The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reference Compound
2.2. Cell Culture
2.3. Experimental Study Design
- (i)
- Untreated HL-1, used as a negative control (CTRL);
- (ii)
- HL-1 treated with 5 μg mL−1 of LPS-G for 24 h;
- (iii)
- HL-1 treated with CAR (6.25 μM) for 24 h;
- (iv)
- HL-1 treated with CAR (50 μM) for 24 h;
- (v)
- HL-1 treated with 5 μg mL−1 of LPS-G for 24 h and CAR (6.25 μM);
- (vi)
- HL-1 treated with 5 μg mL−1 of LPS-G for 24 h and CAR (50 μM).
2.4. Cell Viability Assay
2.5. Confocal Microscopy (CLSM)
2.6. Western Blotting Analysis
2.7. Reactive Oxygen Species (ROS) Evaluation
2.8. Statistical Analysis
3. Results
3.1. Effects of CAR Alone or in Co-Treatment with LPS-G on HL-1 Cells’ Metabolic Activity
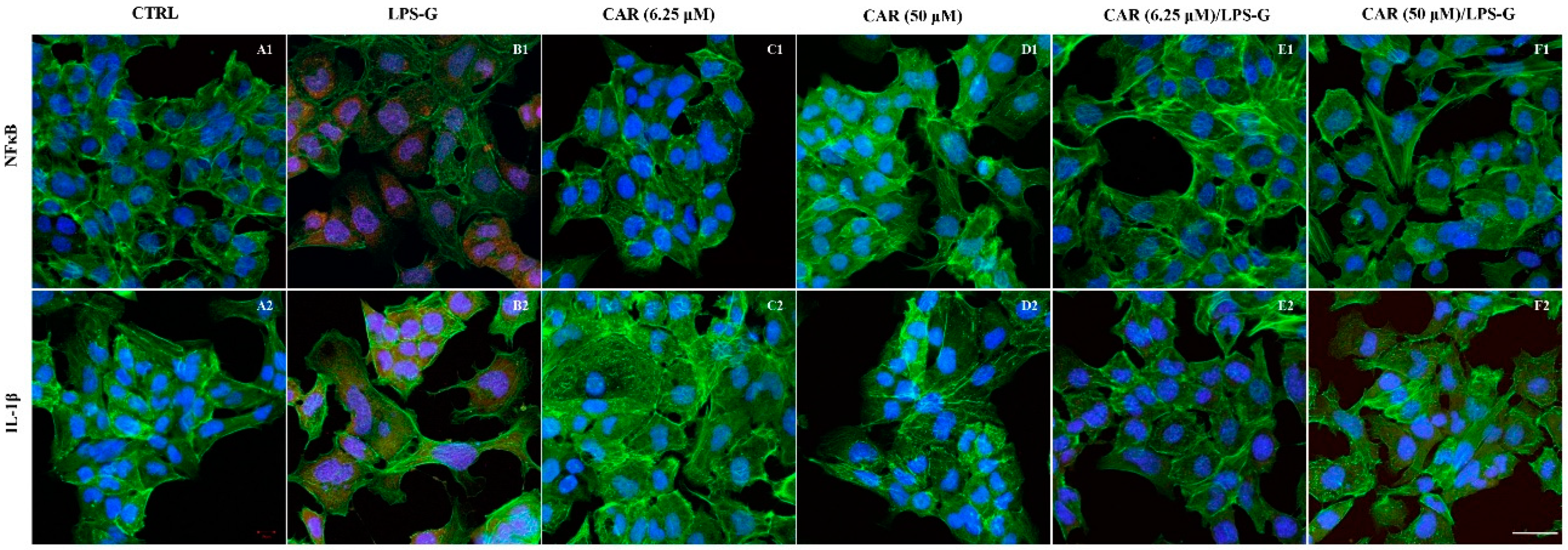
3.2. Expression Levels of TLR4/NFκB/NALP3/IL-1β in CAR, LPS-G, and in Combination CAR/LPS-G-Treated Cells
3.3. CAR Reduces Reactive Oxygen Species Production in LPS-G-Treated HL-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parnas, M.; Peters, M.; Dadon, D.; Lev, S.; Vertkin, I.; Slutsky, I.; Minke, B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 2009, 45, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisto, F.; Carradori, S.; Guglielmi, P.; Traversi, C.B.; Spano, M.; Sobolev, A.P.; Secci, D.; Di Marcantonio, M.C.; Haloci, E.; Grande, R.; et al. Synthesis and Biological Evaluation of Carvacrol-Based Derivatives as Dual Inhibitors of H. pylori Strains and AGS Cell Proliferation. Pharmaceuticals 2020, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Contreras, M.D.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Mouwakeh, A.; Telbisz, A.; Spengler, G.; Mohacsi-Farkas, C.; Kisko, G. Antibacterial and Resistance Modifying Activities of Nigella sativa Essential Oil and its Active Compounds Against Listeria monocytogenes. In Vivo 2018, 32, 737–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskabady, M.H.; Alitaneh, S.; Alavinezhad, A. Carum copticum L.: A Herbal Medicine with Various Pharmacological Effects. BioMed Res. Int. 2014, 2014, 569087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Fratini, F.; Casella, S.; Leonardi, M.; Pisseri, F.; Ebani, V.V.; Pistelli, L.; Pistelli, L. Antibacterial activity of essential oils, their blends and mixtures of their main constituents against some strains supporting livestock mastitis. Fitoterapia 2014, 96, 1–7. [Google Scholar] [CrossRef]
- Baser, K.H.C. Biological and Pharmacological Activities of Carvacrol and Carvacrol Bearing Essential Oils. Curr. Pharm. Design. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Hou, N.; Mai, Y.P.; Qiu, X.X.; Yuan, W.C.; Li, Y.L.; Luo, C.F.; Liu, Y.; Zhang, G.P.; Zhao, G.J.; Luo, J.D. Carvacrol Attenuates Diabetic Cardiomyopathy by Modulating the PI3K/AKT/GLUT4 Pathway in Diabetic Mice. Front. Pharmacol. 2019, 10, 998. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, T.U.; Brasil, G.A.; Endringer, D.C.; da Nobrega, F.R.; de Sousa, D.P. Cardiovascular Activity of the Chemical Constituents of Essential Oils. Molecules 2017, 22, 1539. [Google Scholar] [CrossRef] [Green Version]
