A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action including the Role of PON3 in Health and Disease
Abstract
:1. Introduction
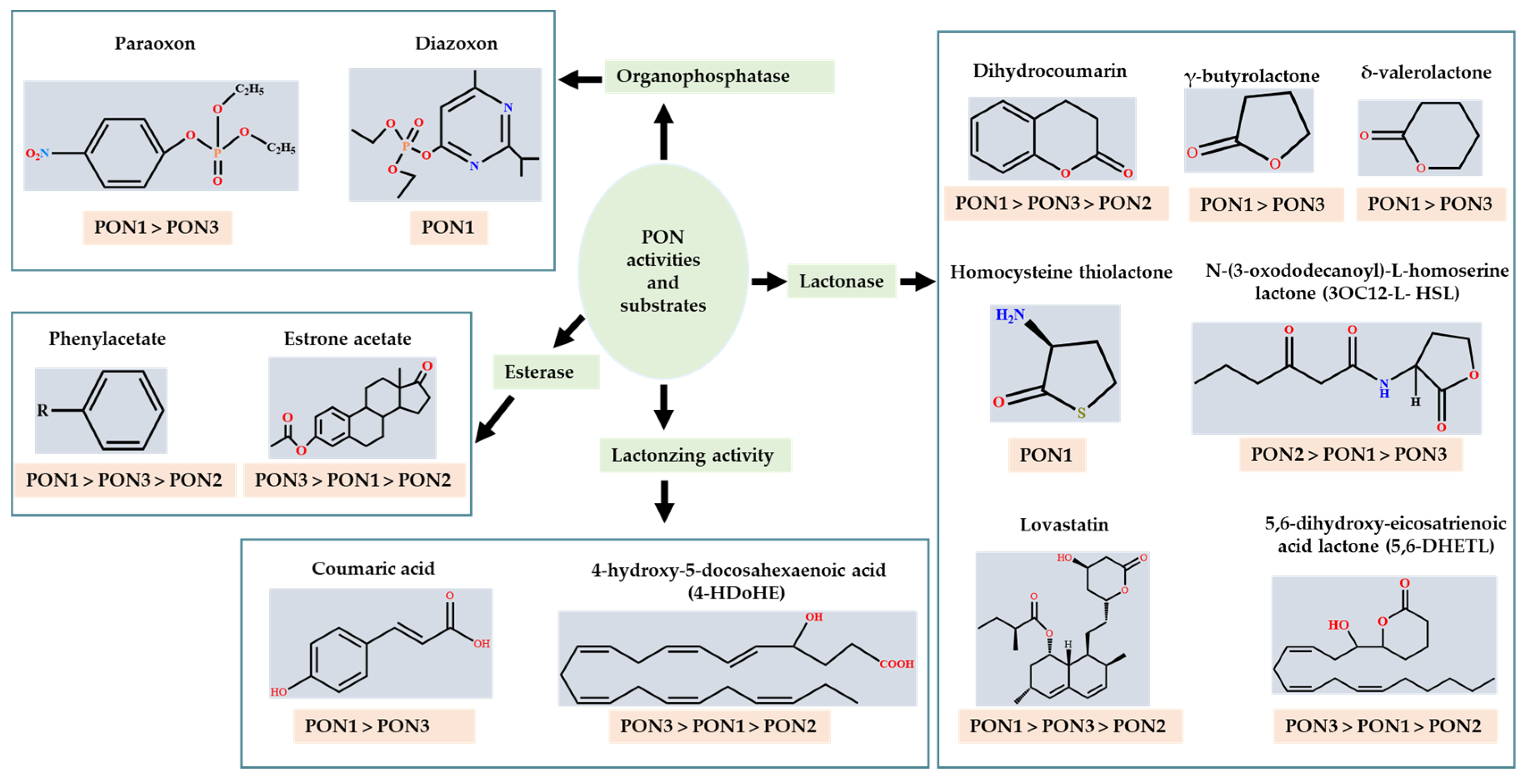
1.1. Substrates
1.1.1. Organophosphates (OP)
1.1.2. Aryl Esters
1.1.3. Lactones
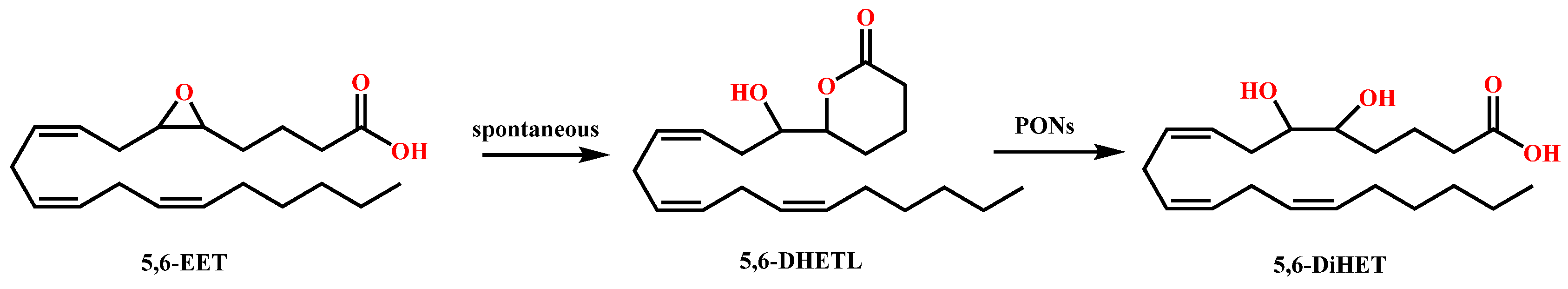
1.2. PONs and Bioactive Lipids of Arachidonic Acid
1.3. PON Activity Assays
1.4. PON Concentration Assays
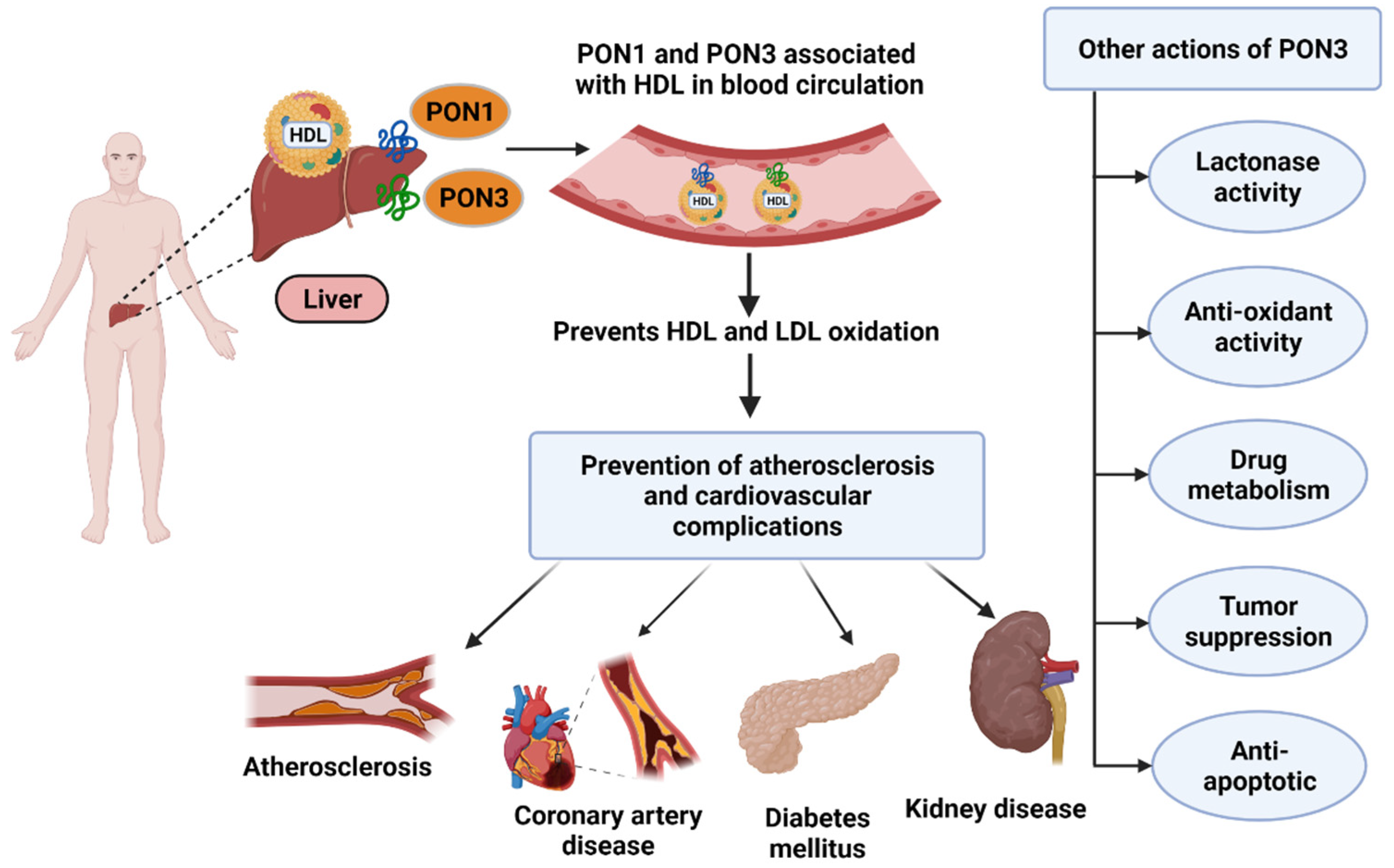
1.5. PON3 in Cardiovascular Disease
1.6. PON3 in Other Disease States
2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Harel, M.; Aharoni, A.; Gaidukov, L.; Brumshtein, B.; Khersonsky, O.; Meged, R.; Dvir, H.; Ravelli, R.B.; McCarthy, A.; Toker, L.; et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat. Struct. Mol. Biol. 2004, 11, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Grdic Rajkovic, M.; Rumora, L.; Barisic, K. The paraoxonase 1, 2 and 3 in humans. Biochem. Med. 2011, 21, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hamby, S.E.; Hirst, J.D. Prediction of glycosylation sites using random forests. BMC Bioinform. 2008, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draganov, D.I.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunahara, R.; La Du, B.N. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draganov, D.I.; Stetson, P.L.; Watson, C.E.; Billecke, S.S.; La Du, B.N. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J. Biol. Chem. 2000, 275, 33435–33442. [Google Scholar] [CrossRef] [Green Version]
- Litvinov, D.; Mahini, H.; Garelnabi, M. Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N. Am. J. Med. Sci. 2012, 4, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Priyanka, K.; Singh, S.; Gill, K. Paraoxonase 3: Structure and its role in pathophysiology of coronary artery disease. Biomolecules 2019, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Socha, E.; Milnerowicz, H. Review: The role of paraoxonase in cardiovascular diseases. Ann. Clin. Lab. Sci. 2015, 45, 226–233. [Google Scholar]
- Camps, J.; Marsillach, J.; Joven, J. The paraoxonases: Role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 2009, 46, 83–106. [Google Scholar] [CrossRef]
- Primo-Parmo, S.L.; Sorenson, R.C.; Teiber, J.; La Du, B.N. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 1996, 33, 498–507. [Google Scholar] [CrossRef]
- Shih, D.M.; Xia, Y.-R.; Yu, J.M.; Lusis, A.J. Temporal and tissue-specific patterns of Pon3 expression in mouse: In situ hybridization analysis. In Paraoxonases in Inflammation, Infection, and Toxicology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 73–87. [Google Scholar]
- Ceron, J.J.; Tecles, F.; Tvarijonaviciute, A. Serum paraoxonase 1 (PON1) measurement: An update. BMC Vet. Res. 2014, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Macharia, M.; Hassan, M.S.; Blackhurst, D.; Erasmus, R.T.; Matsha, T.E. The growing importance of PON1 in cardiovascular health: A review. J. Cardiovasc. Med. 2012, 13, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldridge, W. Serum esterases. 2. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E 600) and its identity with the A-esterase of mammalian sera. Biochem. J. 1953, 53, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draganov, D.; La Du, B.N. Pharmacogenetics of paraoxonases: A brief review. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 78–88. [Google Scholar] [CrossRef] [PubMed]
- La Du, B.N.; Furlong, C.E.; Reiner, E. Recommended nomenclature system for the paraoxonases. Chem. Biol. Interact. 1999, 119–120, 599–601. [Google Scholar] [CrossRef]
- Davies, H.G.; Richter, R.J.; Keifer, M.; Broomfield, C.A.; Sowalla, J.; Furlong, C.E. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 1996, 14, 334–336. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie 2017, 132, 19–27. [Google Scholar] [CrossRef]
- Wu, D.; Wu, C.; Zhong, Y. The association between paraoxonase 1 activity and the susceptibilities of diabetes mellitus, diabetic macroangiopathy and diabetic microangiopathy. J. Cell. Mol. Med. 2018, 22, 4283–4291. [Google Scholar] [CrossRef]
- Arenas, M.; Rodríguez, E.; Sahebkar, A.; Sabater, S.; Rizo, D.; Pallisé, O.; Hernández, M.; Riu, F.; Camps, J.; Joven, J. Paraoxonase-1 activity in patients with cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 127, 6–14. [Google Scholar] [CrossRef]
