The Antinociceptive Potential of Camellia japonica Leaf Extract, (−)-Epicatechin, and Rutin against Chronic Constriction Injury-Induced Neuropathic Pain in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Experimental Animals
2.3. Chronic Constriction Injury (CCI) Surgery
2.4. Experimental Design
2.5. Measurement of Pain-Associated Behavior
2.5.1. Punctate Allodynia
Withdrawal Frequency Elicited by 2 and 10 g von Frey Filaments
Mechanical Withdrawal Threshold (MWT)
2.5.2. Dynamic Allodynia
Brush-Evoked Dynamic Allodynia
Cotton Swab Assay
2.5.3. Cold Allodynia
2.5.4. Spontaneous Pain-Related Behaviors
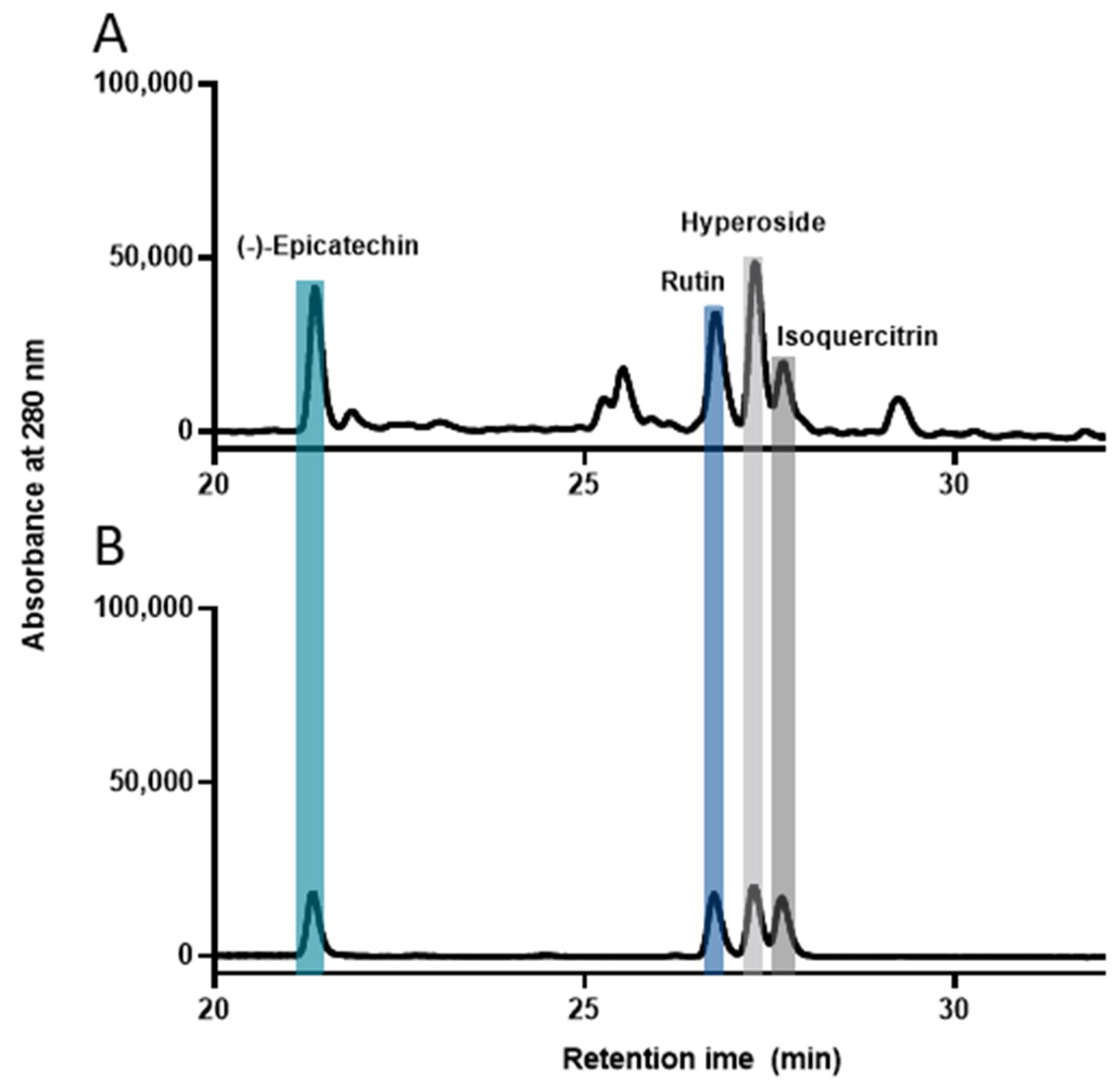
2.6. High-Performance Liquid Chromatography (HPLC) Analysis
2.7. Western Blotting
2.8. Total RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assays
2.9. Immunofluorescence
2.10. Statistics
3. Results
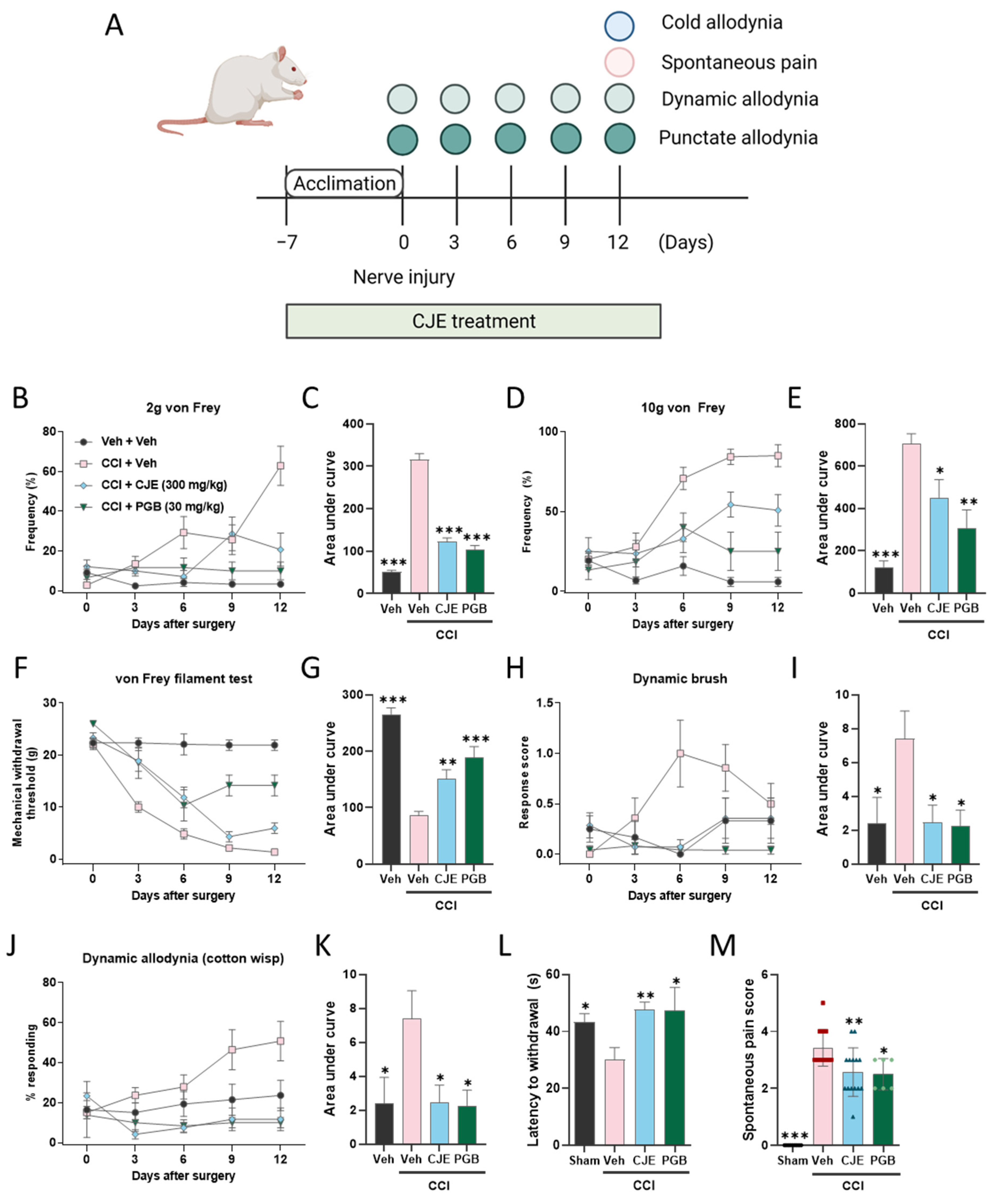
3.1. C. japonica Leaf Extract Attenuates Chronic Constriction Injury-Induced Pain Sensitivity
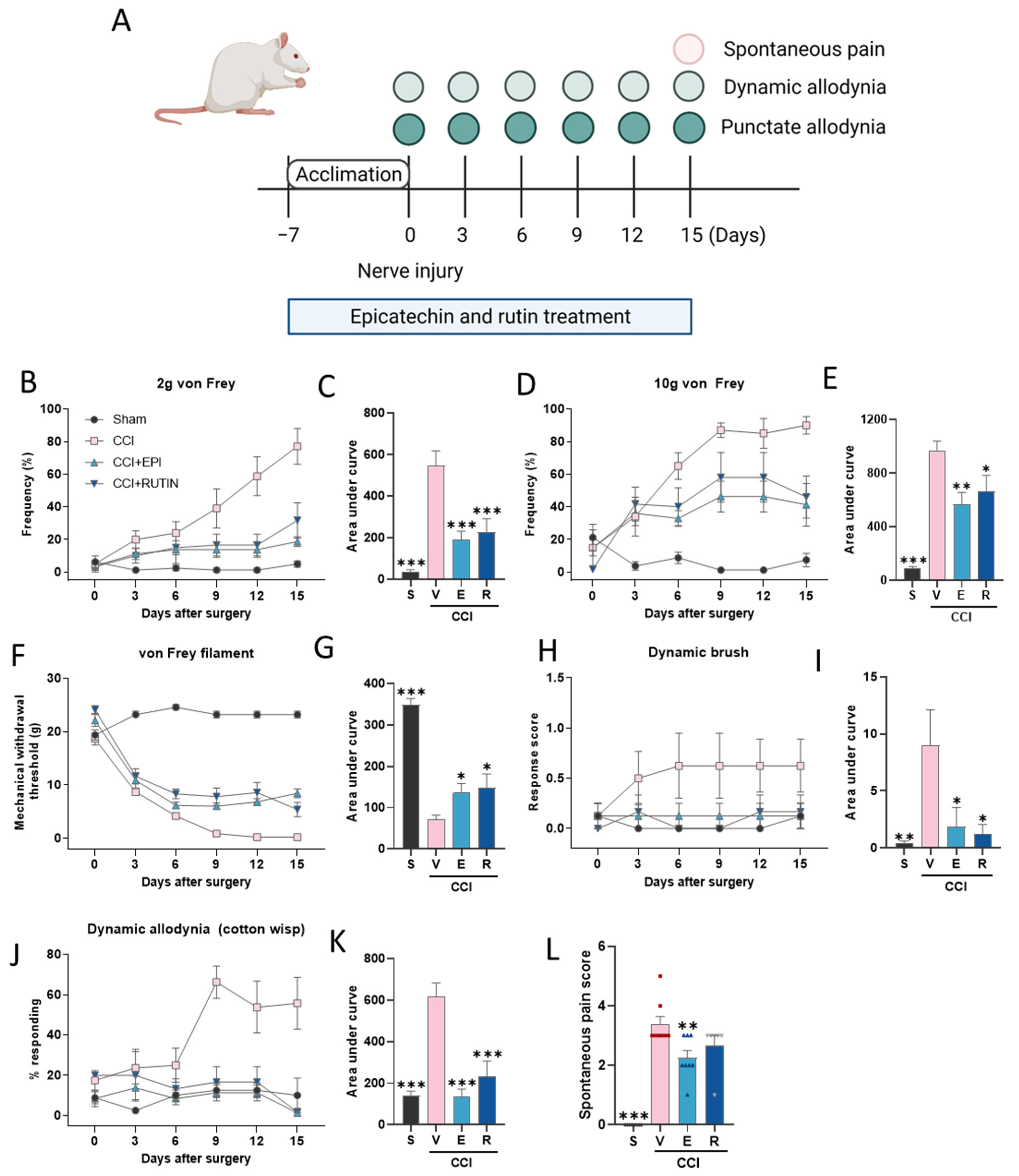
3.2. (−)-Epicatechin and Rutin Attenuates Chronic Constriction Injury-Induced Pain Sensitivity
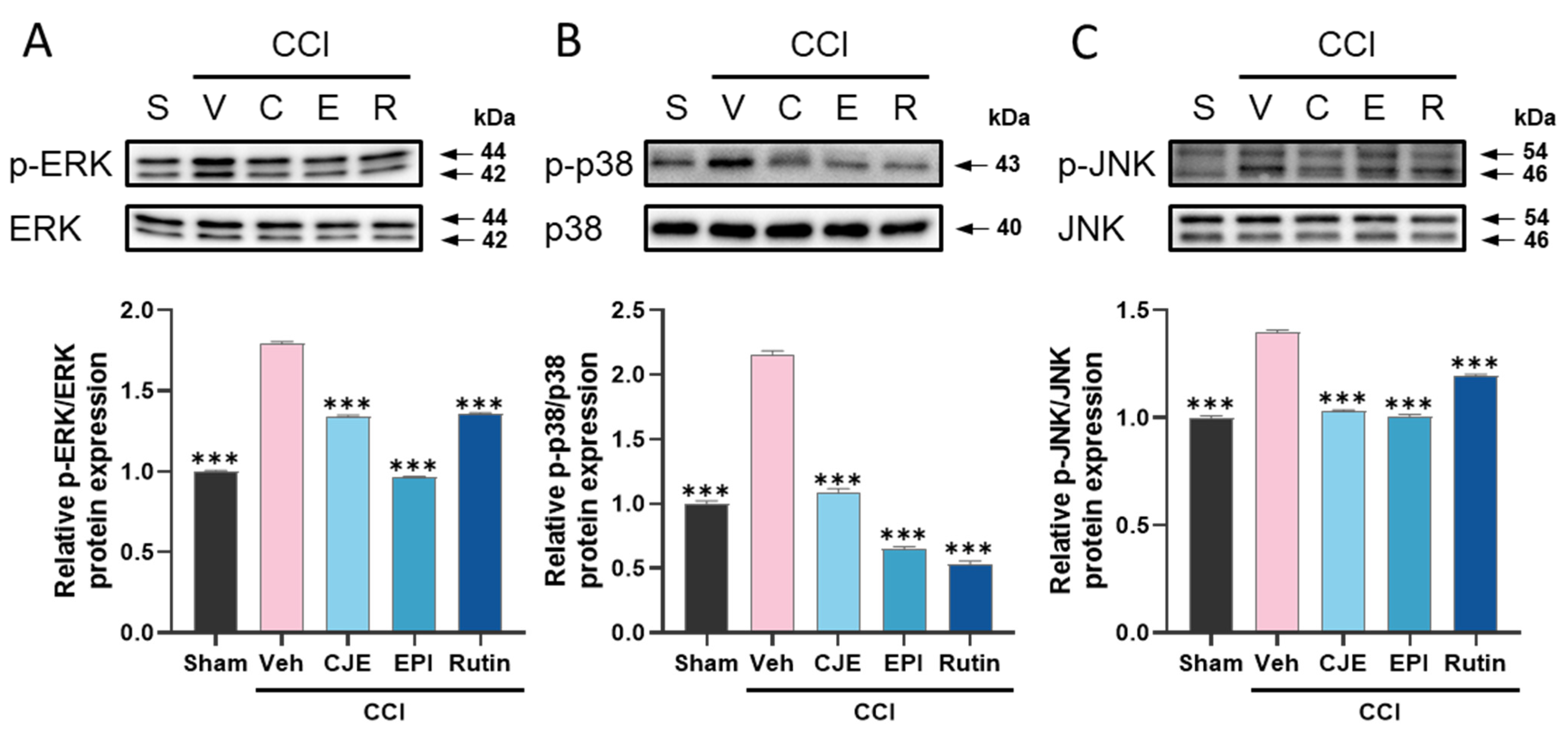
3.3. C. japonica Leaf Extract, (−)-Epicatechin, and Rutin Inhibit the Chronic Constriction Injury-Induced Upregulation of MAPKs Phosphorylation in the Dorsal Root Ganglion
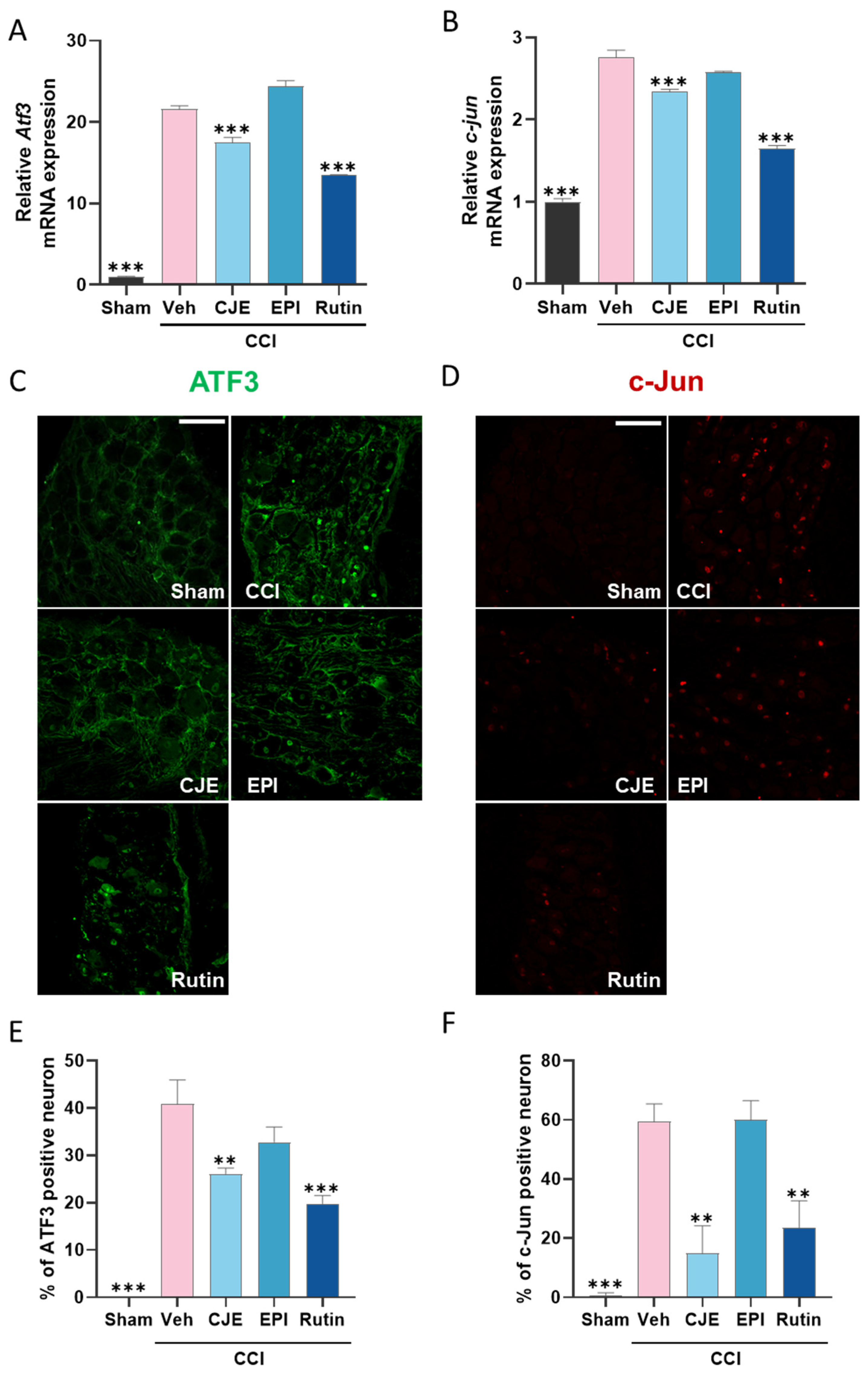
3.4. C. japonica Leaf Extract and Rutin Inhibit the Chronic Constriction Injury-Induced Increase in the ATF3 and c-Jun Expression in the Dorsal Root Ganglion 3 Days after Chronic Constriction Injury in Rats
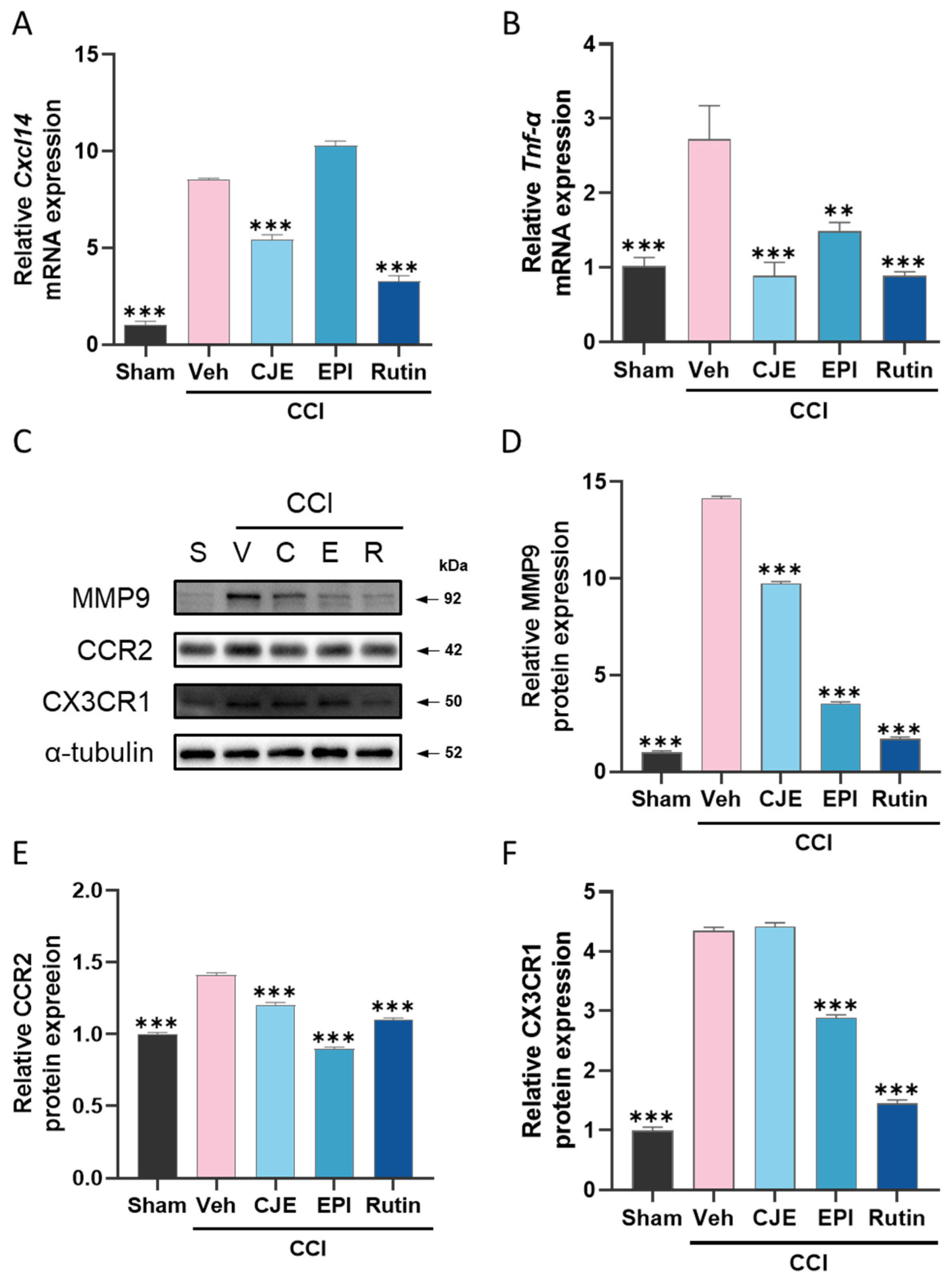
3.5. C. japonica Leaf Extract, (−)-epicatechin, and Rutin Inhibit the Chronic Constriction Injury-Induced Increase in Inflammatory Mediators in the Dorsal Root Ganglion 3 Days after Chronic Constriction Injury in Rats
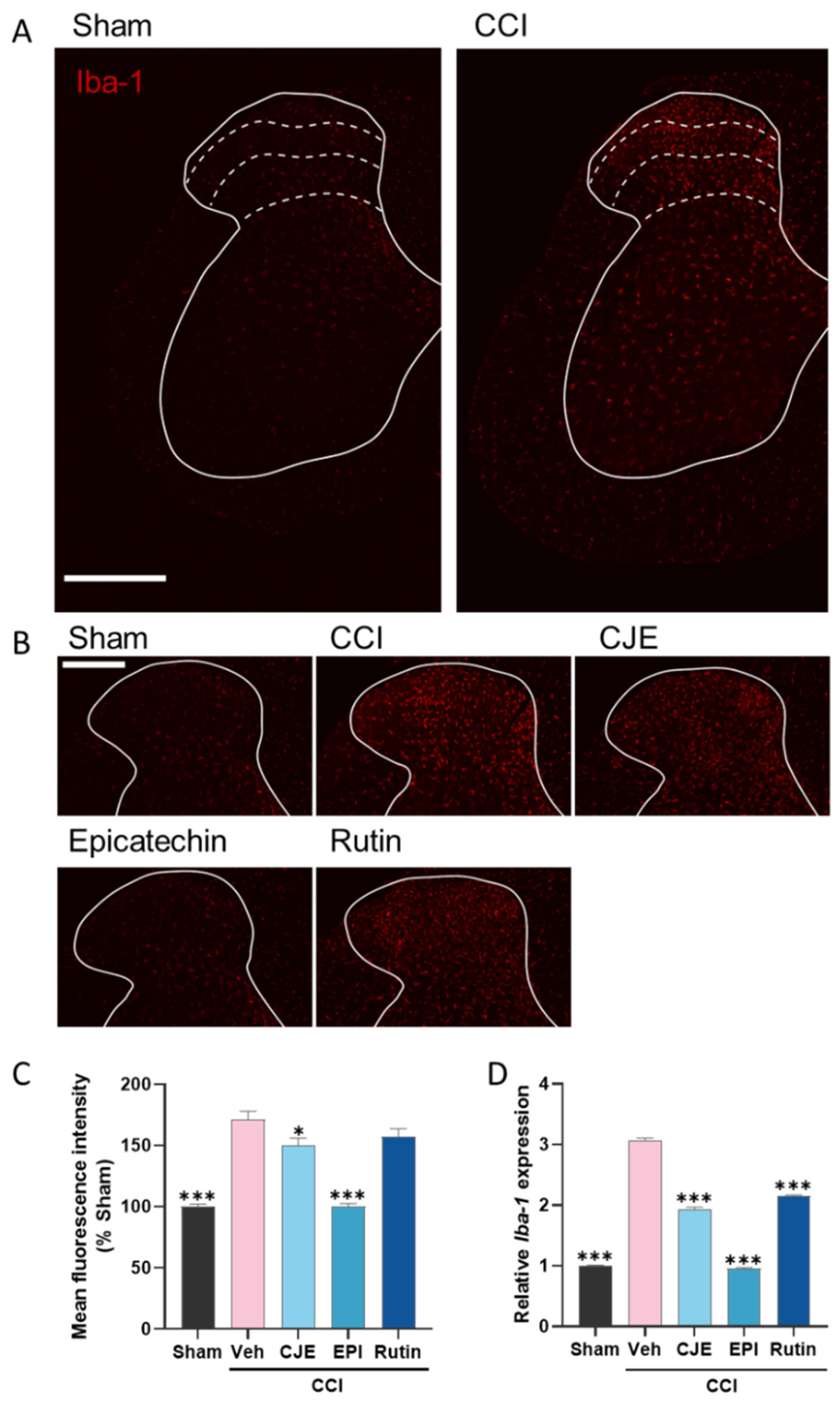
3.6. C. japonica Leaf Extract, (−)-Epicatehin, and Rutin Inhibit Microglial Activation in the Spinal Dorsal Horn
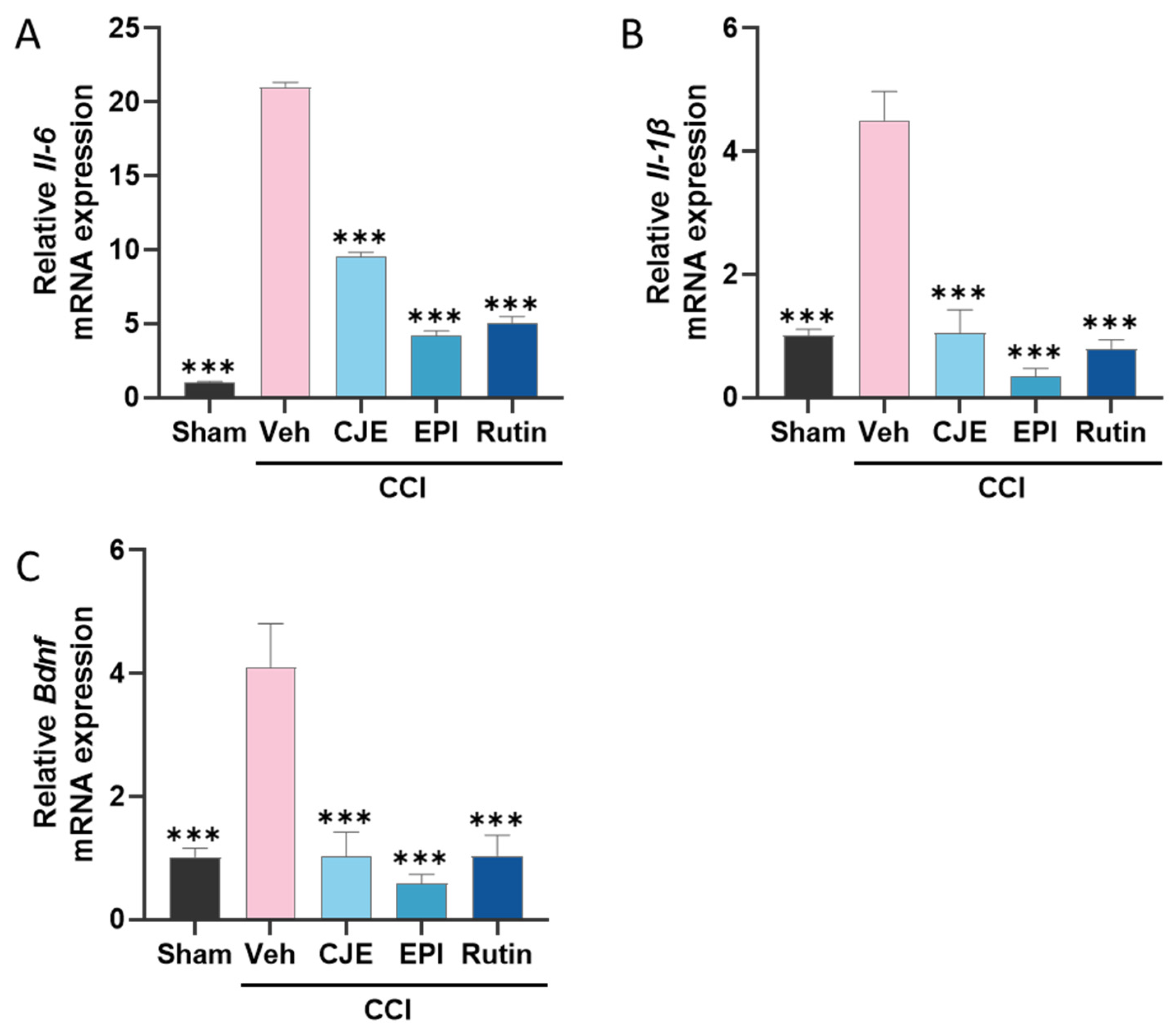
3.7. C. japonica Leaf Extract, (−)-Epicatehin, and Rutin Inhibits Inflammatory Mediator Expression in the Spinal Cord
