Bioactive Compounds and Antioxidant Activities in Different Fractions of Mango Fruits (Mangifera indica L., Cultivar Tommy Atkins and Keitt)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material Sampling and Preparation
2.3. Determination of Mango Fruit Flesh Firmness and Total Soluble Solids
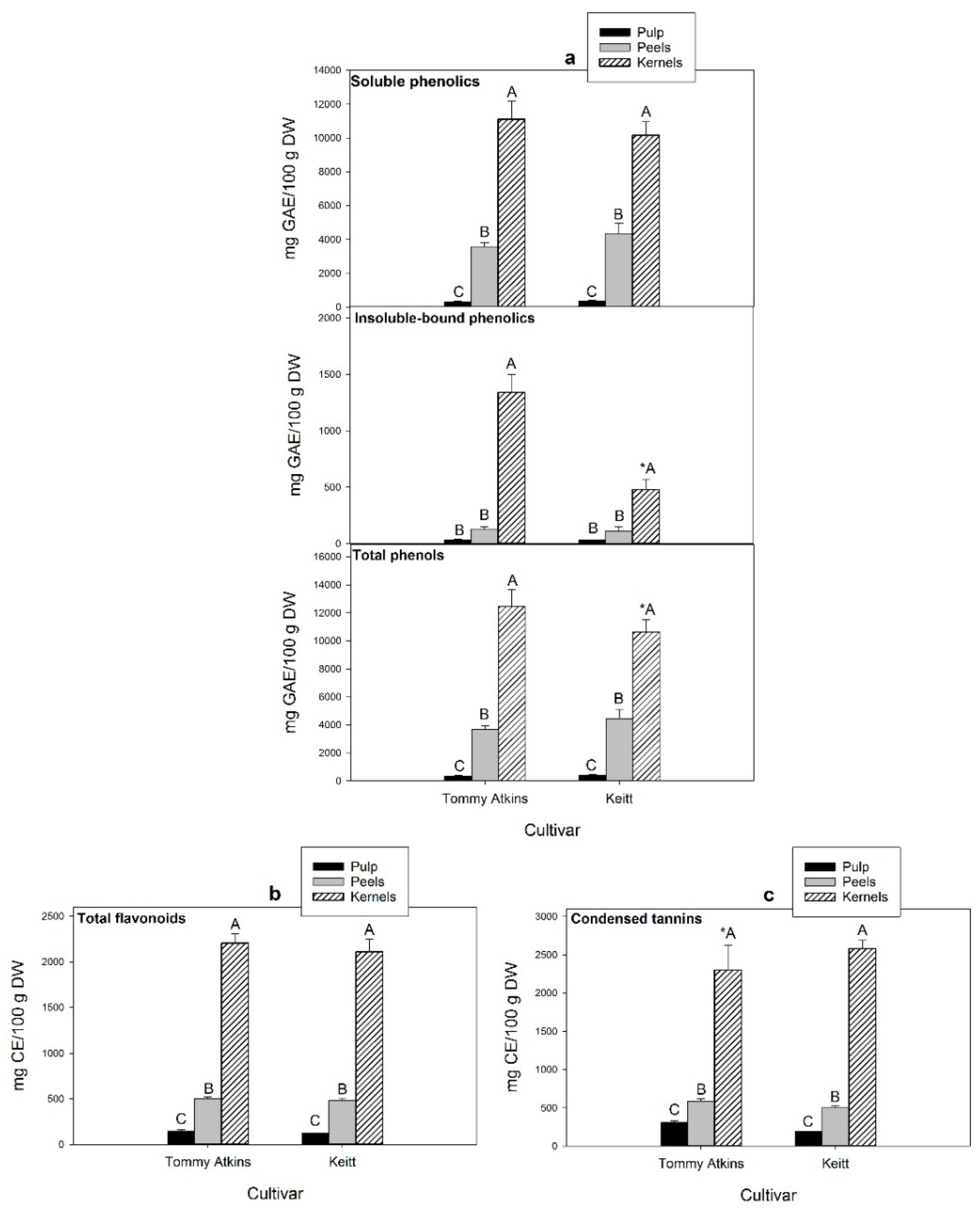
2.4. Extraction and Determination of Soluble and Insoluble-Bound Phenolic Compounds
2.5. Extraction and Determination of Flavonoids and Condensed Tannins
2.6. Extraction and Determination of Ascorbic Acid (AsA) and Dehydroascorbic Acid (DHA) Contents
2.7. Phytosterols and Pentacyclic Triterpenes Determination
2.8. Isoprenoids (Tocopherols, Carotenoids and Chlorophylls) Determination
2.9. Assay of Hydrophilic and Lipophilic Antioxidant Activities
2.10. Fatty Acids Determination
2.11. Statistical Analysis
3. Results and Discussion
3.1. Polyphenolic Composition
3.2. Ascorbic (AsA) and Dehydroascorbic (DHA) Acid Contents
3.3. Lipophilic Bioactives
3.4. Antioxidant Properties
3.5. Principal Component Analysis
3.6. Fatty Acids Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, E.A.; Ballen, F.H.; Siddiq, M. Mango Production, Global Trade, Consumption Trends, and Postharvest Processing and Nutrition. In Handbook of Mango Fruit: Production, Postharvest Science, Processing Technology and Nutrition; Wiley-Blackwell: Hoboken, NJ, USA, 2017; Chapter 1. [Google Scholar]
- Pandey, S.N. Mango cultivars: Nomenclature and registration. Acta Hortic. 1986, 182, 259–264. [Google Scholar] [CrossRef]
- National Mango Database Indian Status of Mango: Area, Production and Productivity-Growth Pattern. National Mango Database. Available online: https://mangifera.res.in/ (accessed on 28 October 2021).