- Goswami, B.; Tayal, D.; Gupta, N.; Mallika, V. Paraoxonase: A multifaceted biomolecule. Clin. Chim. Acta 2009, 410, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1,-2 and-3: What are their functions? Chem. Biol. Interact. 2016, 259, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Senanayake, N.; Karalliedde, L. Neurotoxic effects of organohosphorus insecticides. N. Engl. J. Med. 1987, 316, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, O.; Tawfik, D.S. Structure− reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005, 44, 6371–6382. [Google Scholar] [CrossRef] [PubMed]
- Kanamori-Kataoka, M.; Seto, Y. Paraoxonase activity against nerve gases measured by capillary electrophoresis and characterization of human serum paraoxonase (PON1) polymorphism in the coding region (Q192R). Anal. Biochem. 2009, 385, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Teiber, J.F.; Billecke, S.S.; La Du, B.N.; Draganov, D.I. Estrogen esters as substrates for human paraoxonases. Arch. Biochem. Biophys. 2007, 461, 24–29. [Google Scholar] [CrossRef]
- Draganov, D.I. Lactonases with oragnophosphatase activity: Structural and evolutionary perspectives. Chem. Biol. Interact. 2010, 187, 370–372. [Google Scholar] [CrossRef]
- Draganov, D.; Teiber, J. PONs’ natural substrates–The key for their physiological roles. In The Paraoxonases: Their Role in Disease Development and Xenobiotic Metabolism; Springer: Dordrecht, The Netherlands, 2008; pp. 297–305. [Google Scholar]
- Ben-David, M.; Elias, M.; Filippi, J.J.; Dunach, E.; Silman, I.; Sussman, J.L.; Tawfik, D.S. Catalytic versatility and backups in enzyme active sites: The case of serum paraoxonase 1. J. Mol. Biol. 2012, 418, 181–196. [Google Scholar] [CrossRef]
- Billecke, S.; Draganov, D.; Counsell, R.; Stetson, P.; Watson, C.; Hsu, C.; La Du, B.N. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos. 2000, 28, 1335–1342. [Google Scholar]
- Teiber, J.F.; Xiao, J.; Kramer, G.L.; Ogawa, S.; Ebner, C.; Wolleb, H.; Carreira, E.M.; Shih, D.M.; Haley, R.W. Identification of biologically active δ-lactone eicosanoids as paraoxonase substrates. Biochem. Biophys. Res. Commun. 2018, 505, 87–92. [Google Scholar] [CrossRef]
- Teiber, J.F.; Horke, S.; Haines, D.C.; Chowdhary, P.K.; Xiao, J.; Kramer, G.L.; Haley, R.W.; Draganov, D.I. Dominant role of paraoxonases in inactivation of the Pseudomonas Aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect. Immun. 2008, 76, 2512–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Undas, A.; Perla, J.; Lacinski, M.; Trzeciak, W.; Kazmierski, R.; Jakubowski, H. Autoantibodies against N-homocysteinylated proteins in humans: Implications for atherosclerosis. Stroke 2004, 35, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domagala, T.B.; Lacinski, M.; Trzeciak, W.H.; Mackness, B.; Mackness, M.I.; Jakubowski, H. The correlation of homocysteine-thiolactonase activity of the paraoxonase (PON1) protein with coronary heart disease status. Cell. Mol. Biol. 2006, 52, 4–10. [Google Scholar] [PubMed]
- Teiber, J.F.; Draganov, D.I.; La Du, B.N. Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem. Pharmacol. 2003, 66, 887–896. [Google Scholar] [CrossRef]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, J.; Zhang, Y.; Zuo, Z. Role of esterase mediated hydrolysis of simvastatin in human and rat blood and its impact on pharmacokinetic profiles of simvastatin and its active metabolite in rat. J. Pharm. Biomed. Anal. 2019, 168, 13–22. [Google Scholar] [CrossRef]
- Agrawal, S.; Agrawal, N.; Garg, J.; Gupta, T.; Mohandas, R.; Segal, M. Heart failure and chronic kidney disease: Should we use spironolactone? Am. J. Med. Sci. 2015, 350, 147–151. [Google Scholar] [CrossRef]
- Schoner, W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur. J. Biochem. 2002, 269, 2440–2448. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Role of endogenous cardiotonic steroids in sodium homeostasis. Nephrol. Dial. Transplant. 2008, 23, 2723–2729. [Google Scholar] [CrossRef] [Green Version]