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, T.S.; Baron, R.; Haanpaa, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Gilron, I.; Baron, R.; Jensen, T. Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin. Proc. 2015, 90, 532–545. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, C.; Sadosky, A.; Mann, R.; Daniel, S.; Parsons, B.; Tuchman, M.; Anschel, A.; Stacey, B.R.; Nalamachu, S.; Nieshoff, E. Pain severity and the economic burden of neuropathic pain in the United States: BEAT neuropathic pain observational study. Clin. Outcomes Res. 2014, 6, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Barohn, R.J.; Gajewski, B.; Pasnoor, M.; Brown, A.; Herbelin, L.L.; Kimminau, K.S.; Mudaranthakam, D.P.; Jawdat, O.; Dimachkie, M.M.; The Patient Assisted Intervention for Neuropathy: Comparison of Treatment in Real Life Situations (PAIN-CONTRoLS) Study Team. Patient assisted intervention for neuropathy: Comparison of treatment in real life situations (PAIN-CONTRoLS): Bayesian adaptive comparative effectiveness randomized trial. JAMA Neurol. 2021, 78, 68–76. [Google Scholar] [CrossRef]
- Krames, E.S. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014, 15, 1669–1685. [Google Scholar] [CrossRef]
- Lessans, S.; Lassiter, C.B.; Carozzi, V.; Heindel, P.; Semperboni, S.; Oggioni, N.; Chiorazzi, A.; Thompson, C.; Wagner, M.; Holden, J.; et al. Global transcriptomic profile of dorsal root ganglion and physiological correlates of cisplatin-induced peripheral neuropathy. Nurs. Res. 2019, 68, 145–155. [Google Scholar] [CrossRef]
- Martin, S.L.; Reid, A.J.; Verkhratsky, A.; Magnaghi, V.; Faroni, A. Gene expression changes in dorsal root ganglia following peripheral nerve injury: Roles in inflammation, cell death and nociception. Neural Regen. Res. 2019, 14, 939–947. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Inoue, K.; Salter, M.W. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 2005, 28, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jeon, S.; Suk, K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw. 2012, 12, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hald, A.; Nedergaard, S.; Hansen, R.R.; Ding, M.; Heegaard, A.M. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur. J. Pain 2009, 13, 138–145. [Google Scholar] [CrossRef]
