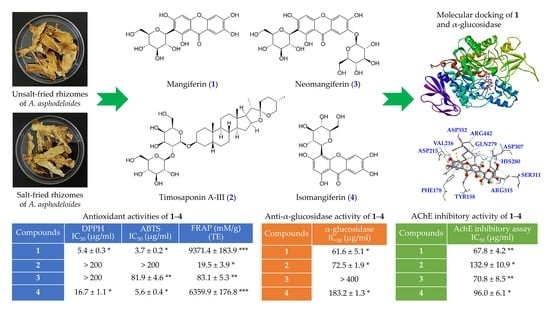
Comparison of Various Solvent Extracts and Major Bioactive Components from Unsalt-Fried and Salt-Fried Rhizomes of Anemarrhena asphodeloides for Antioxidant, Anti-α-Glucosidase, and Anti-Acetylcholinesterase Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of A. asphodeloides Extract
2.3. Preparation of Active Components
2.4. Reverse-Phase HPLC
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. DPPH Radical Scavenging Activity
2.8. ABTS Radical Scavenging Activity
2.9. Superoxide Radical Scavenging Activity
2.10. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.11. α-Glucosidase Inhibitory Activity Assay
2.12. Acetylcholinesterase Inhibitory Activity Assay
2.13. Molecular Modeling Docking Study
2.14. Statistical Analysis
3. Results
3.1. Determination of Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Yields in Each Solvent Extract
3.2. DPPH Free-Radical Scavenging Activity
3.3. ABTS Free-Radical Scavenging Activity
3.4. Superoxide Radical Scavenging Activity
3.5. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.6. Anti-α-Glucosidase Activity Assay
3.7. Acetylcholinesterase (AChE) Inhibitory Activity Assay
3.8. Quantitation of Active Components in Different Solvent Extracts
3.9. Antioxidant Activities of Isolated Components
3.10. Anti-α-Glucosidase Activities of Isolated Components
3.11. Acetylcholinesterase (AChE) Inhibitory Assays of Isolated Components
3.12. Molecular Docking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamache, W.; Amira, S.; Souici, C.B.; Laouer, H.; Benchikh, F. In vitro antioxidant, anticholinesterases, anti-α-amylase, and anti-α-glucosidase effects of Algerian Salvia aegyptiaca and Salvia verbenaca. J. Food Biochem. 2020, 44, e13472. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Oniszczuk, T.; Waksmundzka-Hajnos, M. The influence of common free radicals and antioxidants on development of Alzheimer’s disease. Biomed. Pharmacother. 2016, 78, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.O.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ros production and activity. J. Physiol. Lond. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforsch. C 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Köse, L.P.; Gülcin, I.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crop. Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Helvaci, M.R.; Aydin, Y.; Varan, G.; Abyad, A.; Pocock, L. Acarbose versus metformin in the treatment of metabolic syndrome. World Fam. Med. 2018, 16, 10–15. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Chang, C.-H.; Liao, H.-R.; Fu, S.-L.; Chen, J.-J. Anti-cancer and anti-inflammatory activities of three new chromone derivatives from the marine-derived Penicillium citrinum. Mar. Drugs 2021, 19, 408. [Google Scholar] [CrossRef]
- Iida, Y.; Oh, K.-B.; Saito, M.; Matsuoka, H.; Kurata, H.; Natsume, M.; Abe, H. Detection of antifungal activity in Anemarrhena asphodeloides by sensitive BCT method and isolation of its active compound. J. Agric. Food Chem. 1999, 47, 584–587. [Google Scholar] [CrossRef]