- Nikitina, E.R.; Mikhailov, A.V.; Nikandrova, E.S.; Frolova, E.V.; Fadeev, A.V.; Shman, V.V.; Shilova, V.Y.; Tapilskaya, N.I.; Shapiro, J.I.; Fedorova, O.V.; et al. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J. Hypertens. 2011, 29, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Hamlyn, J.M.; Manunta, P. Endogenous cardiotonic steroids in kidney failure: A review and an hypothesis. Adv. Chronic Kidney Dis. 2015, 22, 232–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, Y.; Dong, X.H.; Nishimura, N.; Masaki, H.; Yoshika, M.; Masuda, M.; Takahashi, H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 2005, 38, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, F.K.; Dube, P.; Mohamed, A.; Tian, J.; Malhotra, D.; Haller, S.T.; Kennedy, D.J. Cardiotonic Steroids and the Sodium Trade Balance: New Insights into Trade-Off Mechanisms Mediated by the Na+/K+-ATPase. Int. J. Mol. Sci. 2018, 19, 2576. [Google Scholar] [CrossRef] [Green Version]
- Stepniewska, J.; Dolegowska, B.; Puchalowicz, K.; Golembiewska, E.; Ciechanowski, K. Bioactive lipids derived from arachidonic acid metabolism in different types of renal replacement therapy. Chem. Phys. Lipids 2017, 206, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gaidukov, L.; Tawfik, D.S. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry 2005, 44, 11843–11854. [Google Scholar] [CrossRef]
- Chang, J.; Blazek, E.; Kreft, A.F.; Lewis, A.J. Inhibition of platelet and neutrophil phospholipase A2 by hydroxyeicosatetraenoic acids (HETES). A novel pharmacological mechanism for regulating free fatty acid release. Biochem. Pharmacol. 1985, 34, 1571–1575. [Google Scholar] [CrossRef]
- Oltman, C.L.; Weintraub, N.L.; VanRollins, M.; Dellsperger, K.C. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ. Res. 1998, 83, 932–939. [Google Scholar] [CrossRef] [Green Version]
- Kujal, P.; Chabova, V.C.; Skaroupkova, P.; Huskova, Z.; Vernerova, Z.; Kramer, H.J.; Walkowska, A.; Kompanowska-Jezierska, E.; Sadowski, J.; Kitada, K.; et al. Inhibition of soluble epoxide hydrolase is renoprotective in 5/6 nephrectomized Ren-2 transgenic hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2014, 41, 227–237. [Google Scholar] [CrossRef]
- Egger, J.; Bretscher, P.; Freigang, S.; Kopf, M.; Carreira, E.M. Discovery of a highly potent anti-inflammatory epoxyisoprostane-derived lactone. J. Am. Chem. Soc. 2014, 136, 17382–17385. [Google Scholar] [CrossRef]
- Bretscher, P.; Egger, J.; Shamshiev, A.; Trotzmuller, M.; Kofeler, H.; Carreira, E.M.; Kopf, M.; Freigang, S. Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO Mol. Med. 2015, 7, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Tecles, F.; Caldin, M.; Tasca, S.; Cerón, J. Validation of spectrophotometric assays for serum paraoxonase type-1 measurement in dogs. Am. J. Vet. Res. 2012, 73, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ferre, N.; Camps, J.; Prats, E.; Vilella, E.; Paul, A.; Figuera, L.; Joven, J. Serum paraoxonase activity: A new additional test for the improved evaluation of chronic liver damage. Clin. Chem. 2002, 48, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Blatter Garin, M.C.; Abbott, C.; Messmer, S.; Mackness, M.; Durrington, P.; Pometta, D.; James, R.W. Quantification of human serum paraoxonase by enzyme-linked immunoassay: Population differences in protein concentrations. Biochem. J. 1994, 304 (Pt. 2), 549–554. [Google Scholar] [CrossRef] [Green Version]
- Richter, R.J.; Jarvik, G.P.; Furlong, C.E. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicol. Appl. Pharmacol. 2009, 235, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.G.; Batuca, J.R.; Marinho, A.T.; Caixas, U.; Monteiro, E.C.; Antunes, A.M.M.; Pereira, S.A. Quantification of the arylesterase activity of paraoxonase-1 in human blood. Anal. Methods 2014, 6, 289–294. [Google Scholar] [CrossRef]
- Horke, S.; Xiao, J.; Schutz, E.M.; Kramer, G.L.; Wilgenbus, P.; Witte, I.; Selbach, M.; Teiber, J.F. Novel Paraoxonase 2-Dependent Mechanism Mediating the Biological Effects of the Pseudomonas Aeruginosa Quorum-Sensing Molecule N-(3-Oxo-Dodecanoyl)-L-Homoserine Lactone. Infect. Immun. 2015, 83, 3369–3380. [Google Scholar] [CrossRef] [Green Version]
- Suchocka, Z.; Swatowska, J.; Pachecka, J.; Suchocki, P. RP-HPLC determination of paraoxonase 3 activity in human blood serum. J. Pharm. Biomed. Anal. 2006, 42, 113–119. [Google Scholar] [CrossRef]
- Shih, D.M.; Xia, Y.R.; Wang, X.P.; Wang, S.S.; Bourquard, N.; Fogelman, A.M.; Lusis, A.J.; Reddy, S.T. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ. Res. 2007, 100, 1200–1207. [Google Scholar] [CrossRef] [Green Version]
