Abstract
Cocoa (Theobroma cacao) is a food product used worldwide and a key raw material for chocolate manufacturing. Cocoa possesses bioactive compounds such as methylxanthines, flavonoids, procyanidins, and related molecules with medicinal or health-promoting properties. Cocoa shell and pod husk have been proposed as a by-product with several interesting bioactivities, and the gummy residue or glue (a sticky, gluey by-product known as “mucilage” in Spanish) is used to produce liquors and is eaten as a food in Perú. However, little is known about the chemical composition and bioactivity of flours made from Peruvian cocoa ecotype wastes such as those from the vein and pod husk of the fruits. This study aimed to characterize the in vitro antioxidant properties and nutritional values of flours made from the waste from a special ecotype of cocoa (CCN-51). The chemical fingerprinting was performed using UHPLC–HESI orbitrap mass spectrometry and allowed the detection of 51 compounds. GC-FID was used for the determination of individual fatty acid contents, and the antioxidant activity was assessed by several assays (DPPH, FRAP, and ABTS). The flours obtained were composed of a good amount of dietary fiber, carbohydrates, and minerals, as well as several bioactive polyphenolic compounds, fatty acids, and amino acids with nutraceutical properties, making the flours a rich and promising food as well as a good source for the preparation of functional foods or nutraceuticals.
1. Introduction
In recent years, fruit and vegetable by-products or food wastes have been shown to be a good source of bioactive compounds that can be extracted and reintroduced into the food chain or in food matrices as natural food additives for the production of functional foods or nutraceuticals [1]. In addition, the reduction of food waste is growing as an important process of environmental and economical welfare. Cocoa by-products, primarily cocoa pod husks, are produced in vast quantities around the world, representing 70–80% of the dry weight of the fruit [2]. They are usually thrown away as a production waste, which has a detrimental environmental impact [3]. Cocoa beans are mainly used to produce cocoa powder and chocolate. It is estimated that a total of around 4–5 million tons of cocoa is produced worldwide per year [4] and 700,000 tons of cocoa bean shells are produced as waste every year [1], being a good source of proteins, fatty acids, and reported polyphenolic compounds [5]. Cocoa-rich products can have beneficial effects on human health due to their beneficial antioxidant components. For instance, it has been proposed that cocoa products can prevent degenerative diseases, metabolic disorders, and cancer, acting as antiobesogenic, antidiabetic, and antihypertensive factors that are associated mainly with the number of phenolic compounds accounting for approximately 8% of cocoa beans [6,7,8]. The stem bark of cocoa has also shown anti-inflammatory properties [9]. Regarding cocoa bean shells, several revalorizations have been proposed for food, livestock feed, or industrial uses, and several health properties have been reported, including anticarcinogenic, antibacterial, anti-inflammatory, antidiabetic, antiviral, neuroprotective, and cardioprotective effects [5]. There are three main varieties of cocoa cultivated in the Amazon region, with the “forastero” variety being the variety that receives the greatest degree of exploitation. This variety is responsible for 96% of worldwide production due to its resistance to pests [10,11]. The main phenolic compounds in cocoa are the group of flavonoids, which include anthocyanins, flavonols, and flavanols. Other phenolic compounds found in cocoa products are amino acid and phenolic acid conjugates (N phenol amino acids, NPAs), stilbenes, and phenolic acids [12]. Finally, cocoa products contain a good quantity of alkaloids known as methylxanthines [13]. The purine alkaloids theobromine (3,7-dimethylxanthine), caffeine (1,3,7-trimethylxanthine), and theophylline (1,3-dimethylxanthine) are the most common methylxanthines found in cocoa [14]. They are biologically active alkaloids responsible for the bitter taste of cocoa. These alkaloids also possess desirable pharmacological effects, e.g., gastric secretion, diuresis, bronchodilation stimulation of the central nervous system, and stimulation of skeletal muscles in high doses. To date, there is little research conducted about nutritional properties and chemical fingerprinting of the metabolites from cocoa waste products; however, cocoa pod husk is considered a cheap and good source of pectin [2]. In the present study, we present the phenolic composition of two flours made from waste (vein and pod husk) from cocoa CCN-51 variety and their antioxidant potential together with nutritional properties, the content of fatty acids, methylxanthines, proximal composition, and mineral content. Therefore, this study aims to describe the chemical fingerprinting by UHPLC–MS analysis and nutritional properties of cocoa waste products, namely, flours made from vein and pod husk (Figure 1) of a selected Peruvian ecotype of cocoa, evaluation of the antioxidant activity, and their potential as a food or food product.

Figure 1.
Pod husk and its flour (left) and vein and its flour (right).
2. Materials and Methods
2.1. Chemicals
Ultra-pure water (<5 µg/L TOC) was obtained from the water purification systems Arium 126 61316-RO, plus an Arium 611 UV unit (Sartorius, Goettingen, Germany). Formic acid (MS grade) and methanol (HPLC grade) were purchased from J.T. Baker (Phillipsburg, NJ, USA). Folin–Ciocalteu (FC) reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric chloride hexahydrate, 2,4,6-tris(2-pyridyl)-s-triazine, quercetin, gallic acid, Amberlite® resin (XAD4), phosphate buffer, trichloroacetic acid, ferric chloride, hydrochloric acid, ascorbic acid, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), gallic acid, potassium hexacyanoferrate(III), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and dimethylsulfoxide (DMSO) were obtained from Sigma-Aldrich; sodium carbonate, ferrous sulfate, sodium persulfate, sodium acetate, sodium sulfate anhydrous, and ethanol were obtained from Merck (Lima, Peru). HPLC standards (with purity higher than 95% by HPLC) were purchased from Sigma Aldrich Chem. Co. (St. Louis, MO, USA) or Extrasynthèse (Genay, France).
