endometrial environments at day 16 were detected when exposed to the conceptus compared to nonpregnant cows or pregnant cows losing embryos (Figure 3). Studies performed in a mouse culture system displayed that melatonin stimulates formation of blastocysts during embryogenesis [41]. From a molecular perspective, melatonin regulates functionality or activation of p53 by inducing p38-dependent phosphorylation of p53 [42]. Damaged DNA leads to activation of p53 phosphorylation, which increases the ability of p53 to bind DNA and mediate transcriptional activation. This p53-dependent DNA-damage response to activate transcription is mediated by MT1 and MT2 in mice [43]. The upregulation of p21 increased p38 activation that favors p53 phosphorylation and increased transcriptional activation [44]. These results indicate that p53 phosphorylation could be a downstream element responsive to MT1–MT2 receptor binding to melatonin. Transcription of BBC3 is increased when exposed to diverse apoptotic stimuli (i.e., DNA-damaging agents) and wild-type p53 induces apoptosis via the mitochondrial pathway. Indeed, BBC3 expression was low in endometrial cells of pregnant cows and increased sequentially in pregnant–embryo-loss and nonpregnant cows. BCL2 prevents BAX/BAK oligomerization, and BAX gene expression was low in all three groups. Furthermore, BCL2 binds to and inactivates BAX as well as other proapoptotic proteins to inhibit apoptosis. These coordinated dynamics would contribute to the differential responses among pregnancy statuses.
LIF expression promotes cell survival by suppressing apoptosis and positively influences embryo implantation [45]. This is supported by parallel increases in MT1, MTBP, MDM2 and LIF gene expressions in luminal epithelial cells from pregnant cows at day 16 compared to nonpregnant cows (Figure 3).
Apoptosis can occur through several pathways, and the present study identified the integrated MDM2-BBC3 and LIF expression pathways as a likely target of melatonin action in bovine endometrial cells. In pregnant cows, increased plasma melatonin and endometrial MT1 expression were associated with increased expressions of MDM2, MTBP and LIF, that directly and indirectly mediated downstream decreases in expression of both apoptotic BBC3 and BAX genes. LIF-induced reductions in gene expression of both BBC3 and BAX are considered important, since each would have ultimate net positive associations to reduced MUC1 expression. In contrast, the upstream MTBP gene may directly target a decrease in MUC1 expression. Net reduction in mucin expression is considered essential for adhesion of trophoblast cells to underlying endometrial cells. Consequently, these associated pathways ultimately target a decrease in mucin expression in pregnancy versus increases of expression in cows losing embryos or nonpregnant cows. Furthermore, increases in ISG15 of blood cells significantly enhanced these negative associations as well. Day 16 is the time associated with the start of the process of pregnancy recognition in dairy cows. Other luminal endometrial epithelial cells throughout the uterine horn not adjacent to the trophoblast cells would remain coated with mucin [46]. Cells harvested locally within the uterine horn of projected pregnancy provided a population of cells differentially expressing a complement of antioxidant/antiapoptotic genes associated with pregnancy statuses (pregnant, pregnant–lost embryos and nonpregnant), and expression of these genes were associated with ISG15 expression + and ─in peripheral blood cells.
MDM2 protein interacts with several intracellular proteins, one of which is MTBP that is expressed highly in the ovary [47]. MTBP inhibits growth and induces G1 arrest in a TP53-independent manner in human lung carcinoma and osteosarcoma cell lines [46], and promotes MDM2 degradation of TP53 protein [48]. The increase in MTBP mRNA expression in pregnant cows in the present study may ultimately potentiate the inhibitory effect of melatonin on MDM2-dependent apoptosis. Furthermore, increased expression of MTBP appears to decrease gene expression of MUC1. We are not aware of any other reports suggesting regulation of MTBP by melatonin in the uterus. Perhaps regulation of the MTBP mRNA expression and interaction with MDM2 are related to cell/tissue remodeling dynamics of the developing endometrium/embryo/conceptus in early pregnancy (e.g., >d16), and this warrants further investigation.