- Thibault, A.; Samid, D.; Cooper, M.R.; Figg, W.D.; Tompkins, A.C.; Patronas, N.; Headlee, D.J.; Kohler, D.R.; Venzon, D.J.; Myers, C.E. Phase I study of phenylacetate administered twice daily to patients with cancer. Cancer 1995, 75, 2932–2938. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Zeng, R.F.; Liu, Y.; Xie, S.S.; Lan, J.S.; Ding, Y.; Yang, Y.T.; Yang, J.; Zhang, T. Design and synthesis of novel 3,4-dihydrocoumarins as potent and selective monoamine oxidase-B inhibitors with the neuroprotection against Parkinson’s disease. Bioorg. Chem. 2021, 109, 104685. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Leung, P.H.; Xu, X.; Wu, C. Homogentisic acid γ-lactone suppresses the virulence factors of Pseudomonas Aeruginosa by quenching its quorum sensing signal molecules. Chin. Chem. Lett. 2018, 29, 313–316. [Google Scholar] [CrossRef]
- Ozer, E.A.; Pezzulo, A.; Shih, D.M.; Chun, C.; Furlong, C.; Lusis, A.J.; Greenberg, E.P.; Zabner, J. Human and murine paraoxonase 1 are host modulators of Pseudomonas Aeruginosa quorum-sensing. FEMS Microbiol. Lett. 2005, 253, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Corban, M.T.; Toya, T.; Sara, J.D.; Lerman, B.; Park, J.Y.; Lerman, L.O.; Lerman, A. Coronary Microvascular Endothelial Dysfunction in Patients With Angina and Nonobstructive Coronary Artery Disease Is Associated with Elevated Serum Homocysteine Levels. J. Am. Heart Assoc. 2020, 9, e0231428. [Google Scholar] [CrossRef]
- Yang, D.J.; Hwang, L.S. Study on the conversion of three natural statins from lactone forms to their corresponding hydroxy acid forms and their determination in Pu-Erh tea. J. Chromatogr. A 2006, 1119, 277–284. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Roest, M.; Voorbij, H. PON1 genotypes and coronary heart disease. In The Paraoxonases: Their Role in Disease Development and Xenobiotic Metabolism; Springer: Berlin/Heidelberg, Germany, 2008; pp. 139–147. [Google Scholar]
- Muthukrishnan, S.; Shete, V.S.; Sanan, T.T.; Vyas, S.; Oottikkal, S.; Porter, L.M.; Magliery, T.J.; Hadad, C.M. Mechanistic insights into the hydrolysis of organophosphorus compounds by paraoxonase-1: Exploring the limits of substrate tolerance in a promiscuous enzyme. J. Phys. Org. Chem. 2012, 25, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Aharoni, A.; Gaidukov, L.; Yagur, S.; Toker, L.; Silman, I.; Tawfik, D.S. Directed evolution of mammalian paraoxonases PON1 and PON3 for bacterial expression and catalytic specialization. Proc. Natl. Acad. Sci. USA 2004, 101, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Masson, P.; Josse, D.; Lockridge, O.; Viguie, N.; Taupin, C.; Buhler, C. Enzymes hydrolyzing organophosphates as potential catalytic scavengers against organophosphate poisoning. J. Physiol. -Paris 1998, 92, 357–362. [Google Scholar] [CrossRef]
- Khersonsky, O.; Rosenblat, M.; Toker, L.; Yacobson, S.; Hugenmatter, A.; Silman, I.; Sussman, J.L.; Aviram, M.; Tawfik, D.S. Directed evolution of serum paraoxonase PON3 by family shuffling and ancestor/consensus mutagenesis, and its biochemical characterization. Biochemistry 2009, 48, 6644–6654. [Google Scholar] [CrossRef]
- Le, Q.A.T.; Kim, S.; Chang, R.; Kim, Y.H. Insights into the Lactonase Mechanism of Serum Paraoxonase 1 (PON1): Experimental and Quantum Mechanics/Molecular Mechanics (QM/MM) Studies. J. Phys. Chem. B 2015, 119, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L.; Gil, F.; Hernandez, A.F.; Lopez, O.; Pla, A. Identification of paraoxonase 3 in rat liver microsomes: Purification and biochemical properties. Biochem. J. 2003, 376, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; Durrington, P.N.; Mackness, M.I. Paraoxonase status in coronary heart disease: Are activity and concentration more important than genotype? Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragones, G.; Garcia-Heredia, A.; Guardiola, M.; Rull, A.; Beltran-Debon, R.; Marsillach, J.; Alonso-Villaverde, C.; Mackness, B.; Mackness, M.; Pedro-Botet, J.; et al. Serum paraoxonase-3 concentration in HIV-infected patients. Evidence for a protective role against oxidation. J. Lipid Res. 2012, 53, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsillach, J.; Becker, J.O.; Vaisar, T.; Hahn, B.H.; Brunzell, J.D.; Furlong, C.E.; de Boer, I.H.; McMahon, M.A.; Hoofnagle, A.N.; Group, D.E.R. Paraoxonase-3 is depleted from the high-density lipoproteins of autoimmune disease patients with subclinical atherosclerosis. J. Proteome Res. 2015, 14, 2046–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, C.J.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Roberts, R. A genetic basis for coronary artery disease. Trends Cardiovasc. Med. 2015, 25, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef] [Green Version]