2.2. Plant Material
The cocoa fruits (Theobroma cacao), CCN-51 variety, were collected in October 2020 directly from the cocoa farm of Mr. Américo Hernández, located in the Pajonal zone, Pardo Miguel district, Rioja province, San Martín region, Peru (05°43’22.5” S and 77°29’20” W, 800 m altitude). The selected fresh cocoa fruits were taken to the laboratory in dark bags where they were washed, brushed, disinfected using a 200-ppm sodium hypochlorite solution for 30 min, and rinsed with water. Then, the pulp was removed, and the fruit opened, wherein the veins were removed, and the pod husks were separated. Then, thermal drying for both waste parts of cocoa fruits was carried out at 70 °C for 48 h. Finally, a blade mill was used (Grindomix GM 200), and the flours were milled using each part of waste and stored. The extract for HPLC analysis and biological activity was prepared by extracting the flour (500 mg), with 10 mL of HPLC-grade methanol with 1% formic acid for 10 min with sonication (3 times). Extractions were combined, the solvent was evaporated under vacuo and filtered thorough Whatman paper number 1, and the yellow gummy residue was obtained (19.5 mg for the vein extract (3.9%) and 12.7 mg for the pod (2.54%) and stored at –20 °C.
2.3. HPLC–MS Parameters
A Thermo Scientific Dionex Ultimate 3000 UHPLC machine that was connected by ESI II probe to a Thermo Q exactive spectrometer was used. For the analysis, 5 mg of the flour extract was dissolved in 2 mL of methanol and filtered (PTFE filter), and 10 μL was injected into the instrument. Liquid chromatography was performed using an UHPLC C18 column (Luna© Omega C18 100 Å, Phenomenex (150 mm × 2.1 mm ID, 1.6 µm)) operated at 30 °C. The detection wavelengths were 330, 254, 280, and 354 nm, and PDA from 200 to 800 nm for characterizing the peaks. Mobile phases were 1% formic aqueous solution (A) and 1% formic acid in acetonitrile (B). The gradient used was (0.00 min, 5% B); (1.00 min, 5% B); (25.00 min, 95% B); (26.00 min, 95% B); (30.00, 5% B); and 20 min for column equilibration before each injection. The flow rate was 0.3 mL/min, and the injection volume was 10 μL. Standards and the flour extract dissolved in methanol were kept at 10 °C during storage in the autosampler. The LC–MS and HESI II and Orbitrap spectrometer parameters were optimized as Full MS scan: AGC target: 5 × 106 resolution: 35,000, maximum IT: 80 ms, range: m/z 100–1500, microscans: 1, parameters for MS2 maximum IT: 100 ms, AGC target: 1 × 106, resolution: 17,500, ionization source parameters: ESI (positive and negative) spray volt: 3.5/2.5 KV, gas heater temp: 280/280 °C, capillary temperature: 260 °C, carrier gas: N2 (sheath gas flow rate: 48, sweep gas flow rate: 2, S-lens RF level: 100).
2.4. HPLC–DAD Analysis of Catechins and Methylxanthines
Catechins and methylxanthines by HPLC were carried out by means of a chromatographic analysis according to the protocol of Oliviero et al. [15] and Brunetto et al. [16], with some modifications. A chromatograph (Hitachi LaCrhom Elite®, Technologies America, Inc., Clarksburg, MD, USA) equipped with a vacuum degasser, a quaternary pump, and a diode-array detector (DAD), calibrated at 280 nm, was employed. The separation of (+)-catechin and (–)-epicatechin was carried out in an RP-18E column whose dimensions were 5 µm in particle size, 250 mm in length, and 4.6 mm in diameter. As mobile phase, methanol (A) acidified with 0.1% formic acid (B) was used, with elution gradients of 0.01 min 60% of A; 5–12 min 80% A; 13–14 min 60% of A. The flow rate of the mobile phase was 1.0 mL/min. The identification of the peaks was carried out by comparing with standards of (+)-catechin and (–)-epicatechin (Sigma-Aldrich®, St. Louis, MO, USA) and theobromine and caffeine standards (Sigma-Aldrich®, St. Louis, MO, USA). The theobromine and caffeine separation were carried out with the same column as above.
2.5. Determination of Proximate Composition
AOAC procedures were used in all determinations [17,18]. The water content was determined by oven-drying the sample up to a constant weight, the crude protein content by the Kjeldahl method (N × 6.25), the fiber content by gravimetric method after acidic hydrolysis of the samples, the total lipid extracted in a Soxhlet apparatus using petroleum ether as solvent, and the ash content by incineration in a muffle furnace at 550 ± 15 °C. Total carbohydrates were calculated as difference: 100 − (g water + g protein + g fiber + g fat + g ash). Results were expressed in grams per 100 g fresh weight (g/100 g fw). The experiments were carried out in triplicate.
2.6. Mineral Analysis
For the mineral analysis, the flours were dried to ash at 550 °C [18]. The ash in each case was boiled with 10 mL of 20% hydrochloric acid in a beaker, and then filtered into a 100 mL standard flask and made up to 100 mL with distilled deionized water. Levels of minerals, potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), zinc (Zn), manganese (Mn), copper (Cu), and iron (Fe) were determined from the resulting solution using atomic absorption spectroscopy (Varian AA240). The values obtained for each parameter are averages of three determinations for a given food sample.