- Altendorf, S. Major Tropical Fruits Market. Review 2018; FAO: Rome, Italy, 2019; pp. 1–9. [Google Scholar]
- Ashoush, I.S.; Gadallah, M.G.E. Utilization of mango peels and seed kernels powders as Sources of phytochemicals in Biscuit. World J. Dairy Food Sci. 2011, 6, 35–42. [Google Scholar]
- Torres-León, C.; Romeo, R.; Juan, C.C.E.; Liliana, S.C.; Ruth, E.B.C.; Cristóbal, N.A. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillòn, J.; Restrepo, B.; Ospina, J.C.G. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant. Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Capili, B.; Anastasi, J.K.; Chang, M. Addressing the Role of Food in Irritable Bowel Syndrome Symptom Management. J. Nurse Pract. 2016, 12, 324–329. [Google Scholar] [CrossRef] [Green Version]
- Bello-Pérez, L.A.; García-Suárez, F.J.; Agama-Acevedo, E. Mango carbohydrates. Food 2007, 1, 36–40. [Google Scholar]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Mirza, B.; Croley, C.R.; Ahmad, M.; Pumarol, J.; Das, N.; Sethi, G.; Bishayee, A. Mango (Mangifera indica L.): A magnificent plant with cancer preventive and anticancer therapeutic potential. Crit. Rev. Food 2021, 61, 2125–2151. [Google Scholar] [CrossRef]
- Kim, H.; Castellon-Chicas, M.J.; Arbizu, S.; Talcott, S.T.; Drury, N.L.; Smith, S.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) Polyphenols: Anti-Inflammatory Intestinal Microbial Health Benefits, and Associated Mechanisms of Actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, N.; Arendt, E.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Leanpolchareanchai, J.; Padois, K.; Falson, F.; Bavovada, R.; Pithayanukul, P. Microemulsion system for topical delivery of Thai mango seed kernel extract: Development, physicochemical characterisation and ex vivo skin permeation studies. Molecules 2014, 19, 17107–17129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative assessment of phenolic content and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef] [Green Version]
- Tokas, J.; Punia, H.; Baloda, S.; Sheokand, R.N. Mango Peel: A Potential Source of Bioactive Compounds and Phytochemicals. Austin Food Sci. 2020, 5, 1035. [Google Scholar] [CrossRef]
- Muchiri, D.R.; Mahungu, S.M.; Gituanja, S.N. Studies on Mango (Mangifera indica, L.) kernel fat of some Kenyan varieties in Meru. J. Am. Oil Chem. Soc. 2012, 89, 1567–1575. [Google Scholar] [CrossRef]
- Jahurul, M.; Zaidul, I.; Ghafoor, K.; Al-Juhaimi, F.; Nyam, K.; Norulaini, N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, D.; Kansal, S.K.; Garg, M.; Krishania, M. Potential cocoa butter substitute derived from mango seed kernel. Food Chem. 2022, 372, 131244. [Google Scholar] [CrossRef]
- Matheyambath, A.C.; Subramanian, J.; Paliyath, G. Mangoes. In Encyclopedia of Food and Health; Paul, B.C., Told, F.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 641–645. [Google Scholar]
- Ferreres, F.; Valentão, P.; Pereira, J.A.; Bento, A.; Noites, A.; Seabra, R.M.; Andrade, P.B. HPLC–DAD–MS/MS-ESI screening of phenolic compounds in Pieris brassicae L. reared on Brassica rapa var. Rapa, L.J. Agric. Food Chem. 2008, 56, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Durante, M.; Minervini, F.; Garbetta, A.; Cardinali, A.; D’Antuono, I.; Caretto, S.; Blanco, A.; Mita, G. Phytochemical Composition and Anti-Inflammatory Activity of Extracts from the Whole-Meal Flour of Italian Durum Wheat Cultivars. Int. J. Mol. Sci. 2015, 16, 3512–3527. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Durante, M.; Tufariello, M.; Tommasi, M.; Lenucci, M.S.; Bleve, G.; Mita, G. Evaluation of bioactive compounds in black table olives fermented with selected microbial starters. J. Sci. Food Agric. 2017, 98, 96–103. [Google Scholar] [CrossRef]