- Campagna, R.; Mateuszuk, Ł.; Wojnar-Lason, K.; Kaczara, P.; Tworzydło, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone methyl displays detrimental effects on endothelial bioenergetics, suppresses endothelial ET-1 release, and increases endothelial permeability in human microvascular endothelium. Oxid. Med. Cell. Longev. 2020, 2020, 4678252. [Google Scholar] [CrossRef] [PubMed]
- García, N.; Zazueta, C.; Aguilera-Aguirre, L. Oxidative Stress and Inflammation in Cardiovascular Disease; Hindawi: London, UK, 2017; Volume 2017. [Google Scholar]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008, 299, 1265–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, D.M.; Lusis, A.J. The roles of PON1 and PON2 in cardiovascular disease and innate immunity. Curr. Opin. Lipidol. 2009, 288–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackness, B.; Mackness, M. Anti-inflammatory properties of paraoxonase-1 in atherosclerosis. Adv. Exp. Med. Biol. 2010, 660, 143–151. [Google Scholar] [CrossRef]
- Borovkova, E.I.; Antipova, N.V.; Komeenko, T.V.; Shakhparonov, M.I.; Borovkov, I.M. Paraoxonase: The Universal Factor of Antioxidant Defense in Human Body. Vestn. Ross. Akad. Med. Nauk. 2017, 72, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Xia, Y.R.; Wang, X.P.; Miller, E.; Castellani, L.W.; Subbanagounder, G.; Cheroutre, H.; Faull, K.F.; Berliner, J.A.; Witztum, J.L.; et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 2000, 275, 17527–17535. [Google Scholar] [CrossRef] [Green Version]
- Tward, A.; Xia, Y.R.; Wang, X.P.; Shi, Y.S.; Park, C.; Castellani, L.W.; Lusis, A.J.; Shih, D.M. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002, 106, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Devarajan, A.; Bourquard, N.; Hama, S.; Navab, M.; Grijalva, V.R.; Morvardi, S.; Clarke, C.F.; Vergnes, L.; Reue, K.; Teiber, J.F.; et al. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid. Redox Signal. 2011, 14, 341–351. [Google Scholar] [CrossRef] [Green Version]
- She, Z.G.; Zheng, W.; Wei, Y.S.; Chen, H.Z.; Wang, A.B.; Li, H.L.; Liu, G.; Zhang, R.; Liu, J.J.; Stallcup, W.B.; et al. Human paraoxonase gene cluster transgenic overexpression represses atherogenesis and promotes atherosclerotic plaque stability in ApoE-null mice. Circ. Res. 2009, 104, 1160–1168. [Google Scholar] [CrossRef] [Green Version]
- Shih, D.M.; Gu, L.; Xia, Y.R.; Navab, M.; Li, W.F.; Hama, S.; Castellani, L.W.; Furlong, C.E.; Costa, L.G.; Fogelman, A.M.; et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998, 394, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Yu, J.M.; Vergnes, L.; Dali-Youcef, N.; Champion, M.D.; Devarajan, A.; Zhang, P.; Castellani, L.W.; Brindley, D.N.; Jamey, C.; et al. PON3 knockout mice are susceptible to obesity, gallstone formation, and atherosclerosis. FASEB J. 2015, 29, 1185–1197. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.J.; Bourquard, N.; Hama, S.Y.; Shih, D.; Grijalva, V.R.; Navab, M.; Fogelman, A.M.; Reddy, S.T. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1368–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, I.; Foerstermann, U.; Devarajan, A.; Reddy, S.T.; Horke, S. Protectors or Traitors: The Roles of PON2 and PON3 in Atherosclerosis and Cancer. J. Lipids 2012, 2012, 342806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweikert, E.M.; Devarajan, A.; Witte, I.; Wilgenbus, P.; Amort, J.; Forstermann, U.; Shabazian, A.; Grijalva, V.; Shih, D.M.; Farias-Eisner, R.; et al. PON3 is upregulated in cancer tissues and protects against mitochondrial superoxide-mediated cell death. Cell Death Differ. 2012, 19, 1549–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golomb, B.A.; Dang, T.T.; Criqui, M.H. Peripheral arterial disease: Morbidity and mortality implications. Circulation 2006, 114, 688–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rull, A.; Garcia, R.; Fernandez-Sender, L.; Garcia-Heredia, A.; Aragones, G.; Beltran-Debon, R.; Marsillach, J.; Alegret, J.M.; Martin-Paredero, V.; Mackness, B.; et al. Serum paraoxonase-3 concentration is associated with insulin sensitivity in peripheral artery disease and with inflammation in coronary artery disease. Atherosclerosis 2012, 220, 545–551. [Google Scholar] [CrossRef]