In contrast to the potential stimulatory effects of melatonin on increased expression of MDM2 and MTBP mRNA in pregnant cows (Figure 3), expression of mRNA encoding the potent proapoptotic protein BBC3 was inhibited by P53 in pregnant cows, as shown Figure 3 and Figure 4. Consequently, expression of BBC3 increased progressively in pregnant cows that lost embryos to even higher expression levels in nonpregnant cows (Figure 3). This protein is the major mediator of TP53-induced apoptosis, and ultimately activates BAX protein at the mitochondrial membrane [49]. However, BAX gene expression in all three reproductive statuses was low (Figure 3). Expression of BAX was weakly associated with BBC3 gene expression in the endometrium (Table 1) and was not altered by ISG15 expression in blood cells. (Table 1 and Table 2).
In present study, mRNA expression of LIF was greater, whereas MUC1 expression was lower at day 16 in pregnant cows, as shown in Figure 3. One of the main estrogen mediators responsible for implantation seems to be leukemia inhibitory factor (LIF), regulated by p53 [50], that supports uterine receptivity during implantation. However, plasma estradiol and estrone concentrations are low in early pregnant cows [51]. Carlomagno et al. (2018) [45] showed implant failure in mice when LIF was suppressed. Mucin (MUC1) is also a regulatory molecule, which is expressed and secreted from endometrial cells to inhibit cell–cell adhesion [52]. Expression of MUC1 in the endometrium has been suggested to create a barrier to embryo adhesion that must be suppressed during implantation [53]. Interestingly, when human blastocysts were allowed to attach to the endometrial epithelial cell monolayer, MUC1 was removed locally at the implantation site. Pregnancy recognition in cattle is associated with adherence and coregulation between trophoblast and endometrial cells [54]. For example, a plethora of endometrial adhesion genes were expressed less in pregnant cows compared to cyclic cows at day 16, see Supplementary file [54]. This would support the idea that cell-to-cell adherence in nonpregnant or pregnant cows that lost embryos in early pregnancy may be partially due to copious gene expression of adhesion molecules. Likewise, a complement of three mucin genes were upregulated and three were downregulated for expression of mucins [54]. Differential expression of these mucin genes co-expressed at the interface of the luminal endometrium with the trophoblast compared to luminal epithelial cells nonadjacent to the trophoblast would be insightful. Indeed, associations of endometrial epithelial gene expressions within apoptotic pathways regulated by melatonin were modulated in association with ISG15 concentrations in blood cells (Table 1 and Table 2; Figure 3). These associations with ISG15 in endometrial cells are due to interferon T secretion by day 16 of pregnancy. This is further substantiated by measurements of ISG15 in blood cells as a predictor of pregnancy status in the present experiment with 200 cows.
Kasimanickam and Kasimanickam, (2021) [55] reported that MUC1 was lower in embryos of grade 1 (excellent) and 2 (fair) compared to 3 (poor) and 4 (unfertilized/dead/degenerate). Similarly, expression of MUC1 was lower in endometrium of cows without subclinical endometritis compared with those that had subclinical endometritis. Kasimanickam et al. (2014) [28] reported greater expression of MUC1 in cows with metritis and endometritis compared to normal cows, which subsequently resulted in poor reproductive performance, possibly due to embryonic death.
The present findings indicate that proper balance and control of OS and ROS are essential for health and productivity of the lactating dairy cow, but there is also a local regulated antioxidative system within the maternal–conceptus unit at the time of pregnancy maintenance or loss in early pregnancy. A major consideration for reproductive management of lactating dairy cows is management of oxidative stress through regulation of melatonin synthesis and secretion, which directly influences antioxidant activity for optimal conceptus development. This system appears to regulate overall metabolism and health under dual control of central nervous system and peripheral tissue metabolism. This is exemplified by the sequential homeorhetic/homeostatic processes occurring postpartum, to ultimately permit a uterine–conceptus dialogue for a successful pregnancy concurrently with meeting the needs of the lactating dairy cow. Further noninvasive strategies of melatonin regulation entail regulation of photoperiod and dietary nutritional supplementation of amino acids (i.e., differential tissue conversion of tryptophan for synthesis of serotonin, the immediate precursor of melatonin) that warrant further investigation. Such strategies may complement and/or advance current antioxidant strategies mediated through vitamin (s) and supplemental nutraceutical inputs, such as feeding organic selenium-methionine and bypassing fatty acid supplements that increase availability of n-3 fatty acids to improve antioxidant status.