2.7. Fatty Acid Profile
Fats were cold extracted using the Bligh and Dyer method [19]. Fatty acid profiles were obtained by gas chromatography of the fatty acid methyl ester derivatives. Derivatives were obtained by esterification with KOH in methanol (2 M). The fatty acid derivatives were extracted with hexane and analyzed through a Varian-450 gas chromatograph (Varian Inc., Palo Alto, CA, USA). The chromatograph was equipped with a VF-WAXms (60 m × 0.25 mm) capillary column and flame ionization detector (FID). Helium was used as the carrier gas, and the temperature program was as follows: 3 min at 130 °C, gradual heating to 220 °C for 9 min, 35 min at 220 °C, cooling to 130 °C, and 130 °C for 5 min. Individual peaks were identified by referring to a fatty acid methyl ester standard solution and analyzed under the same operation conditions [17].
2.8. Antioxidant Activity
2.8.1. DPPH Scavenging Activity
DPPH scavenging activity was determined by the method developed by Brand-Williams et al. [20]. To 3.9 mL of a solution of the DPPH• radical (100 μM) dissolved in 80% methanol, 0.1 mL of the extract (2 mg/mL), previously filtered on a membrane filter (0.45 μm), was added, and the mixture was stirred vigorously and set in the dark for 30 min at 25 °C. After that time, the absorbance at 517 nm was read in a UV-visible Cary60 spectrophotometer. The concentration of DPPH• in the reaction medium was obtained from a calibration curve by linear regression. The control consisted of 0.1 mL of 80% aqueous methanol and 3.9 mL of DPPH • solution (100 µM). The results are expressed in TEAC, that is, antioxidant activity equivalent to Trolox (μmol Trolox/g of extract). The reference synthetic antioxidant Trolox, at a concentration of 5–30 µM in 80% methanol solution, was tested under the same conditions.
2.8.2. ABTS Bleaching Capacity
ABTS bleaching capacity was determined by the method developed by Re et al. [21]. The reaction was started with the addition of 1500 μL of an ABTS•+ solution in PBS buffer (0.70 ± 0.02 at λ = 734 nm) to 500 μL of the extract (2 mg/mL) in a cuvette kept at 30 °C. It was homogenized and allowed to react for 7 min, and then the absorbance reading was made at a wavelength of 734 nm, using a Cary60 UV-visible spectrophotometer. The results are expressed in TEAC (μmol Trolox/g of extract). The calibration curve for TEAC was constructed using different concentrations of Trolox (4–14 µM) in PBS buffer solution under the same conditions.
2.8.3. Ferric-Reducing Antioxidant Power Assay (FRAP)
The ferric-reducing antioxidant power (FRAP) was determined according to the Benzie and Strain method [22]. A volume of 10 μL of the extract (2 mg/mL) was mixed with 90 μL of distilled water and 900 μL of the FRAP reagent (2.5 mL of the 2,4,6-tripyridyl-s-triazine solution at a concentration of 10 μM in HCl 40 mM; 2.5 mL of FeCl3 20 μM and 25 mL of acetate buffer 0.3 μM at a pH of 3.6). The absorbancy was read at 593 nm after 7 min in a UV-visible Cary60 spectrophotometer. The results are expressed in TEAC, that is, antioxidant activity equivalent to Trolox (μmol Trolox/g of extract).
2.8.4. Total Phenolic (TP) Content
The content of total phenols was estimated by a colorimetric method based on the procedures described by Velioglu et al. [23] with some modifications. In essence, 100 µL of the extract (2 mg/mL) was mixed with 750 µL of the Folin–Ciocalteu reagent diluted in a 1/10 proportion of Milli-Q water. After 5 min in the dark, 750 µL of sodium bicarbonate (60 g/L) was added to the mixture. The tubes were kept in the dark for 90 min at 30 °C, then the absorbance was read at 725 nm, using a Cary60 UV-visible spectrophotometer. Gallic acid (10–100 μg) was used for the construction of the standard curve. The results are expressed as milligrams of gallic acid/g extract.
2.9. Statistical Analysis
All the experiments were repeated at least three times. The results were expressed as mean ± standard deviation (SD) using GraphPad Prism 8. Comparison of results was performed using one-way analysis of variance (ANOVA), followed by Tukey’s HSD (honest significant difference) test (p < 0.05).