- Zhishen, J.; MengcHeng, T.; JianMing, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Kampfenkel, K.; van Montagu, M.; Inzè, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissues. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of Response Surface Methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Elisabete, M.; Pinto, S.; Holloway, D.E.; Bramley, P.M. Application of high performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2000, 24, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Lenucci, M.S.; Marrese, P.P.; Rizzi, V.; De Caroli, M.; Piro, G.; Fini, P.; Russo, G.L.; Mita, G. α-Cyclodextrin encapsulation of supercritical CO2 extracted oleoresins from different plant matrices: A stability study. Food Chem. 2016, 199, 684–693. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, R.J.; Goñi, I.; Saura-Calixto, C. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Durante, M.; Montefusco, A.; Marrese, P.P.; Soccio, M.; Pastore, D.; Piro, G.; Mita, G.; Lenucci, M.S. Seeds of pomegranate, tomato and grapes: An underestimated source of natural bioactive molecules and antioxidants from agri-food by-products. J. Food Compost. Anal. 2017, 63, 65–72. [Google Scholar] [CrossRef]
- Liguori, G.; Sortino, G.; Gianguzzi, G.; Inglese, P.; Farina, V. Evaluation of quality attributes and consumer preference of fresh or imported mangoes in Italy. AIMS Agric. Food 2018, 3, 426–440. [Google Scholar] [CrossRef]
- Rovira, L.A.A.; Alvarez, C.R. El Mango (Mangifera indica L.); Editorial America: Caracas, Venezuela, 1990; pp. 401–450. [Google Scholar]
- Afanas’ev, I.B.; Dcrozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Wink, M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. Adv. Bot. Res. 1997, 25, 141–169. [Google Scholar] [CrossRef]
- Hung, L.M.; Mason, S.L.; Bickerstaffe, R. Total phenolic content of Tommy Atkins mangoes imported into New Zealand. Proc. Nutr. Soc. 2010, 34, 34–40. [Google Scholar]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Using drying treatments to stabilize mango peel and seed: Effect on antioxidant activity. LWT-Food Sci. Technol. 2012, 45, 261–268. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity and functional properties of “Tommy Atkins” mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS profiling of Australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef] [Green Version]
- Imeh, U.; Khokhar, S. Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. J. Agric. Food Chem. 2002, 50, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango by-products: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Singh, B.; Negi, S.S. Tannin levels and their degree of polymerisation and specific activity in some agro-industrial by-products. Biol. Wastes 1990, 31, 137–144. [Google Scholar] [CrossRef]
- Ruales, J.; Baenas, N.; Moreno, D.A.; Stinco, C.M.; Melendez-Martinez, A.J.; Garcia-Ruiz, A. Biological Active Ecuadorian Mango ‘Tommy Atkins’ Ingredients—An Opportunity to Reduce Agrowaste. Nutrients 2018, 10, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lounds-Singleton, A.J.; Talcott, S.T. Antioxidant phytochemical and quality changes associated with hot water immersion treatment of mangoes (Mangifera indica L.). Food Chem. 2009, 115, 989–993. [Google Scholar] [CrossRef]