An overarching finding of this experiment is the potential roles of ISG15 secreted locally by the endometrium in apposition to trophoblast cells of the elongating embryo at day 16. This dialogue is essential for maintenance of pregnancy and distal effects such as lifespan of the corpus luteum. A role for ISG15 in regulating oxidative statuses of the maternal–conceptus unit is documented, linking ISG15 gene expression in blood cells with antioxidant and antiapoptotic regulatory genes of uterine luminal epithelial cells recovered with Cytobrush sampling within the potential 16-day-pregnant uterine horn.
The process in which ISG15 catalyzes post-translational conjugation to de novo synthesized intracellular proteins is termed ISGylation, which regulates stability and functional processes [56,57,58,59]. Consequently, a functional role of ISG15 may involve mitochondria, where a plethora of 52-ISGylated proteins were predicted to localize and impact various intracellular systems such as negative regulation of apoptotic signaling pathway, ATP biosynthetic processes, oxidation-reduction status, TCA cycle and glycolysis.
These novel findings emphasize the potential importance of ISG15 in the control of cellular metabolism and mitochondrial responses such as oxidation status and immune responses. A better understanding of how regulation of ISG15 affects downstream oxidation status, immune functions, reproductive processes and their interactions is needed. An overall melatonin-regulatory system may have a profound effect on physiological statuses associated with lactation, environmental heat stress, and nutritional management, genomic differences acquired from genetic selection, production medicine/management programs and reproductive performance. This is particularly important with ongoing global climatic change. In the lactating dairy cow, a localized operational control system involving melatonin appears to be functional in the uterine–ovarian housing system for protection of the developing conceptus and its maintenance during early pregnancy. This system is most likely sustained through the transition period, encompassing parturition and recrudescence of postpartum fertility leading to a pregnancy.
5. Conclusions
During the preimplantation period, oxidative stress negatively influences embryo quality and pregnancy success in lactating dairy cows. At day 16 of pregnancy, peripheral plasma markers of antioxidant status and concentrations of melatonin and localized gene expression of antioxidant cellular markers in uterine luminal cells were associated with subsequent fertility responses. Gene expression responses of uterine luminal cells were associated with gene expression of ISG15 in blood cells. Beneficial responses of melatonin status towards fertility support potential nutraceutical and other strategies to enhance availability and biosynthesis of melatonin, with the goal of enhancing reproductive performance of high producing dairy cows.
Author Contributions
Conceptualization, E.D.; methodology, E.D. and Z.A.-P.; implementation and sampling, E.D. and Z.A.-P.; facility management, E.D.; investigation, E.D.; statistical analysis, E.D. and Z.A.-P.; writing—original draft preparation, E.D. and W.T.; writing—review and editing, E.D. and W.T.; supervision, E.D.; funding acquisition, E.D. and Z.A.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funding from Sari Agricultural Sciences and Natural Resources University (Project No. 03-1396-06). Otherwise, this research received no external funding.
Institutional Review Board Statement
The present study was conducted at the Sari Agricultural Sciences and Natural Resources University (SANRU), Sari, Mazandaran, Iran, in accordance with the guidelines of the Iranian Council of Animal Care (1995) and approved by the ethics committee of Sari Agricultural Sciences and Natural Resources University (protocol # 1998).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings and which are presented in this study are available on request from the corresponding author, Essa Dirandeh, dirandeh@gmail.com. The data is not publicly available as not all data of the study have been published yet.