3. Results and Discussion
3.1. UHPLC–MS Analysis of Cocoa Extract
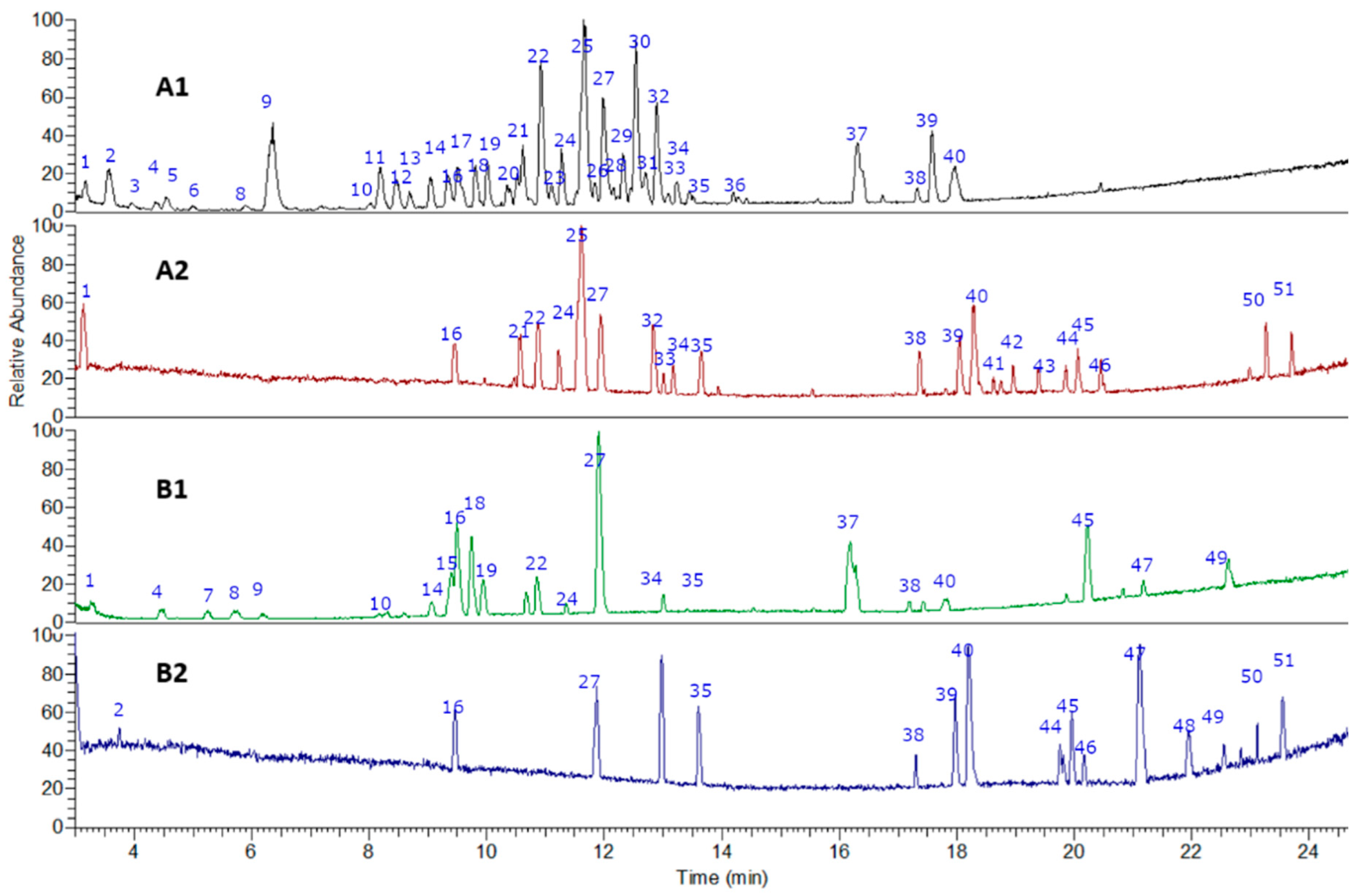
The fingerprinting of the flour from cocoa pod husk and vein were created and investigated by means of UHPLC–high-resolution MS with DAD analysis. The negative mode was used for the identification of phenolic compounds, while the positive mode was used for anthocyanins and methylxanthines. Some of the metabolites identified are reported for the first time in flours made from waste from this species. In total, 51 metabolites were detected and tentatively identified including phenolic acids, amino acids, anthocyanins, flavonoids, alkaloids, terpenes, and fatty acids (See Figure 2 and Figure 3 and Table 1). A detailed analysis is provided below.
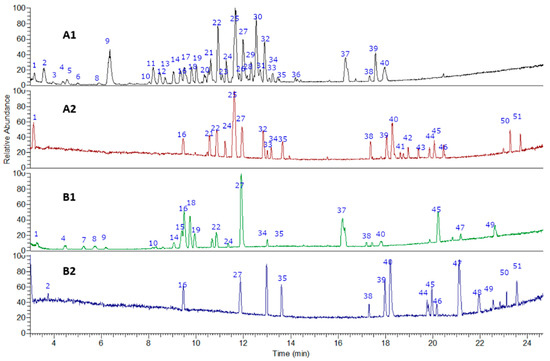
Figure 2.
UHPLC–PDA–ESI–OT–MS/MS chromatograms (TIC, total ion current) of (A1) pod husk flour negative mode, (A2) pod husk flour positive mode, (B1) vein flour negative mode, and (B2) vein flour positive mode.