- Nakashima, N.; Kimura, I.; Kimura, M.; Matsuura, H. Isolation of pseudoprototimosaponin AIII from rhizomes of Anemarrhena asphodeloides and its hypoglycemic activity in streptozotocin-induced diabetic mice. J. Nat. Prod. 1993, 56, 345–350. [Google Scholar] [CrossRef]
- Tobari, A.; Teshima, M.; Koyanagi, J.; Kawase, M.; Miyamae, H.; Yoza, K.; Takasaki, A.; Nagamura, Y.; Saito, S. Spirostanols obtained by cyclization of pseudosaponin derivatives and comparison of anti-platelet agglutination activities of spirostanol glycosides. Eur. J. Med. Chem. 2000, 35, 511–527. [Google Scholar] [CrossRef]
- Kang, Y.J.; Chung, H.J.; Nam, J.W.; Park, H.J.; Seo, E.K.; Kim, Y.S.; Lee, D.; Lee, S.K. Cytotoxic and antineoplastic activity of timosaponin AIII for human cancer cells. J. Nat. Prod. 2011, 74, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Trinh, H.T.; Jung, K.; Han, S.J.; Kim, D.H. Inhibitory effects of steroidal timosaponins isolated from the rhizomes of Anemarrhena asphodeloides against passive cutaneous anaphylaxis reaction and pruritus. Immunopharmacol. Immunotoxicol. 2010, 32, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Jung, K.; Kim, D.-H. Timosaponin AIII, a saponin isolated from Anemarrhena asphodeloides, ameliorates learning and memory deficits in mice. Pharmacol. Biochem. Behav. 2009, 93, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, L.; Lin, D.; Liu, Y.; Zhang, M.; Song, S.J. Determination of the chemical constituents of the different processed products of Anemarrhena asphodeloides rhizomes by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2016, 30, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-W.; Chu, Y.-C.; Huang, C.-Y.; Fu, S.-L.; Chen, J.-J. Evaluation of antioxidant and anti-α-glucosidase activities of various solvent extracts and major bioactive components from the seeds of Myristica fragrans. Molecules 2020, 25, 5198. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Takao, T.; Watanabe, N.; Yagi, I.; Sakata, K. A Simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci. Biotechnol. Biochem. 1994, 58, 1780–1783. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Zhang, Z.-R.; Cheung, H.-Y. Antioxidant activity of rhizoma Smilacis Glabrae Extracts and its key constituent-astilbin. Food Chem. 2009, 115, 297–303. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 2013, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, A.; Pervin, M.; Lim, B.O. Acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of Ganoderma lucidum grown on germinated brown rice. Molecules 2013, 18, 6663–6678. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- BIOVIA. Discovery Studio Client 2021; v.21.1.0; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef]
- Mbeunkui, F.; Grace, M.H.; Lategan, C.; Smith, P.J.; Raskin, I.; Lila, M.A. Isolation and identification of antiplasmodial N-alkylamides from Spilanthes acmella flowers using centrifugal partition chromatography and ESI-ITTOF-MS. J. Chromatogr. B 2011, 879, 1886–1892. [Google Scholar] [CrossRef]
- Yangthong, M.; Hutadilok-Towatana, N.; Phromkunthong, W. Antioxidant activities of four edible seaweeds from the southern coast of Thailand. Plant Food Hum. Nutr. 2009, 64, 218–223. [Google Scholar] [CrossRef]