- Kane, C.J.M.; Menna, J.H.; Sung, C.C.; Yeh, Y.C. Methyl gallate, methyl-3,4,5-trihydoxy-benzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviralactivity of methyl gallate and its derivatives. Biosci. Rep. 1988, 8, 95–102. [Google Scholar] [CrossRef]
- Rastraelli, L.; Selles, A.J.N.; Castro, H.T.V.; Aguero-Aguero, J.; Gonzalez-Gonzalez, J.; Naddeo, F.; Simone, F.D. Isolation and quantitative analysis of phenolic antioxidants, free sugars, and polyols from mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as anutritional Supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef]
- Morgan, R.L.; Baack, B.; Smith, B.D.; Yartel, A.; Pitasi, M.; Falck-Ytter, Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann. Intern. Med. 2013, 158, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, W.C.; Chang, S.P.; Lin, L.C.; Li, C.L.; Richardson, C.D.; Lin, C.C.; Lin, L.T. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antivir. Res. 2015, 118, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, S.; Kumar, N.; Gudipalli, P. Isolation and characterization of gallic acid and methylgallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep. 2015, 2, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Khare, P.; Shanker, K. Mangiferin: A review of sources and interventions for biological activities. BioFactors 2016, 42, 504–514. [Google Scholar] [CrossRef]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suya, D.; Huirong, L.; Tiantian, L.; Xiaofang, X.; Hailian, W.; Xia, H.; Rongsheng, T.; Yi, W. Mangiferin: An effective therapeutic agent against several disorders (Review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Lv, Q.; Zhao, Y.; Hu, G.; Huang, G.; Zhang, J.; Sun, C.; Li, X.; Chen, K. Quantification and purification of mangiferin from Chinese Mango (Mangifera indica L.) cultivars and its protective effect on human umbilical vein endothelial cells under H2O2-induced stress. Int. J. Mol. Sci. 2012, 13, 11260–11274. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Use of HPLC- and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carl, R.; Schieber, A. Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol O- and xanthone C-glycosides, anthocyanins, and pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Barbosa, L.; Queiroz, J.; Knödler, M.; Schieber, A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 2008, 110, 620–626. [Google Scholar] [CrossRef]
- de Almeida Monaco, K.; Costa, S.M.; Minatel, I.O.; Correa, C.R.; Calero, F.A.; Vianello, F.; Lima, G.P.P. Influence of ozonated water sanitation on postharvest quality of conventionally and organically cultivated mangoes after postharvest storage. Postharvest Biol. Technol. 2016, 12, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Chongchatuporn, U.; Ketsa, S.; van Doorn, W.G. Chilling injury in mango (Mangifera indica) fruit peel: Relationship with ascorbic acid concentrations and antioxidant enzyme activities. Postharvest Biol. Technol. 2013, 86, 409–417. [Google Scholar] [CrossRef]
- Carvalho, C.R.L.; Rosseto, C.J.; Mantovani, D.M.B.; Morgano, M.A.; Castro, J.V.; Botoletto, N. Evaluation of mango cultivars selected by “Instituto Agronômico de Campinas” compaired to others of commercial importance. Rev. Bras. Frutic. 2004, 26, 264–271. [Google Scholar] [CrossRef] [Green Version]