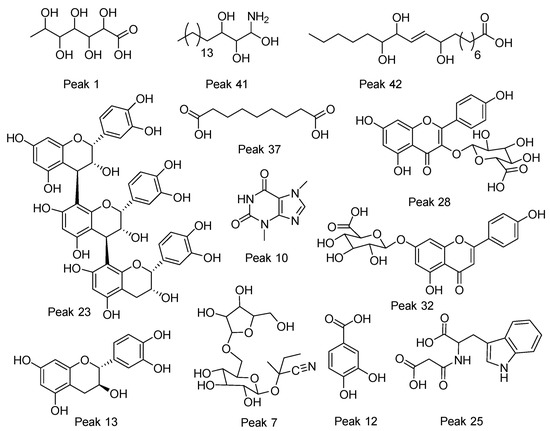
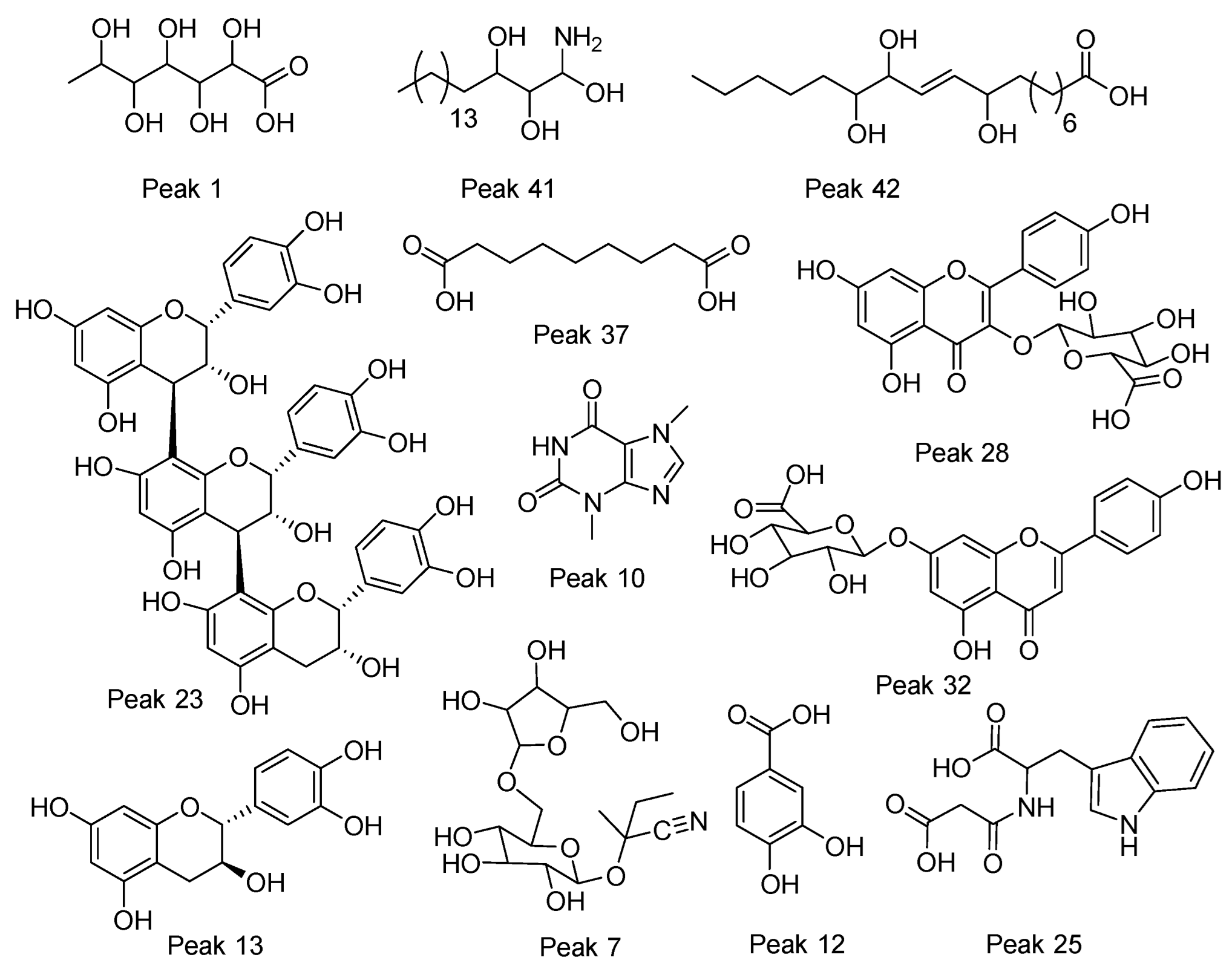
Figure 3.
Structures of some representative compounds in vein and pod husk flours of cocoa.

Table 1.
High resolution UHPLC–PDA-Q orbitrap identification of metabolites in vein and pod husk flours of cocoa.
3.1.1. Saturated Organic Acids
Peak 1 was identified as gluconic acid (C6H12O7), peak 2 as tartaric acid (C4H6O6), peak 5 as succinic acid (C4H6O4), peak 9 as 2-isopropylmalic acid (C7H12O5), peak 11 as citrate (C6H8O7), and peak 7 as 6’-apiosyllotaustralin (C16H27NO10). Peaks 15 and 17 were identified as isomers of everlastoside C (C16H30O10).
3.1.2. Fatty Acids or Derivatives
Peak 37 was identified as the dicarboxylic azelaic acid (C9H16O4), peaks 30 and 40 as the poliols heptadecasphinganine isomers, peak 41 as phytosphingosine, and peaks 42 and 45 as the isomers trihydroxyoctadecenoic acid (m/z 329.2336, C18H34O5). Peaks 46–51 were identified as stearic acid, oleic acid, araquidonic acid, linolenic acid, linoleic acid, and margaric acid, respectively. Their quantities are depicted in Table 2.

Table 2.
Proximal composition in flour from vein and pod husk of cocoa (%).
3.1.3. Procyanidins
Peak 27 was identified as procyanidin type B isomer 1 (C30H26O12), peak 13 as catechin, peak 20 as (-)-epicatechin (C15H14O6), peak 23 as procyanidin C1 (C45H38O18), and peak 35 as procyanidin type B (C30H26O12).
3.1.4. Flavonols
Peak 34 with a M-H ion at m/z 463.0863 and diagnostic MS ions at m/z 300.0276, 178.9982, and 161.0450 was identified as quercetin 3-O-glucoside (C21H20O12); peak 29 as quercetin 3-O-arabinose (C20H18O11); peak 32 as apigenin-7-O-glucuronide (C21H18O11); and peak 19 with a M-H ion at m/z 593.1520 and diagnostic c-glycosyl fragments at 503.1200, 473.1094, 383.0772, and 353.0670 as 6,8-C-dihexosylapigenin (C27H30O15). The same occurred with isomer compound peaks 21 and 22, 8-C-Glucosyl-6-C-arabinosylapigenin and 6-C-Glucosyl-8-C-arabinosylapigenin, respectively (C26H28O14). Peaks 28 and 33 were devoided as kaempferol 3-glucuronide (C27H18O12) and kaempferol 3-O-pentoside (C20H18O10), respectively.