- Gülçin, Ì.; Şat, İ.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.İ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Ferreira, F.R.; Valentim, B.V.; Ramones, E.L.C.; Trevisan, M.T.S.; Olea-Azar, C.; Perez-Cruz, F.; Abreu, F.C.; Goulart, M.O.F. Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. LWT-Food Sci. Technol. 2013, 51, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ma, L.; Chen, W.H.; Park, H.; Ke, Z.; Wang, B. Binding mechanism and synergetic effects of xanthone derivatives as noncompetitive α-glucosidase inhibitors: A theoretical and experimental study. J. Phys. Chem. B 2013, 117, 13464–13471. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, W.; Cai, Y.; Li, C.; Wang, L. HPLC fingerprinting-based multivariate analysis of phenolic compounds in mango leaves varieties: Correlation to their antioxidant activity and in silico α-glucosidase inhibitory ability. J. Pharm. Biomed. Anal. 2020, 191, 113616. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Chakraborty, S.; Mani, S.; Sali, V.K.; Vasanthi, H.R. Mangiferin from Mangifera indica fruits reduces post-prandial glucose level by inhibiting α-glucosidase and α-amylase activity. S. Afr. J. Bot. 2019, 120, 129–134. [Google Scholar] [CrossRef]
- Sramek, J.J.; Frackiewicz, E.J.; Cutler, N.R. Review of the acetylcholinesterase inhibitor galanthamine. Expert Opin. Investig. Drugs 2000, 9, 2393–2402. [Google Scholar] [CrossRef]
- Biradar, S.M.; Joshi, H.; Chheda, T.K. Neuropharmacological effect of mangiferin on brain cholinesterase and brain biogenic amines in the management of Alzheimer’s disease. Eur. J. Pharmacol. 2012, 683, 140–147. [Google Scholar] [CrossRef]









| Extracting Solvents | TPC (mg/g) a (GAE) | TFC (mg/g) b (QCE) | Yields (%) c | |||
|---|---|---|---|---|---|---|
| AA | Salt-Fried AA | AA | Salt-Fried AA | AA | Salt-Fried AA | |
| n-Hexane | 0 | 0 | 42.9 ± 4.9 ** | 8.3 ± 1.4 * | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Chloroform | 23.6 ± 1.9 ** | 38.6 ± 0.8 ** | 20.2 ± 2.5 * | 24.5 ± 2.7 ** | 1.6 ± 0.1 | 0.5 ± 0.1 |
| Dichloromethane | 14.9 ± 3.2 * | 45.4 ± 2.2 ** | 24.2 ± 2.7 ** | 13.3 ± 2.0 * | 1.1 ± 0.1 | 0.3 ± 0.1 |
| Ethyl acetate | 21.1 ± 1.8 ** | 78.2 ± 3.0 *** | 5.8 ± 2.2 * | 34.9 ± 3.0 ** | 2.1 ± 0.1 | 0.6 ± 0.1 |
| Acetone | 45.3 ± 1.2 *** | 55.2 ± 3.7 ** | 16.8 ± 2.7 * | 45.4 ± 4.2 ** | 3.0 ± 0.1 | 1.3 ± 0.1 |
| Ethanol | 13.8 ± 1.4 ** | 33.3 ± 1.1 *** | 10.9 ± 2.3 * | 23.6 ± 3.8 * | 17.7 ± 0.5 | 18.4 ± 0.7 |
| Methanol | 4.8 ± 1.9 * | 33.1 ± 2.7 ** | 8.7 ± 2.2 * | 23.8 ± 3.3 * | 44.0 ± 1.2 | 39.2 ± 1.4 |
| Water | 18.6 ± 3.7 * | 26.3 ± 1.7 ** | 15.4 ± 2.4 * | 23.6 ± 2.6 ** | 76.7 ± 4.4 | 72.9 ± 2.5 |
| Extracting Solvents | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | Superoxide IC50 (μg/mL) | FRAP (mM/g) c (TE) | ||||
|---|---|---|---|---|---|---|---|---|
| AA | Salt-Fried AA | AA | Salt-Fried AA | AA | Salt-Fried AA | AA | Salt-Fried AA | |
| n-Hexane | >400 | >400 | 305.8 ± 10.4 * | >400 | >400 | >400 | 95.9 ± 5.4 ** | 42.6 ± 5.7 * |
| Chloroform | >400 | >400 | 73.8 ± 2.3 ** | 64.3 ± 3.9 ** | >400 | >400 | 278.5 ± 7.2 *** | 346.3 ± 5.3 *** |