- Rojewska, E.; Korostynski, M.; Przewlocki, R.; Przewlocka, B.; Mika, J. Expression profiling of genes modulated by minocycline in a rat model of neuropathic pain. Mol. Pain 2014, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Syngle, A.; Verma, I.; Krishan, P.; Garg, N.; Syngle, V. Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol. Sci. 2014, 35, 1067–1073. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Dowdall, T.; Robinson, I.; Meert, T.F. Comparison of five different rat models of peripheral nerve injury. Pharm. Biochem. Behav. 2005, 80, 93–108. [Google Scholar] [CrossRef]
- Du, H.; Shi, J.; Wang, M.; An, S.; Guo, X.; Wang, Z. Analyses of gene expression profiles in the rat dorsal horn of the spinal cord using RNA sequencing in chronic constriction injury rats. J. Neuroinflammation 2018, 15, 280. [Google Scholar] [CrossRef]
- Stephens, K.E.; Zhou, W.; Ji, Z.; Chen, Z.; He, S.; Ji, H.; Guan, Y.; Taverna, S.D. Sex differences in gene regulation in the dorsal root ganglion after nerve injury. BMC Genom. 2019, 20, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, C.-H.; Kim, J.H.; Choi, G.N.; Kwak, J.H.; Kim, D.-O.; Heo, H.J. Protective effects of extract with phenolics from camellia (Camellia japonica) leaf against oxidative stress-induced neurotoxicity. Food Sci. Biotechnol. 2010, 19, 1347–1353. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, J.H.; Cui, L.; Li, Y.; Yang, J.M.; Yun, J.J.; Jung, J.E.; Choi, W.; Yoon, K.C. Anti-Inflammatory and Antioxidative Effects of Camellia japonica on Human Corneal Epithelial Cells and Experimental Dry Eye: In Vivo and In Vitro Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1196–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Shim, J.H.; Choi, C.Y.; An, K.W.; Kim, E.I.; et al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Ghosh, A.; Bhattacharya, M. Natural anti-inflammatory terpenoids in Camellia japonica leaf and probable biosynthesis pathways of the metabolome. Bull. Natl. Res. Cent. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Azuma, C.M.; Santos, F.C.S.d.; Lago, J.H.G. Flavonoids and fatty acids of Camellia japonica leaves extract. Rev. Bras. Farmacogn. 2011, 21, 1159–1162. [Google Scholar] [CrossRef] [Green Version]