- Balakumar, P.; Maung, U.K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical update: Cardiovascular disease in diabetes mellitus: Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus–mechanisms, management, and clinical considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Rye, K.-A.; Barter, P.J. Cardioprotective functions of HDLs1. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Bacchetti, T.; Ferretti, G.; Carbone, F.; Ministrini, S.; Montecucco, F.; Jamialahmadi, T.; Sahebkar, A. Dysfunctional High-density Lipoprotein: The Role of Myeloperoxidase and Paraoxonase-1. Curr. Med. Chem. 2021, 28, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Wilson Tang, W.; Fan, Y.; Wu, Y.; Mann, S.; Pepoy, M.; Hazen, S.L. Diminished antioxidant activity of high-density lipoprotein–associated proteins in chronic kidney disease. J. Am. Heart Assoc. 2017, 2, e000104. [Google Scholar]
- Mohammed, C.J.; Xie, Y.; Brewster, P.S.; Ghosh, S.; Dube, P.; Sarsour, T.; Kleinhenz, A.L.; Crawford, E.L.; Malhotra, D.; James, R.W. Circulating lactonase activity but not protein level of PON-1 predicts adverse outcomes in subjects with chronic kidney disease. J. Clin. Med. 2019, 8, 1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.B.; Wang, Y.; He, Y.F.; Wang, G.; Wang, W.; Han, X.H.; Sun, Y.B.; Lin, L.; Shan, B.J.; Shen, G.D.; et al. Paraoxonase 3 is involved in the multi-drug resistance of esophageal cancer. Cancer Cell Int. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Shui, I.M.; Wong, C.J.; Zhao, S.S.; Kolb, S.; Ebot, E.M.; Geybels, M.S.; Rubicz, R.; Wright, J.L.; Lin, D.W.; Klotzle, B.; et al. Prostate tumor DNA methylation is associated with cigarette smoking and adverse prostate cancer outcomes. Cancer 2016, 122, 2168–2177. [Google Scholar] [CrossRef]
- Cai, J.; Yuan, S.X.; Yang, F.; Tao, Q.F.; Yang, Y.; Xu, Q.G.; Wang, Z.G.; Yu, J.; Lin, K.Y.; Wang, Z.Y.; et al. Paraoxonase 3 inhibits cell proliferation and serves as a prognostic predictor in hepatocellular carcinoma. Oncotarget 2016, 7, 70045–70057. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Li, Q.; Qiu, J.; Zhao, X.; Zheng, C.; Lv, S.; Bai, Y.; Shan, Y.; Ye, L.C. Downregulation of paraoxonase 3 contributes to aggressive human hepatocellular carcinoma progression and associates with poor prognosis. Tumour Biol. 2016, 37, 14193–14203. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, Y.; Sun, W. Paraoxonase 3 promotes cell proliferation and metastasis by PI3K/Akt in oral squamous cell carcinoma. Biomed. Pharmacother. 2017, 85, 712–717. [Google Scholar] [CrossRef]
- Siegel, M.O.; Borkowska, A.G.; Dubrovsky, L.; Roth, M.; Welti, R.; Roberts, A.D.; Parenti, D.M.; Simon, G.L.; Sviridov, D.; Simmens, S.; et al. HIV infection induces structural and functional changes in high density lipoproteins. Atherosclerosis 2015, 243, 19–29. [Google Scholar] [CrossRef] [Green Version]
- García-Heredia, A.; Marsillach, J.; Aragonès, G.; Guardiola, M.; Rull, A.; Beltrán-Debón, R.; Folch, A.; Mackness, B.; Mackness, M.; Pedro-Botet, J. Serum paraoxonase-3 concentration is associated with the severity of hepatic impairment in patients with chronic liver disease. Clin. Biochem. 2011, 44, 1320–1324. [Google Scholar] [CrossRef]
- Peng, W.; Jiang, X.; Haiqin, L.; Zhang, C.; Zhu, J.; Zhang, J.; Zang, Y.; Qin, J. Protective effects of transgene expressed human PON3 against CCl4-induced subacute liver injury in mice. Biomed. Pharmacother. 2009, 63, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.M.; Al-Shabanah, O.A.; Al-Harbi, N.O.; Al-Harbi, M.M.; Al-Rejaie, S.S.; Alsurayea, S.M.; Sayed-Ahmed, A.M. Association between Paraoxonases Gene Expression and Oxidative Stress in Hepatotoxicity Induced by CCl4. Oxid. Med. Cell. Longev. 2014, 893212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedmaier, S.; Klein, K.; Winter, S.; Hofmann, U.; Schwab, M.; Zanger, U.M. Paraoxonase (PON1 and PON3) Polymorphisms: Impact on Liver Expression and Atorvastatin-Lactone Hydrolysis. Front. Pharmacol. 2011, 2, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Activity | PON1 | PON2 | PON3 |