- López-Cobo, A.; Martín-García, B.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Comparison of Two Stationary Phases for the Determination of Phytosterols and Tocopherols in Mango and Its By-Products by GC-QTOF-MS. Int. J. Mol. Sci. 2017, 18, 1594. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Santos, S.A.; Oliveira, L.; Camacho, J.F.; Cordeiro, N.; Freire, C.S.R.; Silvestre, A.J.D. The ripe pulp of Mangifera indica L.: A rich source of phytosterols and other lipophilic phytochemicals. Food Res. Int. 2013, 54, 1535–1540. [Google Scholar] [CrossRef]
- Jin, J.; Warda, P.; Mu, H.; Zhang, Y.; Jie, L.; Mao, J.; Xie, D.; Huang, J.; Jin, Q.; Wang, X. Characteristics of mango kernel fats extracted from 11 China-specific varieties and their typically fractionated fruit parts. J. Am. Oil Chem. Soc. 2016, 93, 1115–1125. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Vieira-Júnior, G.M.; Chaves, M.H.; Almeida, F.R.; Florêncio, M.G.; Lima, R.C., Jr.; Silva, R.M.; Santos, F.A.; Rao, V.S. Gastroprotective and anti-inflammatory effects of resin from Protium heptaphyllum in mice and rats. Pharmacol. Res. 2004, 49, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannowetz, N.; Miller, M.R.; Lishko, P.V. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl. Acad. Sci. USA 2017, 114, 5743–5748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Montañez, G.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Velázquez-de la Cruz, G.; Ramírez de León, J.A.; Navarro-Ocaña, A. Evaluation of extraction methods for preparative scale obtention of mangiferin and lupeol from mango peels (Mangifera indica L.). Food Chem. 2014, 159, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Jyotshna Srivastava, P.; Bharti, K.; Karuna, S. Uni-dimensional double development HPTLC-densitometry method for simultaneous analysis of mangiferin and lupeol content in mango (Mangifera indica) pulp and peel during storage. Food Chem. 2015, 176, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chaurasia, A.K.; Bari, R.; Sane, V.A. Tocopherol levels in different mango varieties correlate with MiHPPD expression and its over-expression elevates tocopherols in transgenic Arabidopsis and tomato. 3 Biotech 2017, 7, 352. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodriguez-Amaya, D.B.; Britton, G. HPLC and mass spectrometric analysis of carotenoids from mango. J. Agric. Food Chem. 1997, 45, 120–123. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodriguez-Amaya, D.B. Effects of ripening, cultivar differences, and processing on the carotenoid composition of mango. J. Agric. Food Chem. 1998, 46, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Adiletta, G.; Di Matteo, M.; Panfili, G.; Niro, S.; Gentile, C.; Farina, V.; Cinquanta, L.; Corona, O. Evolution of Carotenoid Content, Antioxidant Activity and Volatiles Compounds in Dried Mango Fruits (Mangifera indica L.). Foods 2020, 9, 1424. [Google Scholar] [CrossRef]
- Ajila, C.M.; Rao, L.J.; Prasada Rao, U.J.S. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food Chem. Toxicol. 2010, 48, 3406–3411. [Google Scholar] [CrossRef]
- Cuttriss, A.J.; Cazzonelli, C.I.; Wurtzel, E.T.; Pogson, B.J. Carotenoids. In Advances in Botanical Research; Rébeillé, F., Douce, R., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 58, pp. 1–36. [Google Scholar]
- Rumainum, I.M.; Worarad, K.; Srilaong, V.; Yamane, K. Fruit quality and antioxidant capacity of six Thai mango cultivars. Agric. Nat. Resour. 2018, 52, 208–214. [Google Scholar] [CrossRef]