Acknowledgments
The authors thank the personnel of the Mahdasht dairy farm for permission to use their cows and for their assistance during the study. The authors also thank the director and technical committee members of Pars Agriculture and Livestock Investing Holding Co. for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sartori, R.; Sartor-Bergfelt, R.; Mertens, S.A.; Guenther, J.N.; Parish, J.J.; Wiltbank, M.C. Fertilization and Early Embryonic Development in Heifers and Lactating Cows in Summer and Lactating and Dry Cows in Winter. J. Dairy Sci. 2002, 85, 2803–2812. [Google Scholar] [CrossRef]
- Spencer, T.E.; Forde, N.; Lonergan, P. Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod. Fertil. Dev. 2016, 29, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Dirandeh, E.; Sayyar, M.A.; Ansari, Z.; Deldar, H.; Thatcher, W.W. Peripheral leucocyte molecular indicators of inflammation and oxidative stress are altered in dairy cows with embryonic loss. Sci Rep. 2021, 11, 12771. [Google Scholar] [CrossRef] [PubMed]
- Dirandeh, E.; Ansari, Z.; Deldar, H.; Shohreh, B.; Ghaffari, J. Endocannabinoid system and early embryonic loss in Holstein dairy cows. Anim. Sci. Pap. Rep. 2020, 2, 135–144. [Google Scholar]
- Moraes, J.G.N.; Behura, S.K.; Geary, T.W.; Hansen, P.J.; Neibergs, H.L.; Spencer, T.E. Uterine influences on conceptus development in fertility-classified animals. Proc. Natl. Acad. Sci. USA 2018, 115, E1749–E1758. [Google Scholar] [CrossRef] [Green Version]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human placental trophoblasts synthesize melatonin and express its receptors. J. Pineal Res. 2008, 45, 50–60. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Soliman, A.; Vaillancourt, C. Maternal and placental melatonin: Actions and implication for successful pregnancies. Minerva Ginecol. 2014, 66, 251–266. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a naturally against oxidative stress: A physicochemical examination. J. Pineal Res. 2013, 51, 1–16. [Google Scholar] [CrossRef]
- Martin, M.; Macias, M.; Escames, G.; Leon, J.; Acuna-Castroviejo, D. Melatonin but not vitamins c and e maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Lopez-Burillo, S.; Sainz, R.M.; Mayo, J.C. Melatonin: Detoxification of oxygen and nitrogen-based toxic reactants. Adv. Exp. Med. Biol. 2003, 527, 539–548. [Google Scholar]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Kivela, A. Serum melatonin during human pregnancy. Acta Endocrinol. 1991, 124, 233–237. [Google Scholar] [CrossRef]
- Bharti, V.K.; Srivastava, R.S.; Kumar, H.; Bag, S.; Majumdar, A.C.; Singh, G.; Pandi-Perumal, S.R.; Brown, G.M. Effects of melatonin and epiphyseal proteins on fluoride-induced adverse changes in antioxidant status of heart, liver, and kidney of rats. Adv. Pharmacol. Sci. 2014, 532969. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilly, K.I.; Banerjee, S.; Banerjee, P.P.; Tilly, J.L. Expression of the p53 and Wilms’ tumor suppressor genes in the rat ovary: Gonadotropin repression in vivo and immunohistochemical localization of nuclear p53 protein to apoptotic granulosa cells of atretic follicles. Endocrinology 1995, 136, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Qin, J.; Srivenugopal, K.S.; Wang, M.; Zhang, R. The MDM2-p53 pathway revisited. J. Biomed. Res. 2013, 27, 254–271. [Google Scholar]
- Portela, V.M.; Dirandeh, E.; Zamberlam, G.; Barreta, M.H.; Gotten, A.F.; Price, C.A. Fibroblast growth factor-18 increases apoptosis in bovine granulosa cells through an estrogen-dependent pathway involving mouse double-minute homolog-2. Biol. Reprod. 2014, 92, 1–8. [Google Scholar]
- Speidel, D. Transcription-independent p53 apoptosis: An alternative route to death. Trends Cell Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy of Science: Washington, DC, USA, 2001. [Google Scholar]