- Van der Wal, S.; Cornelissen, L.; Behet, M.; Vaneker, M.; Steegers, M.; Vissers, K. Behavior of neuropathic pain in mice following chronic constriction injury comparing silk and catgut ligatures. SpringerPlus 2015, 4, 225. [Google Scholar] [CrossRef] [Green Version]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Cheng, L.; Duan, B.; Huang, T.; Zhang, Y.; Chen, Y.; Britz, O.; Garcia-Campmany, L.; Ren, X.; Vong, L.; Lowell, B.B.; et al. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat. Neurosci. 2017, 20, 804–814. [Google Scholar] [CrossRef] [Green Version]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kuhnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 2018, 10, eaat9897. [Google Scholar] [CrossRef] [Green Version]
- Garrison, S.R.; Dietrich, A.; Stucky, C.L. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J. Neurophysiol. 2012, 107, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, J.T.; LoCoco, P.M.; Furr, A.R.; Bendele, M.R.; Tram, M.; Li, Q.; Chang, F.-M.; Colley, M.E.; Samenuk, G.M.; Arris, D.A. Elevated dietary ω-6 polyunsaturated fatty acids induce reversible peripheral nerve dysfunction that exacerbates comorbid pain conditions. Nat. Metab. 2021, 3, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, Z.; Zhang, H.; Atianjoh, F.E.; Zhao, J.Y.; Liang, L.; Wang, W.; Guan, X.; Kao, S.C.; Tiwari, V.; et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 2013, 16, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Jazat, F.; Kayser, V.; Guilbaud, G. Further evidence for ‘pain-related’ behaviours in a model of unilateral peripheral mononeuropathy. Pain 1990, 41, 235–251. [Google Scholar] [CrossRef]
- Miyakawa, T.; Terashima, Y.; Takebayashi, T.; Tanimoto, K.; Iwase, T.; Ogon, I.; Kobayashi, T.; Tohse, N.; Yamashita, T. Transient receptor potential ankyrin 1 in spinal cord dorsal horn is involved in neuropathic pain in nerve root constriction rats. Mol. Pain 2014, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, Y.T. Erythronium japonicum alleviates inflammatory pain by inhibiting MAPK activation and by suppressing NF-kappaB activation via ERK/Nrf2/HO-1 signaling pathway. Antioxidants 2020, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Richner, M.; Jager, S.B.; Siupka, P.; Vaegter, C.B. Hydraulic extrusion of the spinal cord and isolation of dorsal root ganglia in rodents. J. Vis. Exp. 2017, 119, e55226. [Google Scholar] [CrossRef] [Green Version]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural antioxidant control of neuropathic pain-exploring the role of mitochondrial SIRT3 pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Jug, U.; Naumoska, K.; Vovk, I. (−)-Epicatechin—An important contributor to the antioxidant activity of Japanese knotweed rhizome bark extract as determined by antioxidant activity-guided fractionation. Antioxidants 2021, 10, 133. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. Int. J. Mol. Med. 2013, 32, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes Lda, S.; Marques, R.B.; Fernandes, H.B.; Pereira Sda, S.; Ayres, M.C.; Chaves, M.H.; Almeida, F.R. Mechanisms of the antinociceptive action of (−) epicatechin obtained from the hydroalcoholic fraction of Combretum leprosum Mart & Eic in rodents. J. Biomed. Sci. 2012, 19, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinonez-Bastidas, G.N.; Pineda-Farias, J.B.; Flores-Murrieta, F.J.; Rodriguez-Silverio, J.; Reyes-Garcia, J.G.; Godinez-Chaparro, B.; Granados-Soto, V.; Rocha-Gonzalez, H.I. Antinociceptive effect of (−)-epicatechin in inflammatory and neuropathic pain in rats. Behav. Pharmacol. 2018, 29, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Quinonez-Bastidas, G.N.; Cervantes-Duran, C.; Rocha-Gonzalez, H.I.; Murbartian, J.; Granados-Soto, V. Analysis of the mechanisms underlying the antinociceptive effect of epicatechin in diabetic rats. Life Sci. 2013, 93, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Al-Enazi, M.M. Ameliorative potential of rutin on streptozotocin-induced neuropathic pain in rat. Afr. J. Pharm. Pharmacol. 2013, 7, 2743–2754. [Google Scholar] [CrossRef]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.; Wong, D.V.T.; Lima-Júnior, R.C.; de Albuquerque Ribeiro, R.; Vale, M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 2013, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Kim, S.S. Therapeutic strategies for neuropathic pain: Potential application of pharmacosynthetics and optogenetics. Mediat. Inflamm. 2016, 2016, 5808215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Qu, Y.J.; Jia, L.; Zhang, X.; Wei, H.; Yue, S.W. MAPK pathways are involved in neuropathic pain in rats with chronic compression of the dorsal root ganglion. Evid.-Based Complement. Altern. Med. 2016, 2016, 6153215. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, L.; Xu, Z.; Cao, N.; Tang, X.; Gao, R.; Zhang, J.; Deng, S.; Tan, C.; Zhang, M.; et al. The Role of TMEM16A/ERK/NK-1 signaling in dorsal root ganglia neurons in the development of neuropathic pain induced by Spared Nerve Injury (SNI). Mol. Neurobiol. 2021, 58, 5772–5789. [Google Scholar] [CrossRef]