- Costa, H.A.; Dias, C.J.M.; Martins, V.D.; Araujo, S.A.; da Silva, D.P.; Mendes, V.S.; Oliveira, M.N.S.; Mostarda, C.T.; Borges, A.C.R.; Ribeiro, R.M.; et al. Effect of treatment with carvacrol and aerobic training on cardiovascular function in spontaneously hypertensive rats. Exp. Physiol. 2021, 106, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.J.; Costa, H.A.; Alves Dias-Filho, C.A.; Ferreira, A.C.; Rodrigues, B.; Irigoyen, M.C.; Romao Borges, A.C.; de Andadre Martins, V.; Branco Vidal, F.C.; Ribeiro, R.M.; et al. Carvacrol reduces blood pressure, arterial responsiveness and increases expression of MAS receptors in spontaneously hypertensive rats. Eur. J. Pharmacol. 2022, 917, 174717. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Hua, C.; Pan, X.Q.; Fu, X.J.; Wu, W. Carvacrol Exerts Neuroprotective Effects Via Suppression of the Inflammatory Response in Middle Cerebral Artery Occlusion Rats. Inflammation 2016, 39, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Canbek, M.; Uyanoglu, M.; Bayramoglu, G.; Senturk, H.; Erkasap, N.; Koken, T.; Uslu, S.; Demirustu, C.; Aral, E.; Baser, K.H.C. Effects of carvacrol on defects of ischemia-reperfusion in the rat liver. Phytomed. Int. J. Phytother. Phytopharm. 2008, 15, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Ba, L.N.; Huang, W.; Liu, Y.; Pan, H.; Ming Yao, E.; Shi, P.L.; Wang, Y.; Li, S.Z.; Qi, H.P.; et al. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur. J. Pharmacol. 2017, 796, 90–100. [Google Scholar] [CrossRef]
- Mezzaroma, E.; Abbate, A.; Toldo, S. NLRP3 Inflammasome Inhibitors in Cardiovascular Diseases. Molecules 2021, 26, 976. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Guarnieri, S.; Cavalcanti, M.F.X.B.; Franchi, S.; Gatta, V.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Ascorbic Acid: A New Player of Epigenetic Regulation in LPS-gingivalis Treated Human Periodontal Ligament Stem Cells. Oxid. Med. Cell. Longev. 2021, 2021, 6679708. [Google Scholar] [CrossRef]
- Chen, T.S.; Kuo, C.H.; Battsengel, S.; Pan, L.F.; Day, C.H.; Shen, C.Y.; Chung, L.C.; Padma, V.V.; Yao, C.K.; Lin, Y.M.; et al. Adipose-derived stem cells decrease cardiomyocyte damage induced by Porphyromonas gingivalis endotoxin through suppressing hypertrophy, apoptosis, fibrosis, and MAPK markers. Environ. Toxicol. 2018, 33, 508–513. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Marconi, G.D.; De Colli, M.; Zara, S.; Zavan, B.; Salini, V.; Fontana, A.; Cataldi, A.; Piattelli, A. In Vitro Behavior of Primary Human Osteoblasts Onto Microrough Titanium Surface. Implant. Dent. 2015, 24, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Trubiani, O.; Toniato, E.; Di Iorio, D.; Diomede, F.; Merciaro, I.; D’Arcangelo, C.; Caputi, S. Morphological analysis and interleukin release in human gingival fibroblasts seeded on different denture base acrylic resins. Int. J. Immunopathol. Pharmacol. 2012, 25, 637–643. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated with 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Diomede, F.; Pizzicannella, J.; Fonticoli, L.; Merciaro, I.; Pierdomenico, S.D.; Mazzon, E.; Piattelli, A.; Trubiani, O. Enhanced VEGF/VEGF-R and RUNX2 Expression in Human Periodontal Ligament Stem Cells Cultured on Sandblasted/Etched Titanium Disk. Front. Cell Dev. Biol. 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Gallorini, M.; Carradori, S.; Guglielmi, P.; Cataldi, A.; Zara, S. The Up-Regulation of Oxidative Stress as a Potential Mechanism of Novel MAO-B Inhibitors for Glioblastoma Treatment. Molecules 2019, 24, 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diomede, F.; Marconi, G.D.; Guarnieri, S.; D’Attilio, M.; Cavalcanti, M.; Mariggio, M.A.; Pizzicannella, J.; Trubiani, O. A Novel Role of Ascorbic Acid in Anti-Inflammatory Pathway and ROS Generation in HEMA Treated Dental Pulp Stem Cells. Materials 2019, 13, 130. [Google Scholar] [CrossRef] [Green Version]