- Lee, F.Y.; Vo, G.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Mango rejects and mango waste: Characterization and quantification of phenolic compounds and their antioxidant potential. J. Food Process. Preserv. 2021, 45, e15618. [Google Scholar] [CrossRef]
- Ma, X.; Wu, H.; Liu, L.; Yao, Q.; Wang, S.; Zhan, R.; Xing, S.; Zhou, Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Puravankara, D.; Boghra, V.; Sharma, R.S. Effect of antioxidant principles isolated from mango (Mangifera indica L.) seed kernels on oxidative stability of buffalo ghee (butter-fat). J. Sci. Food Agric. 2000, 80, 522–526. [Google Scholar] [CrossRef]
- Soong, Y.; Barlow, J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Ji, Y.; Qu, Y.; Jiang, Z.; Yan, J.; Chu, J.; Xu, M.; Su, X.; Yuan, H.; Wang, A. The mechanism for brassinosteroids suppressing climacteric fruit ripening. Plant. Physiol. 2021, 4, 1875–1893. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Paczkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Häggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front. Plant. Sci. 2019, 10, 431. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant. Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant. Sci. 2020, 296, 110471. [Google Scholar] [CrossRef] [PubMed]
- Awolu, O.O.; Manohar, B. Quantitative and qualitative characterization of mango kernel seed oil extracted using supercritical CO2 and solvent extraction techniques. Heliyon 2019, 12, e03068. [Google Scholar] [CrossRef] [Green Version]
- Kittiphoom, S.; Sutasinee, S. Mango seed kernel oil and its physicochemical properties. Int. Food Res. J. 2013, 20, 1145–1149. [Google Scholar]
- Nzikou, J.M.; Kimbonguila, A.; Matos, L.; Loumouamou, B.; Pambou-Tobi, N.P.G.; Ndangui, C.B.; Desobry, S. Extraction and characteristics of seed kernel oil from mango (Mangifera indica). Res. J. Environ. Earth Sci. 2010, 2, 31–35. [Google Scholar]
- Chen, B.; McClements, D.J.; Decker, E.A. Design of foods with bioactive lipids for improved health. Annu. Rev. Food Sci. 2013, 4, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol. Res. 2004, 37, 263–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Yoo, M.J.; Carius, B.M. Mango Dermatitis after Urushiol Sensitization. Clin. Pract. Cases Emerg. Med. 2019, 30, 361–363. [Google Scholar] [CrossRef]
- Berghea, E.C.; Craiu, M.; Ali, S.; Corcea, S.L.; Bumbacea, R.S. Contact Allergy Induced by Mango (Mangifera indica): A Relevant Topic? Medicina 2021, 57, 1240. [Google Scholar] [CrossRef]




| Soluble-Phenols | Fruit Fractions | |||||
|---|---|---|---|---|---|---|
| Pulp | Peels | Kernels | ||||
| mg/100 g dw | ||||||
| Tommy Atkins | Keitt | Tommy Atkins | Keitt | Tommy Atkins | Keitt | |
| Free | ||||||
| Gallic acid | nd | nd | nd | nd | 14.6 ± 0.9 | 104.2 ± 7.5 |
| Methyl gallate | 6.6 ± 0.2 C | 9.3 ± 0.2 C | 31.9 ± 1.9 B | 62.9 ± 0.1 B | 2126.5 ± 130.7 A | 1885.2 ± 66.6 A |
| Propyl gallate | nd | nd | 27.3 ± 0.4 B | 101.1 ± 0.9 B | 1420.8 ± 63.5 A | 1375.2 ± 17.5 A |
| Total | 6.6 ± 0.2 C | 9.3 ± 0.2 C | 59.2 ± 2.3 B | 164.0 ± 1.0 B | 3561.9 ± 195.1 A | 3364.6 ± 91.6 A |
| Conjugated | ||||||
| Gallic acid | 197.1 ± 5.0 C | 189.2 ± 1.6 B | 129.7 ± 1.7 B | 214.6 ± 3.9 C | 851.3 ± 3.1 A | 338.2 ± 6.5 A |
| Methyl gallate | 23.6 ± 0.1 C | 3.8 ± 0.1 C | 367.7 ± 23.3 B | 559.4 ± 3.5 A | 387.4 ± 35.9 A | nd |
| Propyl gallate | nd | nd | nd | nd | nd | nd |
| Mangiferin | nd | nd | 411.0 ± 2.4 A | nd | 185.8 ± 1.7 B | 57.9 ± 0.2 |
| Rutin | nd | nd | 65.2 ± 1.6 A | 30.2 ± 0.3 A | 40.3 ± 0.1 B | 25.5 ± 6.1 B |