|---|---|---|---|
| Organophosphate | Yes [18] | No [4] | No (except paraoxonase) [4] |
| Arylesterase | Yes [25,27] | Yes, but very low [4,27] | Yes, but mostly low [5] |
| Lactonase | Yes [25] | Yes [33] | Yes, high [36] |
| Eicosanoid | Yes [4] | Little or no [4] | Yes [4,32] |
| Substrate | PON1 | PON2 | PON3 | Source | Ref. | Biological Role |
|---|---|---|---|---|---|---|
| Organophosphate (μmol/min/mg) | Purified recombinant human PONs | [4] | Inhibition of acetylcholinesterase, leading to cholinergic syndrome [24] | |||
| Paraoxon (1 mM) | 1.94 | ND | 0.205 | |||
| Chlorpyrifos oxon (0.32 mM) | 40.9 | ND | ND | |||
| Diazoxon (1 mM) | 113 | ND | ND | |||
| Esters (μmol/min/mg) | Purified recombinant human PONs | [4] | Phenyl acetate can be used as anticancer drug [62] | |||
| Phenyl acetate (1 mM) | 1120 | 0.086 | 4.1 | |||
| p-NO2-acetate (1 mM) | 15.0 | 0.7 | 39.0 | |||
| p-NO2-propionate (1 mM) | 13.6 | 0.96 | 20.7 | |||
| p-NO2-butyrate (1 mM) | 1.3 | 1.4 | 11.4 | |||
| Thiophenyl acetate (1 mM) | 259 | ND | 0.48 | Purified rabbit serum | [5] | |
| β-Napthyl acetate (0.5 mM) | 139 | ND | 4.6 | |||
| Estrone acetate (25 µM) | 0.137 | 0.004 | 0.515 | Purified recombinant human PONs | [27] | Estrogens are antioxidants [27] |
| Estrone propionate (25 µM) | 0.057 | ND | 0.220 | |||
| 17β-Estradiol acetate (25 µM) | 0.125 | 0.005 | 1.06 | |||
| 17β-Estradiol diacetate (10 µM) | 1.00 | 0.509 | 55.4 | |||
| Estrone Enol diacetate (10 µM) | 0.987 | 0.955 | 31.8 | |||
| 17β-Estradiol 3-Ac 17-cyclopentyl-propionate (10 µM) | 0.024 | 0.114 | 1.60 | |||
| Aromatic lactones (μmol/min/mg) | Purified recombinant human PONs | [4] | 3,4 DHC exhibits neuroprotective activity [63] Homogentisic acid lactone prevents infection against Pseudomonas Aeruginosa [64] | |||
| Dihydrocoumarin (1 mM) | 129.9 | 3.1 | 126.1 | |||
| 2-Coumaronone (1 mM) | 135.7 | 10.9 | 40.7 | |||
| Homogentisic acid lactone (HgAL) (1 mM) | 329.5 | ND | ND | |||
| γ-lactones (μmol/min/mg) | Human recombinant PON | [4] | ||||
| γ-Butyrolactone (1 mM) | 32.1 | ND | 0.81 | |||
| γ-Valerolactone (1 mM) | 45.0 | ND | 6.2 | |||
| γ-Hexalactone (1 mM) | 51.7 | ND | 23.9 | |||
| γ-Heptalactone (1 mM) | 57.2 | ND | 27.7 | |||
| γ-Octalactone (1 mM) | 69.2 | ND | 25.6 | |||
| γ-Nonalactone (1 mM) | 144.7 | ND | 30.9 | |||
| γ-Decanolactone (1 mM) | 173.8 | ND | 45.6 | |||
| γ-Undecanolactone (1 mM) | 127.6 | ND | 71.4 | |||
| α-Angelica lactone (1 mM) | 183.0 | ND | 20.7 | |||
| γ-Phenyl-γ-butyrolactone (0.5 mM) | 63.0 | 0.68 | 11.4 | |||
| δ-lactones (μmol/min/mg) | ||||||
| δ-Valerolactone (1 mM) | 671 | ND | 14.5 | |||
| δ-Hexalactone (1 mM) | 72 | ND | 11.7 | |||
| δ-Nonalactone (1 mM) | 150 | ND | 11.1 | |||
| δ-Decanolactone (1 mM) | 251 | ND | 44.3 | |||
| δ-Undecanolactone (1 mM) | 287 | ND | 84.4 | |||
| δ-Tetradecanolactone (0.5 mM) | 154 | ND | 22.7 | |||
| DL-3-Oxo-hexanoyl-HSL (250 µM) | 0.0334 | 0.2683 | ND | |||
| L-3-Oxo-hexanoyl-HSL (250 µM) | N | 0.5080 | ND | |||
| DL-Heptanoyl-HSL (25 µM) | 0.0036 | 0.0311 | 0.0049 | |||
| DL-Dodecanoyl-HSL (25 µM) | 0.0167 | 0.4588 | 0.0877 | |||
| DL-Tetradecanoyl-HSL (25 µM) | 0.0035 | 0.4239 | 0.0255 | |||
| Eicosanoid lactones (µmol/min/mg) | Human recombinant PON a Recombinant PON | a [4] [32] | Control homeostatic and inflammatory processes, potent vasodilators [50] | |||
| 5-HETEL (10 µM) | 75.4 a | 1.83 a | 27.5 a | |||
| (192Q) | ||||||
| (192R) | ||||||
| Cyclo-EC | 0.007 0.008 | <0.002 | 19.2 | |||
| (±)−5 (6)-DHETL | 0.62 0.67 | 0.67 | 12.7 | |||
| Homoserine lactones (nmol/min/mg) | (192Q) (192R) | Purified human PON | [33] | Prevents bacterial infection [65] | ||
| 5-HL (10 µM) | 29,500 | |||||
| 3OC12-L-HSL (10 µM) | 36,800 | 3100 | 22,100 | |||
| 224 334 | 7647 | 100 | ||||
| Amino acid derived lactones (µmol/min/mg) Homocysteine thiolactone (10 mM) | (192Q) (192R) 0.02 0.03 | ND | ND | Purified human PON1 Q and R | [31] | Risk factor for atherosclerosis [66] |
| Lactone drugs (pmol/min/mg) | (192Q) b (192R) b | Purified human PON1 Q and R b Human recombinant PON c | b [31] c [4] | Lipid lowering drugs; used as cardiovascular medicine [67] | ||
| Mevastatin (12.8 µM) b | 485.1 461.8 | ND | ND | |||
| Lovastatin (12.8 µM) b (25 µM) c | 489.6 473.4 | ND | (2.66 × 105) c | |||