- Heidari, F.; Dirandeh, E.; Ansari Pirsaraei, Z.; Colazo, M.G. Modifications of the G6G timed-AI protocol improved pregnancy per AI and reduced pregnancy loss in lactating dairy cows. Animal 2017, 11, 2002–2009. [Google Scholar] [CrossRef] [Green Version]
- Mohtashamipour, F.; Dirandeh, E.; Ansari-Pirsaraei, Z.; Colazo, M.G. Postpartum health disorders in lactating dairy cows and its associations with reproductive responses and pregnancy status after first timed-AI. Theriogenology 2020, 141, 98–104. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Quinlan, G.J. Malondialdehyde formation from lipid peroxides in the thiobarbituric acid test: The role of lipid radicals, iron salts, and metal chelators. J. Appl. Biochem. 1983, 5, 293–299. [Google Scholar]
- Cardoso, B.; Oliveira, M.L.; Pugliesi, G.; Batista, E.O.S.; Binelli, M. Cytobrush: A tool for sequential evaluation of gene expression in bovine endometrium. Reprod. Dom. Anim. 2017, 52, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Dirandeh, E.; Ghaffari, J. Effects of feeding a source of omega-3 fatty acid during the early postpartum period on the endocannabinoid system in the bovine endometrium. Theriogenology 2018, 121, 141–146. [Google Scholar] [CrossRef]
- Saxena, V.K.; Jha, B.K.; Meena, A.S.; Naqvi, S.M.K. Sequence analysis and identification of new variations in the coding sequence of melatonin receptor gene (MTNR1A) of Indian Chokla sheep breed. Meta Gene 2014, 2, 450–458. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V.; Kastelic, J.P. Mucin 1 and cytokines mRNA in endometrium of dairy cows with postpartum uterine disease or repeat breeding. Theriogenology 2014, 81, 952–958. [Google Scholar] [CrossRef]
- Lam, L.; Dance, A.; Thundathil, J.; Dobrinski, I. Effects of culture medium and substrate on attachment of in vitro produced bovine embryos. Anim. Reprod. 2014, 11, 33–542. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines—Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2011, 25, 402–408. [Google Scholar] [CrossRef]
- Yuan, J.S.; Wang, D.; Stewart, C.N., Jr. Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnol. J. 2008, 3, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Dirandeh, E.; Ansari-Pirsaraei, Z.; Deldar, H. Antioxidant levels, copper and zinc concentrations were associated with postpartum luteal activity, pregnancy loss and pregnancy status in Holstein dairy cows. Theriogenology 2019, 15, 97–103. [Google Scholar] [CrossRef]
- Geisert, R.; Fazleabas, A.; Lucy, M.; Mathew, D. Interaction of the conceptus and endometrium to establish pregnancy in mammals: Role of interleukin 1β. Cell Tissue Res. 2012, 349, 825–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Shu, S.; Bai, Y.; Wang, D.; Xia, C.; Xu, C. Plasma Protein Comparison between Dairy Cows with Inactive Ovaries and Estrus. Sci. Rep. 2019, 9, 13709. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Takahashi, M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 2012, 58, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Han, H.B.; Tian, X.Z.; Tan, D.X.; Wang, L.; Zhou, G.B.; Zhu, S.E.; Liu, G.S. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J. Pineal Res. 2012, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; Silavin, S.L.; Wentworth, R.A.; Figueroa, J.P.; Honnebier, B.O.; Fishburne, J.I., Jr.; Nathanielsz, P.W. Different patterns of myometrial activity and 24-h rhythms in myometrial contractility in the gravid baboon during the second half of pregnancy. Biol. Reprod. 1992, 46, 1158–1164. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, Y.; Lee, J.; Lee, J.Y.; Kim, H.; Lee, S.; Oh, C.-M. A Systems Biology Approach to Investigating the Interaction between Serotonin Synthesis by Tryptophan Hydroxylase and the Metabolic Homeostasis. Int. J. Mol. Sci. 2021, 22, 245. [Google Scholar] [CrossRef]