| Luteolin 7-O-glucoside | nd | nd | 13.1 ± 0.1 | 5.3 ± 0.3 | nd | nd |
| Total | 220.7 ± 5.1 C | 193.0 ± 1.7 C | 986.7 ± 29.1 B | 809.5 ± 8.0 B | 1464.8 ± 40.8 A | 421.6 ± 12.8 A |
| Fruit Fractions | ||||||
|---|---|---|---|---|---|---|
| Pulp | Peels | Kernels | ||||
| mg/100 g dw | ||||||
| Tommy Atkins | Keitt | Tommy Atkins | Keitt | Tommy Atkins | Keitt | |
| Phytosterols | ||||||
| Campesterol | 11.4 ±0.2 A | 11.5 ± 0.8 A | 3.0 ± 0.2 B | 1.4 ± 0.1 B | nd | nd |
| Stigmasterol | 11.8 ± 0.5 B | 20.8 ± 0.2 A | 13.9 ± 1.6 A | 15.3 ± 1.4 B | nd | nd |
| ß-sitosterol | 128.4 ± 16.2 A | 117.6 ± 19.2 A | 66.2 ± 0.6 B | 39.2 ± 3.6 B | 106.9 ± 7.6 A | 135.5 ± 18.2 A |
| Total | 151.5 ± 16.9 A | 150.0 ± 20.1 A | 83.0 ± 2.4 B | 55.9 ± 5.1 B | 106.9 ± 7.6 B | 135.5 ± 18.2 A |
| Pentacyclic triterpenes | ||||||
| Lupeol | 2.3 ± 0.3 A | 4.6 ± 0.2 B | 9.3 ± 0.4 B | 7.9 ± 0.4 A | nd | nd |
| α -amyrin | 4.0 ± 0.2 B | 4.6 ± 0.3 B | 8.4 ± 0.3 A | 6.3 ± 0.6 A | nd | nd |
| Total | 6.3 ± 0.4 B | 9.1 ± 0.5 B | 17.7 ± 0.7 A | 14.2 ± 1.3 A | nd | nd |
| Tocopherols | ||||||
| α -tocopherol | 2.3 ± 0.3 C | 3.7 ± 0.1 B | 7.5 ± 0.3 A | 8.7 ± 0.3 A | 0.2 ± 0.1 B | 0.1 ± 0.1 C |
| ß-tocopherols | nd | nd | 3.0 ± 0.2 A | 3.6 ± 0.4 A | 0.3 ± 0.1 B | 0.1 ± 0.1 B |
| Total | 2.3 ± 0.3 B | 3.7 ± 0.1 B | 10.6 ± 0.5 A | 12.3 ± 0.8 A | 0.5 ± 0.2 C | 0.2 ± 0.2 C |
| Carotenoids | ||||||
| Violaxanthin | nd | nd | nd | 0.04 ± 0.00 | nd | nd |
| Lutein | 0.02 ± 0.01 B | nd | 0.69 ± 0.03 A | 2.56 ± 0.11 A | 0.01 ± 0.00 B | 0.01 ± 0.00 B |
| Zeaxanthin | 0.03 ± 0.01 A | 0.02 ± 0.01 B | 0.03 ± 0.01 A | 0.05 ± 0.01 A | nd | nd |
| α-carotene | nd | nd | 0.08 ± 0.00 | 0.11 ± 0.00 | nd | nd |
| β-carotene | 0.60 ± 0.10 B | 1.08 ± 0.03 A | 0.83 ± 0.07 A | 0.88 ± 0.08 B | 0.01 ± 0.00 C | 0.01 ± 0.00 C |
| 9-cis-β-carotene | 0.07 ± 0.01 | 0.07 ± 0.01 | nd | nd | nd | nd |
| 13-cis-β-carotene | 0.03 ± 0.01 B | 0.05 ± 0.00 B | 0.12 ± 0.01 A | 0.16 ± 0.01 A | nd | nd |
| Total | 0.71 ± 0.14 B | 1.22 ± 0.05 B | 1.75 ± 0.11 A | 3.76 ± 0.20 A | 0.02 ± 0.00 C | 0.01 ± 0.00 C |
| Chlorophylls | ||||||
| Chlorophyll a | nd | nd | 0.48 ± 0.03 | 1.59 ± 0.01 | nd | nd |
| Chlorophyll b | nd | nd | 1.39 ± 0.02 | 6.01 ± 0.22 | nd | nd |
| Total | nd | nd | 1.87 ± 0.05 | 7.60 ± 0.23 | nd | nd |
| Fruit Fractions | ||||||
|---|---|---|---|---|---|---|
| Pulp | Peels | Kernels | ||||
| mM TE/g dw | ||||||
| Tommy Atkins | Keitt | Tommy Atkins | Keitt | Tommy Atkins | Keitt | |
| HAA | 5.1 ± 0.1 C | 5.1 ± 0.1 C | 24.9 ± 0.8 B | 35.9 ± 3.7 B | 122.4 ± 0.5 A | 116.2 ± 9.8 A |
| LAA | 2.7 ± 0.3 C | 2.5 ± 0.2 C | 6.2 ± 0.4 B | 9.2 ± 1.0 B | 28.6 ± 1.6 A | 19.7 ± 1.6 A |
| TAA | 7.8 ± 0.4 C | 7.6 ± 0.3 B | 31.1 ± 1.2 B | 45.1 ± 4.7 B | 151.0 ± 2.1 A | 135.9 ± 11.4 A |
| Antioxidants | HAA | LAA | ||
|---|---|---|---|---|
| r | p | r | p | |
| TPC | 0.990 | <0.001 | 0.915 | 0.01 |
| TFC | 0.994 | <0.0001 | 0.958 | 0.002 |
| TCT | 0.984 | <0.001 | 0.982 | <0.001 |
| AsA | 0.703 | 0.119 | 0.620 | 0.190 |
| DHA | −0.396 | 0.437 | −0.401 | 0.431 |
| TP | −0.141 | 0.789 | −0.017 | 0.974 |
| TPT | −0.723 | 0.104 | −0.749 | 0.086 |
| TT | −0.500 | 0.312 | −0.555 | 0.253 |
| TC | −0.511 | 0.300 | −0.553 | 0.255 |
| TCh | −0.207 | 0.694 | −0.264 | 0.613 |
| Variables | Contribution (%) | |
|---|---|---|
| PC1 | PC2 | |
| TSP | 3.350 | 3.983 |
| TIBP | 3.446 | 1.491 |
| TF | 4.334 | 2.059 |
| TCT | 4.447 | 1.610 |
| fGA | 2.483 | 0.232 |
| fMG | 4.855 | 1.074 |