| Dichloromethane | >400 | >400 | 95.4 ± 5.5 ** | 40.9 ± 2.5 ** | >400 | >400 | 264.5 ± 14.4 ** | 355.6 ± 5.1 *** |
| Ethyl acetate | 241.2 ± 15.4 * | 219.4 ± 6.4 * | 75.3 ± 3.7 ** | 24.1 ± 1.2 ** | >400 | >400 | 282.7 ± 7.3 *** | 485.5 ± 4.3 *** |
| Acetone | 123.6 ± 3.9 * | 169.8 ± 8.6 * | 48.5 ± 2.9 ** | 41.9 ± 0.7 ** | >400 | >400 | 381.5 ± 8.3 *** | 481.6 ± 19.0 ** |
| Methanol | 193.3 ± 9.6 * | 181.2 ± 5.2 * | 216.0 ± 3.9 * | 119.4 ± 4.2 * | >400 | >400 | 152.2 ± 12.8 ** | 255.2 ± 4.6 *** |
| Ethanol | 150.1 ± 1.1 * | 194.2 ± 4.3 * | 177.8 ± 3.4 * | 112.4 ± 3.6 * | >400 | >400 | 203.2 ± 12.4 ** | 296.0 ± 11.7 ** |
| Water | 127.7 ± 2.3 * | 113.9 ± 8.6 * | 117.9 ± 0.6 * | 97.5 ± 1.6 * | 369.9 ± 4.3 * | 295.4 ± 6.1 * | 230.0 ± 13.8 ** | 323.7 ± 14.9 ** |
| BHT a | 35.5 ± 0.6 ** | 39.7 ± 1.4 ** | 20.6 ± 0.2 ** | 19.6 ± 0.4 ** | N.A. b | N.A. b | 4297.0 ± 48.4 *** | 4216.1 ± 66.5 *** |
| Extracting Solvents | α-Glucosidase IC50 (μg/mL) | |
|---|---|---|
| AA | Salt-Fried AA | |
| n-Hexane | 25.9 ± 2.0 * | 34.8 ± 3.0 * |
| Chloroform | 23.4 ± 2.3 * | 34.7 ± 4.7 * |
| Dichloromethane | 21.8 ± 1.4 ** | 32.0 ± 2.5 * |
| Ethyl acetate | 55.5 ± 2.8 * | 26.0 ± 0.6 ** |
| Acetone | 101.7 ± 15.9 | 105.0 ± 2.6 * |
| Methanol | >200 | >200 |
| Ethanol | >200 | >200 |
| Water | >200 | >200 |
| Acarbose a | 319.5 ± 17.3 * | 305.0 ± 4.6 * |
| Extracting Solvents | AChE Inhibitory Assay IC50 (μg/mL) | |
|---|---|---|
| AA | Salt-Fried AA | |
| n-Hexane | 163.7 ± 6.7 * | 134.2 ± 5.2 * |
| Chloroform | 133.3 ± 6.8 | 135.6 ± 6.0 * |
| Dichloromethane | 129.5 ± 6.2 * | 79.7 ± 2.6 * |
| Ethyl acetate | 169.9 ± 4.0 * | 133.8 ± 6.5 * |
| Acetone | 130.2 ± 3.7 * | 162.3 ± 6.1 * |
| Methanol | 126.5 ± 4.6 * | 126.8 ± 4.6 * |
| Ethanol | 117.1 ± 2.6 * | 85.1 ± 6.9 ** |
| Water | 139.4 ± 5.2 | 73.6 ± 2.8 * |
| Galanthamine a | 0.5 ± 0.1 ** | 0.5 ± 0.1 ** |
| Extracting Solvents | Neomangiferin (mg/kg) | Timosaponin A-III (mg/kg) | Isomangiferin (mg/kg) | Mangiferin (mg/kg) | Total Amount (mg/kg) |
|---|---|---|---|---|---|
| Water (AA) | 2.5 ± 0.5 * | 5.4 ± 0.7 ** | 2.8 ± 0.4 ** | 4.4 ± 0.6 ** | 15.1 ± 0.6 *** |
| Methanol (AA) | 2.0 ± 0.4 * | 3.6 ± 0.4 ** | 3.3 ± 0.4 ** | 3.8 ± 0.4 ** | 12.8 ± 1.6 ** |
| Ethanol (AA) | 1.6 ± 0.7 | 3.1 ± 0.4 ** | 1.0 ± 0.4 * | 3.8 ± 0.2 *** | 9.6 ± 0.5 *** |
| Acetone (AA) | 7.9 ± 1.1 ** | 4.8 ± 1.3 * | 9.6 ± 1.2 * | 6.5 ± 1.9 * | 28.8 ± 1.2 *** |
| Ethyl acetate (AA) | 6.2 ± 0.9 ** | 2.4 ± 0.9 * | 2.2 ± 0.8 * | 4.2 ± 1.0 * | 15.1 ± 0.7 *** |
| Chloroform (AA) | 4.4 ± 1.5 * | 13.9 ± 1.2 ** | 23.7 ± 1.9 ** | 5.5 ± 1.2 * | 47.5 ± 1.5 *** |
| Dichloromethane (AA) | 8.7 ± 1.4 ** | 4.3 ± 1.4 * | 3.3 ± 0.9 * | 7.2 ± 1.3 * | 23.5 ± 1.3 ** |
| n-Hexane (AA) | 2.7 ± 1.2 | 21.3 ± 1.1 *** | 12.3 ± 1.9 ** | 7.6 ± 1.2 ** | 43.9 ± 1.4 *** |
| Water (salt-fried AA) | 0.9 ± 0.5 | 3.4 ± 0.8 * | 3.8 ± 0.6 ** | 4.4 ± 1.1 * | 12.6 ± 0.8 ** |
| Methanol (salt-fried AA) | 3.0 ± 0.7 * | 5.6 ± 0.9 ** | 5.3 ± 0.8 ** | 7.8 ± 0.7 ** | 21.8 ± 0.8 *** |
| Ethanol (salt-fried AA) | 1.9 ± 0.9 | 3.1 ± 0.9 * | 3.0 ± 0.8 * | 5.8 ± 1.2 * | 13.8 ± 1.0 ** |