- Asgari, Z.; Ghasemian, F.; Ramezani, M.; Bahadori, M.H. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012, 14, 203–208. [Google Scholar]
- Mediavilla, M.D.; Cos, S.; Sanchez-Barcelo, E.J. Melatonin increases p53 and p21waf1 expression in mcf-7 human breast cancer cells in vitro. Life Sci. 1999, 65, 415–420. [Google Scholar] [CrossRef]
- Santoro, R.; Mori, F.; Marani, M.; Grasso, G.; Cambria, M.A.; Blandino, G.; Muti, P.; Strano, S. Blockage of melatonin receptors impairs p53-mediated prevention of DNA damage accumulation. Carcinogenesis 2013, 34, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; Dobrowolny, G.; D’Anselmi, F.; Dinicola, S.; Masiello, M.G.; Pasqualato, A.; Palombo, A.; Morini, V.; Reiter, R.J.; et al. Melatonin down-regulates mdm2 gene expression and enhances p53 acetylation in mcf-7 cells. J. Pineal Res. 2014, 57, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.A.; Bazer, F.W.; Burghardt, R.C.; Wu, G.; Sen, H.; Kramer, A.C. Cellular events during ovine implantation and impact for gestation. Anim. Reprod. 2018, 15 (Suppl. 1), 843–855. [Google Scholar] [CrossRef] [Green Version]
- Boyd, M.T.; Vlatkovic, N.; Haines, D.S. A novel cellular protein (MTBP) binds to MDM2 and induces a G1 arrest that is suppressed by MDM2. J. Biol. Chem. 2000, 275, 31883–31890. [Google Scholar] [CrossRef] [Green Version]
- Brady, M.; Vlatković, N.; Boyd, M.T. Regulation of p53 and MDM2 activity by MTBP. Mol. Cell. Biol. 2005, 25, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, L. PUMA, a potent killer with or without p53. Oncogene 2009, 27, S71–S83. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.J.; Tomasini, R.; McKeon, F.D.; Mak, T.W.; Melino, G. The p53 family: Guardians of maternal reproduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 259–265. [Google Scholar] [CrossRef]
- Eley, R.M.; Thatcher, W.W.; Bazer, F.W. Hormonal and physical changes associated with bovine conceptus development. J. Reprod. Fert. 1979, 55, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Wesseling, J.; van der Valk, S.W.; Hilkens, J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell. Biol. 1995, 129, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meseguer, M.; Aplin, J.D.; Caballero-Campo, P.; O’Connor, J.E.; Martín, J.C.; Remohí, J.; Pellicer, A.; Simón, C. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol. Reprod. 2001, 64, 590–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerri, R.L.A.; Thompson, I.M.; Kim, I.H.; Ealy, A.D.; Hansen, P.J.; Staples, C.R.; Li, J.L.; Santos, L.E.P.; Thatcher, W.W. Effects of lactation and pregnancy on gene expression of endometrium of Holstein cows at day 17 of the estrous cycle or pregnancy. J. Dairy Sci. 2012, 95, 5657–5675. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Kasimanickam, V.R. mRNA Expressions of Candidate Genes in Gestational Day 16 Conceptus and Corresponding Endometrium in Repeat Breeder Dairy Cows with Suboptimal Uterine Environment Following Transfer of Different Quality Day 7 Embryos. Animals 2021, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Baldanta, S.; Fernández-Escobar, M.; Acin-Perez, R.; Albert, M.; Camafeita, E.; Jorge, I.; Vazquez, J.; Enriquez, A.; Guerra, S. ISG15 governs mitochondrial function in macrophages following vaccinia virus infection. PLoS Pathog. 2017, 13, e1006651. [Google Scholar] [CrossRef]
- Albert, M.; Bécares, M.; Falqui, M.; Fernández-Lozano, C.; Guerra, S. ISG15, a Small Molecule with Huge Implications: Regulation of Mitochondrial Homeostasis. Viruses 2018, 10, 629. [Google Scholar] [CrossRef] [Green Version]
- Marine, J.-C.; Lozano, G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 2010, 17, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Zac, S.; da Costa, I.C.; Schmidt, C.K. More than Meets the ISG15: Emerging Roles in the DNA Damage Response and Beyond. Biomolecules 2020, 10, 1557. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).