| Simvastatin (12.4 µM) b (25 µM) c | 684.5 568.3 | ND | (1.1 × 104) c | |||
| Spironolactone (12.0 µM) b (25 µM) c | 234.8 262.9 | ND | (1.3 × 104) c | |||
| Lactonizing activity (μmol/min/mg) | Recombinant PON | [4] | p-coumaric acid has antioxidant, and anti-inflammatory properties [68] 4-HDoHE triggers inflammation [69] | |||
| Coumaric acid (100 µM) | 0.047 | ND | 0.013 | |||
| 4-HDoHE (10 µM) | 1.51 | 0.52 | 13.7 |
| Substrate | Structure | Kinetic Constants | PON1 | PON3 | Source | Ref. |
|---|---|---|---|---|---|---|
| Paraoxon |  | kcat (s−1) | 126 a | 0.001 b | rePON1G2E6 a RabPON3 b | a [70] b [71] |
| KM (mM) | 0.9 a | 1.3 b | ||||
| kcat/KM (s−1 M−1) | 1.4 × 105 a | 0.73 b | ||||
| Chlorpyrifos oxon | 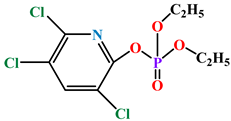 | kcat (s−1) | 7800 | ND | PON1Q192 | [72] |
| KM (mM) | 0.075 | ND | ||||
| kcat/KM (s−1 M−1) | 1.0 × 108 | ND | ||||
| Sarin |  | kcat (s−1) | 190 | ND | PON1Q192 | [31] |
| KM (mM) | 0.21 | ND | ||||
| kcat/KM (s−1 M−1) | 9.0 × 105 | ND | ||||
| Soman |  | kcat (s−1) | 1150 | ND | PON1Q192 | [31] |
| KM (mM) | 0.42 | ND | ||||
| kcat/KM (s−1 M−1) | 2.7 × 106 | ND | ||||
| 2-napthylacetate | 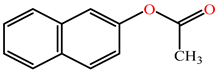 | kcat (s−1) | ND | 0.66 | RabPON3 | [71] |
| KM (mM) | ND | 0.211 | ||||
| kcat/KM (s−1 M−1) | ND | 3100 | ||||
| Phenylacetate | 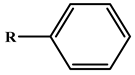 | kcat (s−1) | 698 | ND | rePON1G2E6 | [25] |
| KM (mM) | 1.2 | ND | ||||
| kcat/KM (s−1 M−1) | 5.95 × 105 | ND | ||||
| 5-thiobutil butyrolactone | 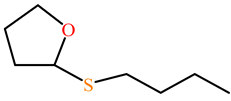 | kcat (s−1) | 116 | 42 | PON1 variant P2E6, rePON3 | [73] |
| KM (mM) | 0.27 | 0.44 | ||||
| kcat/KM (s−1 M−1) | 4.4 × 105 | 99,200 | ||||
| γ-nonanoic lactone | 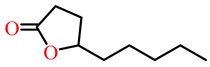 | kcat (s−1) | 31 | 22 | PON1 variant P2E6, rePON3 | [73] |
| KM (mM) | 0.39 | 1.1 | ||||
| kcat/KM (s−1 M−1) | 7.8 × 104 | 20,550 | ||||
| γ-undecanoic lactone | 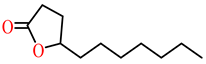 | kcat (s−1) | 62 | 21 | PON1 variant P2E6, rePON3 | [73] |
| KM (mM) | 0.60 | 0.47 | ||||
| kcat/KM (s−1 M−1) | 1.03 × 105 | 43,700 | ||||
| δ-undecanoic lactone | 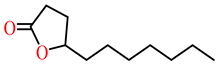 | kcat (s−1) | ND | 21 | PON1 variant P2E6, rePON3 | [73] |
| KM (mM) | ND | 0.8 | ||||
| kcat/KM (s−1 M−1) | ND | 28,700 | ||||
| γ-butyrolactone | 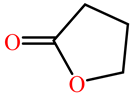 | kcat (s−1) | 147.209 | ND | G3C9 PON1 wild Type | [74] |
| KM (mM) | 36.800 | ND | ||||
| kcat/KM (s−1 M−1) | 4 × 103 | ND | ||||
| γ -valerolactone | 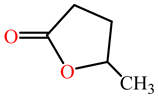 | kcat (s−1) | 36.621 | ND | G3C9 PON1 wild Type | [74] |
| KM (mM) | 1.316 | ND | ||||
| kcat/KM (s−1 M−1) | 2.78 × 104 | ND | ||||
| Dihydrocoumarin | 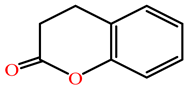 | kcat (s−1) | 152 c | 1321 d | rePON1 | c [25] d [75] |
| KM (mM) | 0.129 c | 0.75 d | G2E6 c | |||
| kcat/KM (s−1 M−1) | 1.19 × 106 c | 1761 d | Purified rat PON d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, C.J.; Lamichhane, S.; Connolly, J.A.; Soehnlen, S.M.; Khalaf, F.K.; Malhotra, D.; Haller, S.T.; Isailovic, D.; Kennedy, D.J. A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action including the Role of PON3 in Health and Disease. Antioxidants 2022, 11, 590. https://doi.org/10.3390/antiox11030590
Mohammed CJ, Lamichhane S, Connolly JA, Soehnlen SM, Khalaf FK, Malhotra D, Haller ST, Isailovic D, Kennedy DJ. A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action including the Role of PON3 in Health and Disease. Antioxidants. 2022; 11(3):590. https://doi.org/10.3390/antiox11030590
Chicago/Turabian StyleMohammed, Chrysan J., Sabitri Lamichhane, Jacob A. Connolly, Sophia M. Soehnlen, Fatimah K. Khalaf, Deepak Malhotra, Steven T. Haller, Dragan Isailovic, and David J. Kennedy. 2022. "A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action including the Role of PON3 in Health and Disease" Antioxidants 11, no. 3: 590. https://doi.org/10.3390/antiox11030590
APA StyleMohammed, C. J., Lamichhane, S., Connolly, J. A., Soehnlen, S. M., Khalaf, F. K., Malhotra, D., Haller, S. T., Isailovic, D., & Kennedy, D. J. (2022). A PON for All Seasons: Comparing Paraoxonase Enzyme Substrates, Activity and Action including the Role of PON3 in Health and Disease. Antioxidants, 11(3), 590. https://doi.org/10.3390/antiox11030590