- Saljoughian, S.; Roohinejad, S.; Bekhit, A.E.A.; Greiner, R.; Omidizadeh, A.; Nikmaram, N.; Khaneghah, A.M. The effects of food essential oils on cardiovascular diseases: A review. Crit. Rev. Food Sci. 2018, 58, 1688–1705. [Google Scholar] [CrossRef]
- Chen, T.S.; Battsengel, S.; Kuo, C.H.; Pan, L.F.; Lin, Y.M.; Yao, C.H.; Chen, Y.S.; Lin, F.H.; Kuo, W.W.; Huang, C.Y. Stem cells rescue cardiomyopathy induced by P-gingivalis-LPS via miR-181b. J. Cell Physiol. 2018, 233, 5869–5876. [Google Scholar] [CrossRef]
- Mei, F.; Xie, M.R.; Huang, X.F.; Long, Y.L.; Lu, X.F.; Wang, X.L.; Chen, L.L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.H.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Diomede, F.; Thangavelu, S.R.; Merciaro, I.; D’Orazio, M.; Bramanti, P.; Mazzon, E.; Trubiani, O. Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells: Role of epigenetic modifications to the inflammation. Eur. J. Histochem. 2017, 61, 2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soundara Rajan, T.; Giacoppo, S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int. J. Immunopathol. Pharmacol. 2017, 30, 238–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wise, L.; Fukuchi, K.I. TLR4 Cross-Talk With NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Muhlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magyar, J.; Szentandrassy, N.; Banyasz, T.; Fulop, L.; Varro, A.; Nanasi, P.P. Effects of terpenoid phenol derivatives on calcium current in canine and human ventricular cardiomyocytes. Eur. J. Pharmacol. 2004, 487, 29–36. [Google Scholar] [CrossRef]
- Magyar, J.; Szentandrassy, N.; Banyasz, T.; Fulop, L.; Varro, A.; Nanasi, P.P. Effects of thymol on calcium and potassium currents in canine and human ventricular cardiomyocytes. Br. J. Pharmacol. 2002, 136, 330–338. [Google Scholar] [CrossRef]
- Lahmar, A.; Akcan, T.; Chekir-Ghedira, L.; Estevez, M. Molecular interactions and redox effects of carvacrol and thymol on myofibrillar proteins using a non-destructive and solvent-free methodological approach. Food Res. Int. 2018, 106, 1042–1048. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconi, G.D.; Della Rocca, Y.; Fonticoli, L.; Guarnieri, S.; Carradori, S.; Rajan, T.S.; Pizzicannella, J.; Diomede, F. The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations. Antioxidants 2022, 11, 386. https://doi.org/10.3390/antiox11020386
Marconi GD, Della Rocca Y, Fonticoli L, Guarnieri S, Carradori S, Rajan TS, Pizzicannella J, Diomede F. The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations. Antioxidants. 2022; 11(2):386. https://doi.org/10.3390/antiox11020386
Chicago/Turabian StyleMarconi, Guya Diletta, Ylenia Della Rocca, Luigia Fonticoli, Simone Guarnieri, Simone Carradori, Thangavelu Soundara Rajan, Jacopo Pizzicannella, and Francesca Diomede. 2022. "The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations" Antioxidants 11, no. 2: 386. https://doi.org/10.3390/antiox11020386
APA StyleMarconi, G. D., Della Rocca, Y., Fonticoli, L., Guarnieri, S., Carradori, S., Rajan, T. S., Pizzicannella, J., & Diomede, F. (2022). The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations. Antioxidants, 11(2), 386. https://doi.org/10.3390/antiox11020386