| fPG | 4.807 | 1.134 |
| cGA | 3.787 | 1.004 |
| cMG | 2.160 | 2.810 |
| cPG | 3.110 | 0.355 |
| M | 0.001 | 1.027 |
| R | 1.462 | 6.131 |
| L7OG | 1.915 | 1.874 |
| AsA | 0.428 | 8.860 |
| DHA | 2.489 | 1.692 |
| CaOL | 0.807 | 8.669 |
| StOL | 4.206 | 1.337 |
| β-SiOL | 2.538 | 5.162 |
| Lp | 4.475 | 0.672 |
| α-Amy | 4.828 | 0.014 |
| α-T | 4.575 | 1.408 |
| β-T | 2.594 | 5.203 |
| Vx | 1.558 | 4.206 |
| Lut | 2.289 | 4.993 |
| Zx | 4.841 | 0.295 |
| α-Car | 2.886 | 4.846 |
| β-Car | 4.553 | 0.791 |
| 9-cis-β-Car | 0.280 | 9.156 |
| 13-cis-β-Car | 4.232 | 2.232 |
| Chla | 2.364 | 5.059 |
| Chlb | 2.195 | 4.933 |
| HAA | 4.020 | 2.691 |
| LAA | 3.685 | 2.998 |
| Fatty Acids | Fruit Fractions | |||||
|---|---|---|---|---|---|---|
| Pulp | Peels | Kernels | ||||
| % of Total Identified Fatty Acids | ||||||
| Tommy Atkins | Keitt | Tommy Atkins | Keitt | Tommy Atkins | Keitt | |
| Myristic acid (C14:0) | 4.8±0.7 A | 3.2±0.4 A | 5.4±0.9 A | 3.4±0.4 A | nd | nd |
| Palmitic acid (C16:0) | 18.5±2.4 AB | 20.1±2.7 A | 20.8 ± 2.7 A | 19.3 ± 2.7 A | 13.8±1.3 B | 11.1±1.1 B |
| Heptadecanoic acid (C17:0) | 1.1±0.1 A | 0.9±0.1 A | 0.6 ± 0.1 B | 0.7 ± 0.1 A | nd | nd |
| Stearic acid (C18:0) | 17.2 ± 2.3 C | 17.1 ± 2.2 B | 6.8±0.8 B | 9.4±1.2 C | 32.8±3.0 A | 37.3±3.1 A |
| Arachidic acid (C20:0) | 1.1±0.1 B | 0.9±0.1 B | 1.2 ± 0.2 B | 1.3 ± 0.2 B | 2.5±0.2 A | 1.8±0.1 A |
| Behenic acid (C22:0) | nd | nd | 1.4 ± 0.3 | 1.2 ± 0.2 | nd | nd |
| Lignoceric acid (C24:0) | nd | nd | 0.9 ± 0.1 | 0.9 ± 0.1 | nd | nd |
| Palmitoleic acid (C16:1) | 8.2 ± 1.1 A | 5.7 ± 0.7 A | 2.7±0.4 B | 2.1±0.3 B | nd | nd |
| Oleic acid (C18:1n-9c) | 10.2±1.3 B | 7.2±1.0 B | 9.5±1.2 B | 6.7±0.8 B | 38.4±3.7 A | 41.5±3.9 A |
| 11-octadecenoic acid (C18:1n-7c) | 8.8 ± 1.2 A | 8.0 ± 1.1 A | 3.3±0.4 B | 2.6±0.3 B | nd | nd |
| Linoleic acid (C18:2n-6) | 14.3±1.7 B | 17.2±1.9 B | 25.8±3.1 A | 29.9±3.9 A | 11.0±1.0 C | 7.6±0.7 C |
| Linolenic acid (C18:3n-3) | 15.9±1.7 B | 19.8±2.6 B | 21.6±2.7 A | 22.5±2.7 A | 1.5±0.1 C | 0.6±0.06 C |
| SFA | 42.7 ± 5.6 AB | 42.1 ± 5.5 AB | 37.1 ± 5.1 B | 36.2 ± 4.9 B | 49.1 ± 4.5 A | 50.2 ± 4.3 A |
| MUFA | 27.1±3.6 B | 20.9±2.8 B | 15.5±2.0 C | 11.4±1.4 C | 38.4 ± 3.7 A | 41.5 ± 3.9 A |
| PUFA | 30.2±3.4 B | 37.0±4.5 B | 47.4 ± 5.8 A | 52.4 ± 6.6 A | 12.5±1.1 C | 8.2± 0.76 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenucci, M.S.; Tornese, R.; Mita, G.; Durante, M. Bioactive Compounds and Antioxidant Activities in Different Fractions of Mango Fruits (Mangifera indica L., Cultivar Tommy Atkins and Keitt). Antioxidants 2022, 11, 484. https://doi.org/10.3390/antiox11030484
Lenucci MS, Tornese R, Mita G, Durante M. Bioactive Compounds and Antioxidant Activities in Different Fractions of Mango Fruits (Mangifera indica L., Cultivar Tommy Atkins and Keitt). Antioxidants. 2022; 11(3):484. https://doi.org/10.3390/antiox11030484
Chicago/Turabian StyleLenucci, Marcello Salvatore, Riccardo Tornese, Giovanni Mita, and Miriana Durante. 2022. "Bioactive Compounds and Antioxidant Activities in Different Fractions of Mango Fruits (Mangifera indica L., Cultivar Tommy Atkins and Keitt)" Antioxidants 11, no. 3: 484. https://doi.org/10.3390/antiox11030484
APA StyleLenucci, M. S., Tornese, R., Mita, G., & Durante, M. (2022). Bioactive Compounds and Antioxidant Activities in Different Fractions of Mango Fruits (Mangifera indica L., Cultivar Tommy Atkins and Keitt). Antioxidants, 11(3), 484. https://doi.org/10.3390/antiox11030484