3.1.5. Phenolic Acids
Peak 3 with a M-H ion at m/z 329.0883 and diagnostic ions at m/z 167.0345, 152.0109, and 123.0444 was identified as vanillic acid 4-hexoside (C14H18O9); in the same manner, peak 4 as 1-O-syringoyl-glucopyranose (C15H20O10), peak 12 as protocatechuic acid (C7H6O4), peak 14 as benzyl O-[pentosyl-hexoside] (C18H26O10), and peak 36 as gentisic acid 5-O-hexoside (C13H16O9) showing ions at 165.0187, 152.0109, 108.0208, and 85.0285.
3.1.6. Amino Acids
Peak 18 was identified as the amino acid derivative methoxytyrosine (C10H13NO4); in the same way, peaks 25 and 26 were regarded as malonyltryptophan (C14H14N2O5) and N-acetyltryptophan (C13H14N2O3), respectively.
3.1.7. Alkaloids
Peaks 10 and 16 were identified as theobromine (C7H8N4O2) and caffeine (C8H10N4O2), respectively.
3.2. Chemical Composition and Nutritional Properties of Flours
The chemical composition and nutritional properties of flours were performed according to previous methodologies [15,16,17,19]. This included proximal analysis (Table 2), mineral content (Table 3), and content of fatty acids (Table 4). Table 2 shows proximal composition such as the humidity, ashes, protein, lipids, carbohydrates, and fiber, while Table 3 shows the mineral contents of two flours for cocoa waste material. The results of physicochemical properties showed that the proximal composition and the caloric value of these flours made of cocoa waste had a high fiber content that is good for use as a supplement (35.48 ± 1.47 and 7.26 ± 0.17% in pod husk and vein flour, respectively) and carbohydrate content (41.89 and 57.96%, respectively), which make them two highly caloric flours. The composition showed differences with other flours derived from foods, such as banana flour (Musa paradisiacal L.), which showed total starch of 73.36% and dietary fiber of 14.52% [24], while in buckwheat, for instance, in the flour, these values were dietary fiber: 6.77%, ashes: 1.82%, starch: about 78.4%, protein: 10%, and lipid content: 2%, but in its bran, starch: 40.7%, ashes: 4%, protein: above 21%, and lipid content: around 7% [25]. Soy flours collected at Cedar Rapids showed protein at 52%, fat at 0.8%, and ashes at 6.31% [26], while a group of flours obtained from the species of Prosopis showed protein: 7.17–11.2%, crude fiber: 2.7–3.4%, and carbohydrates: all around 8.2% [27]. The composition of cocoa pod husk in the literature showed great variability; however, our results are in accordance with those reported by Mariatti et al. [28]. Briefly, ashes: 5.9–13.0 (8.62%), lipids: 0.6–4.7 (0.51%), proteins: 2.9–9.1 (8.50%), fiber: 18.3–59.0 (35.48%), and carbohydrates: 17.4–47.0 (41.89%) (Table 2). The flours were analyzed for mineral content (Ca, Na, K, Mg, Cu, Mn, Zn, and Fe) and were high in K (112.04 and 54.03 mg K/100 g for pod husk and vein, respectively) and low in sodium (0.47 and 0.09 mg Na/100 g for pod husk and vein, respectively), which is also good for hypertensive people. The pod husk had the highest contents of Mg, Ca, Mn, Zn, and Fe (Table 3). The content of Ca makes the flour a good supplement for bones. The cocoa pod husks have been studied for use as fertilizers due to their high mineral content [2]. The fatty acid profile of the flours was investigated using a standardized protocol [19]. Saturated fatty acids were 44.99% and 39.40% in vein and pod husk flours, respectively (Table 4). While the pod husk showed more polyunsaturated fatty acids (52.54%), the vein showed more monounsaturated fatty acids (37.11%), and thus the pod husk flour has more healthy fatty acids. Indeed, the pod husk is richest in dietary poly-UFAs, and it is more suitable as a food or food supplement for its nutritional values. Because of its high values in proteins, crude fats, fibers, and mineral levels, cocoa pod husk has been widely explored as feed for poultry and/or animals [3]. Moreover, the main methylxanthine alkaloid compounds: theobromine, caffeine, and the two main catechins were quantified, and the results are shown in Table 5. The pod husk showed the highest content of theobromine (10.21 µg/g), while the vein showed the highest content of caffeine, epicatechin, and catechin (1.11 µg/g, 3.40 µg/g, and 3.09 mg/g, respectively; Table 5).

Table 3.
Mineral content (mg/100 g) in flour from vein and pod husk of cocoa.

Table 4.
Fatty acids profile in flour from vein and pod husk of cocoa (%).

Table 5.
Main compound quantitative analyses by HPLC in flour from vein and pod husk of cocoa.