- Dai, W.L.; Yan, B.; Bao, Y.N.; Fan, J.F.; Liu, J.H. Suppression of peripheral NGF attenuates neuropathic pain induced by chronic constriction injury through the TAK1-MAPK/NF-kappaB signaling pathways. Cell Commun. Signal. 2020, 18, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokhilko, A.; Nash, A.; Cader, M.Z. Common transcriptional signatures of neuropathic pain. Pain 2020, 161, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Sisignano, M.; Baron, R.; Scholich, K.; Geisslinger, G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 2014, 10, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Rivat, C.; Sar, C.; Mechaly, I.; Leyris, J.P.; Diouloufet, L.; Sonrier, C.; Philipson, Y.; Lucas, O.; Mallie, S.; Jouvenel, A.; et al. Inhibition of neuronal FLT3 receptor tyrosine kinase alleviates peripheral neuropathic pain in mice. Nat. Commun. 2018, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Kan, H.W.; Chang, C.H.; Chang, Y.S.; Ko, Y.T.; Hsieh, Y.L. Genetic loss-of-function of activating transcription factor 3 but not C-type lectin member 5A prevents diabetic peripheral neuropathy. Lab. Investig. 2021, 101, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Lee, K.M.; Jeon, S.M.; Park, E.S.; Park, K.M.; Cho, H.J. Activation of transcription factor c-jun in dorsal root ganglia induces VIP and NPY upregulation and contributes to the pathogenesis of neuropathic pain. Exp. Neurol. 2007, 204, 467–472. [Google Scholar] [CrossRef]
- Wlaschin, J.J.; Gluski, J.M.; Nguyen, E.; Silberberg, H.; Thompson, J.H.; Chesler, A.T.; Le Pichon, C.E. Dual leucine zipper kinase is required for mechanical allodynia and microgliosis after nerve injury. Elife 2018, 7, e33910. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Sacerdote, P.; Franchi, S.; Trovato, A.E.; Valsecchi, A.E.; Panerai, A.E.; Colleoni, M. Transient early expression of TNF-alpha in sciatic nerve and dorsal root ganglia in a mouse model of painful peripheral neuropathy. Neurosci. Lett. 2008, 436, 210–213. [Google Scholar] [CrossRef]
- Ogawa, N.; Kawai, H.; Terashima, T.; Kojima, H.; Oka, K.; Chan, L.; Maegawa, H. Gene therapy for neuropathic pain by silencing of TNF-alpha expression with lentiviral vectors targeting the dorsal root ganglion in mice. PLoS ONE 2014, 9, e92073. [Google Scholar] [CrossRef] [Green Version]