| Acetone (salt-fried AA) | 2.9 ± 1.0 * | 3.8 ± 1.2 * | 9.6 ± 1.3 ** | 5.5 ± 1.2 * | 21.8 ± 1.2 ** |
| Ethyl acetate (salt-fried AA) | 3.2 ± 0.9 * | 9.6 ± 1.3 ** | 4.2 ± 1.6 * | 10.2 ± 1.2 ** | 27.3 ± 1.3 *** |
| Chloroform (salt-fried AA) | 3.4 ± 1.2 * | 12.9 ± 1.4 ** | 23.7 ± 1.7 ** | 6.5 ± 1.2 * | 46.5 ± 1.4 *** |
| Dichloromethane (salt-fried AA) | 2.7 ± 1.6 | 10.3 ± 1.4 ** | 3.6 ± 1.2 * | 6.2 ± 1.6 * | 22.8 ± 1.5 ** |
| n-Hexane (salt-fried AA) | 12.7 ± 1.2 ** | 3.3 ± 1.1 * | 21.3 ± 1.9 ** | 4.6 ± 1.2 * | 41.9 ± 1.4 *** |
| Compounds | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | Superoxide IC50 (μg/mL) | FRAP (mM/g) c (TE) |
|---|---|---|---|---|
| Mangiferin | 5.4 ± 0.3 * | 3.7 ± 0.2 * | 53.8 ± 2.4 * | 9371.4 ± 183.9 *** |
| Timosaponin A-III | >200 | >200 | >200 | 19.5 ± 3.9 * |
| Neomangiferin | >200 | 81.9 ± 4.6 ** | >200 | 83.1 ± 5.3 ** |
| Isomangiferin | 16.7 ± 1.1 * | 5.6 ± 0.4 * | 155.3 ± 13.8 * | 6359.9 ± 176.8 *** |
| BHT a | 27.2 ± 1.9 * | 15.4 ± 0.9 * | N.A. b | 3963.9 ± 104.0 *** |
| Compounds | α-Glucosidase IC50 (μg/mL) |
|---|---|
| Mangiferin | 61.6 ± 5.1 ** |
| Timosaponin A-III | 72.5 ± 1.9 * |
| Neomangiferin | >400 |
| Isomangiferin | 183.2 ± 1.3 * |
| Acarbose a | 322.0 ± 18.7 * |
| Compounds | AchE Inhibitory Assay IC50 (μg/mL) |
|---|---|
| Mangiferin | 67.8 ± 4.2 ** |
| Timosaponin A-III | 132.9 ± 10.9 * |
| Neomangiferin | 70.8 ± 8.5 ** |
| Isomangiferin | 96.0 ± 6.1 * |
| Galanthamine a | 0.5 ± 0.1 ** |
| Compounds | Affinity (kcal/mol) |
|---|---|
| Mangiferin | −10.0 |
| Timosaponin A-III | −8.2 |
| Neomangiferin | −2.0 |
| Isomangiferin | −7.7 |
| Acarbose a | −2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.-C.; Yang, C.-S.; Cheng, M.-J.; Fu, S.-L.; Chen, J.-J. Comparison of Various Solvent Extracts and Major Bioactive Components from Unsalt-Fried and Salt-Fried Rhizomes of Anemarrhena asphodeloides for Antioxidant, Anti-α-Glucosidase, and Anti-Acetylcholinesterase Activities. Antioxidants 2022, 11, 385. https://doi.org/10.3390/antiox11020385
Chu Y-C, Yang C-S, Cheng M-J, Fu S-L, Chen J-J. Comparison of Various Solvent Extracts and Major Bioactive Components from Unsalt-Fried and Salt-Fried Rhizomes of Anemarrhena asphodeloides for Antioxidant, Anti-α-Glucosidase, and Anti-Acetylcholinesterase Activities. Antioxidants. 2022; 11(2):385. https://doi.org/10.3390/antiox11020385
Chicago/Turabian StyleChu, Yi-Cheng, Chang-Syun Yang, Ming-Jen Cheng, Shu-Ling Fu, and Jih-Jung Chen. 2022. "Comparison of Various Solvent Extracts and Major Bioactive Components from Unsalt-Fried and Salt-Fried Rhizomes of Anemarrhena asphodeloides for Antioxidant, Anti-α-Glucosidase, and Anti-Acetylcholinesterase Activities" Antioxidants 11, no. 2: 385. https://doi.org/10.3390/antiox11020385
APA StyleChu, Y. -C., Yang, C. -S., Cheng, M. -J., Fu, S. -L., & Chen, J. -J. (2022). Comparison of Various Solvent Extracts and Major Bioactive Components from Unsalt-Fried and Salt-Fried Rhizomes of Anemarrhena asphodeloides for Antioxidant, Anti-α-Glucosidase, and Anti-Acetylcholinesterase Activities. Antioxidants, 11(2), 385. https://doi.org/10.3390/antiox11020385