3.3. Antioxidant Activity and Total Polyphenol Content
The antioxidant capacity cannot be fully described by only one method; thus, for this study, we employed three different complementary antioxidant methods (ABTS, DPPH, and FRAP) that have been applied to the flours in addition to the phenolic content measured by spectrophotometry. It has been reported that the total phenolic content of cocoa varies on the basis of the growing region and extraction solvent technique [29]. Table 6 shows the antioxidant capacities of the three methods used in this study. The DPPH antiradical activity as well as the reducing power of the flours can serve as a significant indicator of their potential antioxidant activity. For this reason, the reducing power of ferric ions and total polyphenol content was also examined in the two cocoa waste flours (Table 6). The FRAP of the flour from the vein exhibited a weak reducing power compared to the pod husk, but the total phenolic content (111.05 mg GAE/g flour) was higher than those reported for cocoa pod husk flour (CPHF) criollo variety (5.4 to 16.6 mg GAE/g flour) [30]. This difference may have been due to the location growth of cocoa, variety, and the solvent system used in the extraction of phenolics [29]. To show some comparison, antioxidant properties of refined and whole wheat flour by the oxygen radical absorbance capacity (ORAC) test for refined wheat flours ranged from 10.88 to 14.38 µmol TE/g (mean 12.52 µmol TE/g) while showing significantly lower values compared to their whole wheat flour counterparts, which ranged from 27.93 to 44.33 µmol TE/g (mean 35.74 µmol TE/g) from the same brand [31]. In addition the DPPH content reported for those wheat flours ranged among 4–5 μmol equivalent of Trolox/g [31], which is lower than our cocoa waste flours (46 and 87 μmol equivalent of Trolox/g for the vein and husk flours, respectively) (Table 6).

Table 6.
Antioxidant activity of vein and pod husk flours of cocoa.
Furthermore, our values for the TPC (Table 6) were higher than those reported for the TPC of cocoa shell hydroalcoholic extract (51.9 mg/g) [32] and lower than those obtained by Amin et al. (113 mg/g extract) who used ethanol as an extraction solvent [33]. Our DPPH activity was also higher (Table 6) than that reported by Grillo et al. (83.1 ± 5.3 mg/mL) [32] and is in accordance with that reported by Delgado-Ospina et al. (36 to 133 µmol Trolox/g) [30]. Furthermore, strong correlation was found between total phenolic and the three antioxidant assays DPPH (r = 0.9995, p < 0.001), ABTS (r = 0.9970, p < 0.0001), and FRAP (r = 0.9988, p < 0.0001).
In the flours, the antioxidant compound protocatechuic acid, PCA (3,4-dihydroxy benzoic acid), was detected; this is one of the main metabolites produced by anthocyanins and proanthocyanins and has been shown to possess antioxidant activity in vitro and in vivo [34]. In addition, catechin, epicatechin, and procyanidins detected in the flours are present in chocolate made of cocoa and considered the main phenolics in it (30%), being directly related to its antioxidant capacity [35]. The flavanols detected in cocoa food products (such as apigenin-7-O-glucuronide, quercetin 3-O-glucoside, and kaempferol 3-O-pentoside) are also responsible for the antioxidant activity of those products [36]. On the other hand, dietary-important unsaturated and saturated fatty acids were also detected, and saturated ones were measured by GC-FID (Table 4). In the cocoa flours, it was shown that chocolates showed a high concentration of saturated fatty acids, mainly stearic acid (18:0), and palmitic acid (16:0), followed by the unsaturated fatty acids, among which linoleic acid (18:6) and oleic acid (18:1n-9) were the more concentrated [37], having been very important in stopping the development of coronary diseases and high blood pressure [38].
4. Conclusions
In this study, two flours made of the vein and pod husk from a special ecotype of Peruvian cocoa (CCN-51) were investigated regarding their potential as a food or food supplement and their antioxidant capacities. Different compounds were detected in the two flours including methylxanthines, catechin, flavonoids, fatty acids, amino acids, phenolic acids, and other common acids. The principal methylxanthines and catechins were quantified, and the content of fatty acids was quantified individually for each waste material, making it a standardized food waste material. Proximal composition and mineral content were analyzed for the first time in these by-products, and the findings of physicochemical characteristics revealed that these flours generated from cocoa waste had a significant fiber and carbohydrate content, making it an energetic and healthy product. The mineral content (Ca, Na, K, Mg, Cu, Mn, Zn, and Fe) of these flours is shown to be a rich source of potassium and low in sodium, which is good for people suffering from high blood pressure. The pod husk had the highest contents of main dietary minerals, and therefore is better for this purpose. Flours made of cocoa waste have good nutritional properties and can be a good source of dietary phenolic compounds, which are essential for the preparation of nutraceuticals or food supplements. More biological tests and more analyses are necessary to test the health potential of these waste flours.