- Andrade, P.; Hoogland, G.; Del Rosario, J.S.; Steinbusch, H.W.; Visser-Vandewalle, V.; Daemen, M.A. Tumor necrosis factor-alpha inhibitors alleviation of experimentally induced neuropathic pain is associated with modulation of TNF receptor expression. J. Neurosci. Res. 2014, 92, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Strong, J.A.; Xie, W.; Coyle, D.E.; Zhang, J.M. Microarray analysis of rat sensory ganglia after local inflammation implicates novel cytokines in pain. PLoS ONE 2012, 7, e40779. [Google Scholar] [CrossRef] [Green Version]
- Poisbeau, P.; Aouad, M.; Gazzo, G.; Lacaud, A.; Kemmel, V.; Landel, V.; Lelievre, V.; Feron, F. Cholecalciferol (Vitamin D3) reduces rat neuropathic pain by modulating opioid signaling. Mol. Neurobiol. 2019, 56, 7208–7221. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Illias, A.M.; Gist, A.C.; Zhang, H.; Kosturakis, A.K.; Dougherty, P.M. Chemokine CCL2 and its receptor CCR2 in the dorsal root ganglion contribute to oxaliplatin-induced mechanical hypersensitivity. Pain 2018, 159, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Jones, M.; Tanaka, M.; Selvaraj, P.; Symes, A.J.; Cox, B.; Zhang, Y. WWL70 protects against chronic constriction injury-induced neuropathic pain in mice by cannabinoid receptor-independent mechanisms. J. Neuroinflammation 2018, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, H.; Hamel, K.A.; Morvan, M.G.; Yu, S.; Leff, J.; Guan, Z.; Braz, J.M.; Basbaum, A.I. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat. Commun. 2020, 11, 264. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Song, C.; Huang, Y.; Lei, W.; Sun, J. MMP-9 regulates CX3CL1/CX3CR1 in the early phase of neuropathic pain in chronic sciatic nerve constriction injury (CCI) rats. Ann. Palliat. Med. 2020, 9, 2020–2027. [Google Scholar] [CrossRef]
- Amin, B.; Noorani, R.; Marjan Razavi, B.; Hosseinzadeh, H. The effect of ethanolic extract of lippia citriodora on rats with chronic constriction injury of neuropathic pain. Cell J. 2018, 19, 528–536. [Google Scholar] [CrossRef]
- Yao, Y.; Tan, Y.H.; Light, A.R.; Mao, J.; Yu, A.C.; Fu, K.Y. Alendronate attenuates spinal microglial activation and neuropathic pain. J. Pain 2016, 17, 889–903. [Google Scholar] [CrossRef]
- Reeve, A.J.; Patel, S.; Fox, A.; Walker, K.; Urban, L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur. J. Pain 2000, 4, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Gabay, E.; Tal, M.; Yirmiya, R.; Shavit, Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain 2006, 120, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Sugawara, T.; Fujishita, K.; Shinozaki, Y.; Matsukawa, T.; Suzuki, T.; Koizumi, S. The astrocyte-targeted therapy by Bushi for the neuropathic pain in mice. PLoS ONE 2011, 6, e23510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| Atf3 | NM_012912 XM_001068451 | CGCCATCCAGAACAAGCA | TGAGCCCGGACGATACACG |
| c-jun | NM_021835 | TGCAAAGATGGAAACGACCT | CCACTCTCGGACTGGAGGAA |
| Cxcl14 | NM_001013137 XM_225162 | GAGCACTGCCTGCATCCTAA | AAGGCTTTCGCACACAGCTA |
| Tnf-α | NM_012675 | ACTGAACTTCGGGGTGATTG | GCTTGGTGGTTTGCTACGAC |
| Aif1 | NM_017196 XM_346597 | CAACAAGCACTTCCTCGATGATC | TGAAGGCCTCCAGTTTGGACT |
| Il-6 | NM_012589 | TCTCTCCGCAAGAGACTTCC | TCTTGGTCCTTAGCCACTCC |
| Il-1β | NM_031512 | AAAGAAGAAGATGGAAAAGCGGTT | GGGAACTGTGCAGACTCAAACTC |
| Bdnf | NM_001270638 | CTACGAGACCAAGTGCAATCC | AATCGCCAGCCAATTCTCTTT |
| Gapdh | NM_017008 XM_216453 | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, E.Y.; Lee, C.; Kim, Y.T. The Antinociceptive Potential of Camellia japonica Leaf Extract, (−)-Epicatechin, and Rutin against Chronic Constriction Injury-Induced Neuropathic Pain in Rats. Antioxidants 2022, 11, 410. https://doi.org/10.3390/antiox11020410
Lim EY, Lee C, Kim YT. The Antinociceptive Potential of Camellia japonica Leaf Extract, (−)-Epicatechin, and Rutin against Chronic Constriction Injury-Induced Neuropathic Pain in Rats. Antioxidants. 2022; 11(2):410. https://doi.org/10.3390/antiox11020410
Chicago/Turabian StyleLim, Eun Yeong, Changho Lee, and Yun Tai Kim. 2022. "The Antinociceptive Potential of Camellia japonica Leaf Extract, (−)-Epicatechin, and Rutin against Chronic Constriction Injury-Induced Neuropathic Pain in Rats" Antioxidants 11, no. 2: 410. https://doi.org/10.3390/antiox11020410
APA StyleLim, E. Y., Lee, C., & Kim, Y. T. (2022). The Antinociceptive Potential of Camellia japonica Leaf Extract, (−)-Epicatechin, and Rutin against Chronic Constriction Injury-Induced Neuropathic Pain in Rats. Antioxidants, 11(2), 410. https://doi.org/10.3390/antiox11020410