Author Contributions
Conceptualization, G.V.-A. and M.J.S.; methodology, validation, G.V.-A., C.M.-Z. and M.T.; formal UHPLC–PDA–ESI–OT–MS analysis, M.J.S. and M.W.P.; in vitro antioxidant assays, G.V.-A., C.M.-Z. and M.T.; resources, G.V.-A. and M.J.S.; writing—original draft preparation, M.W.P., G.V.-A. and M.J.S.; writing—review and editing, G.V.-A. and M.J.S.; supervision and project administration, G.V.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Budget Program 130: Competitiveness and sustainable use of forest resources and wildlife. COD. CEPLAN: AOI00005300007. M.J.S acknowledge funding from FONDECYT 1220075. G.V.-A. and M.J.S. are members of the CYTED network “P320RT0186—Aprovechamiento sostenible de recursos biomásicos vegetales iberoamericanos en cosmética” (BIOLATES).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article. Raw HPLC data or other data can be available on the authors’ request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rojo-Poveda, O.; Zeppa, G.; Ferrocino, I.; Stévigny, C.; Barbosa-Pereira, L. Chemometric Classification of Cocoa Bean Shells Based on Their Polyphenolic Profile Determined by RP-HPLC-PDA Analysis and Spectrophotometric Assays. Antioxidants 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, Z.S.; Neto, D.P.C.; Pereira, G.V.M.; Vandergberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Neto, A.G.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and its by-products: Identification and utilization. In Chocolate in Health and Nutrition; Watson, R., Preedy, V., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 7, pp. 23–37. [Google Scholar] [CrossRef]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Pérez, L.A.; Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Artisanal cocoa bean fermentation: From cocoa bean proteins to bioactive peptides with potential health benefits. J. Funct. Foods 2020, 73, 104134. [Google Scholar] [CrossRef]
- Keen, C.L.; Holt, R.R.; Oteiza, P.I.; Fraga, C.G.; Schmitz, H.H. Cocoa antioxidants and cardiovascular health. Am. J. Clin. Nutr. 2005, 81, 298S–303S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, M.Á.; Ramos, S. Health beneficial effects of cocoa phenolic compounds: A mini-review. Curr. Opin. Food Sci. 2017, 14, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Oyeleke, S.A.; Ajayi, A.M.; Umukoro, S.; Aderibigbe, A.O.; Ademowo, O.G. Anti-inflammatory activity of Theobroma cacao L. stem bark ethanol extract and its fractions in experimental models. J. Ethnopharmacol. 2018, 222, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cambrai, A.; Marchioni, E.; Julien-David, D.; Marcic, C. Discrimination of Cocoa Bean Origin by Chocolate Polyphenol Chromatographic Analysis and Chemometrics. Food Anal. Methods 2017, 10, 1991–2000. [Google Scholar] [CrossRef]
- Peláez, P.; Bardón, I.; Camasca, P. Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci. Agropecu. 2016, 7, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and quantification of free and bound phenolic compounds contained in the high-molecular weight melanoidin fractions derived from two different types of cocoa beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Santos, H.M.; Coutinho, J.P.; Lôbo, I.P.; da Silva Junior, A.L.S.; Santos, A.G.; de Jesus, R.M. Optimization of chromatographic separation and classification of artisanal and fine chocolate based on its bioactive compound content through multivariate statistical techniques. Microchem. J. 2020, 152, 104342. [Google Scholar] [CrossRef]
- Bartella, L.; Di Donna, L.; Napoli, A.; Siciliano, C.; Sindona, G.; Mazzotti, F. A rapid method for the assay of methylxanthines alkaloids: Theobromine, theophylline and caffeine, in cocoa products and drugs by paper spray tandem mass spectrometry. Food Chem. 2019, 278, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Capuano, E.; Cämmerer, B.; Fogliano, V. Influence of Roasting on the Antioxidant Activity and HMF Formation of a Cocoa Bean Model Systems. J. Agric. Food Chem. 2009, 57, 147–152. [Google Scholar] [CrossRef]
- Del Rosario Brunetto, M.; Gutiérrez, L.; Delgado, Y.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G.; Romero, C. Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chem. 2007, 100, 459–467. [Google Scholar] [CrossRef]
- Darnet, S.H.; da Silva, L.H.M.; da Rodrigues, A.M.C.; Lins, R.T. Nutritional composition, fatty acid and tocopherol contents of buriti (Mauritia flexuosa) and patawa (Oenocarpus bataua) fruit pulp from the amazon region. Ciência Tecnol. Aliment. 2011, 31, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Arana, G.; Merino-Zegarra, C.; Riquelme-Penaherrera, M.; Nonato-Ramirez, L.; Delgado-Wong, H.; Pertino, M.W.; Parra, C.; Simirgiotis, M.J. Antihyperlipidemic and antioxidant capacities, nutritional analysis and uhplc-pda-ms characterization of cocona fruits (Solanum sessiliflorum dunal) from the peruvian amazon. Antioxidants 2021, 10, 1566. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrinia, N.; Proteggente, A.; Pannalaa, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Juarez-Garcia, E.; Agama-Acevedo, E.; Sáyago-Ayerdi, S.G.; Rodríguez-Ambriz, S.L.; Bello-Pérez, L.A. Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum. Nutr. 2006, 61, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Porter, M.A.; Jones, A.M. Variability in soy flour composition. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 557–562. [Google Scholar] [CrossRef]
- Felker, P.; Takeoka, G.; Dao, L. Pod Mesocarp Flour of North and South American Species of Leguminous Tree Prosopis (Mesquite): Composition and Food Applications. Food Rev. Int. 2013, 29, 49–66. [Google Scholar] [CrossRef]
- Mariatti, F.; Gunjević, V.; Boffa, L.; Cravotto, G. Process intensification technologies for the recovery of valuable compounds from cocoa by-products. Innov. Food Sci. Emerg. Technol. 2021, 68, 102601. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Lucas-González, R.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A.; Martuscelli, M.; Chaves-López, C. Bioactive compounds and techno-funtional properties of high-fiber co-products of the cacao agro-industrial chain. Heliyon 2021, 7, e06799. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Yee, C. Antioxidative effects of extracts of cocoa shell, roselle seeds and a combination of both extracts on the susceptibility of cooked beef to lipid oxidation. J. Food Technol. 2006, 4, 10–15. [Google Scholar]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid.-Based Complement. Alternat. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.; Miller, K.B.; Payne, M.J.; Hurst, W.J.; Stuart, D.A. Comparison of antioxidant activity and flavanol content of cacao beans processed by modern and traditional Mesoamerican methods. Herit. Sci. 2013, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- De Melo, C.W.B.; de Bandeira, M.J.; Maciel, L.F.; da Bispo, E.S.; de Souza, C.O.; Soares, S.E. Chemical composition and fatty acids profile of chocolates produced with different cocoa (Theobroma cacao L.) cultivars. Food Sci. Technol. 2020, 40, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).