Fuzhuan Brick Tea Boosts Melanogenesis and Prevents Hair Graying through Reduction of Oxidative Stress via NRF2-HO-1 Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Preparation of Tea Extract and HPLC Analysis
2.3. Cell Culture and Cell Viability Assay
2.4. Cellular Melanin Contents in Melan-A Cells
2.5. Zymography Analysis of Intracellular Tyrosinase
2.6. Reverse-Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.7. Western Blot Analysis
2.8. Animals and Care
2.9. Hydroquinone-Induced Grey Hair Model
2.10. Melanin Content Analysis in Hair Shaft
2.11. Fontana–Masson Staining
2.12. Image Analyses and Quantification
2.13. Statistical Analysis
3. Results
3.1. HPLC Analysis of FBTH
3.2. Attenuation of Cellular Oxidative Stress by FBTH
3.3. Effects of FBTH on Antioxidant Enzyme Expression in Melan-A Cells
3.4. Effects of the FBTH on Melanogenesis in Melan-A Cells
3.5. Effect of FBTH on the Expression of Melanogenesis-Related Proteins
3.6. Effects of FBTH on the Melanogenesis-Associated Signaling Pathways
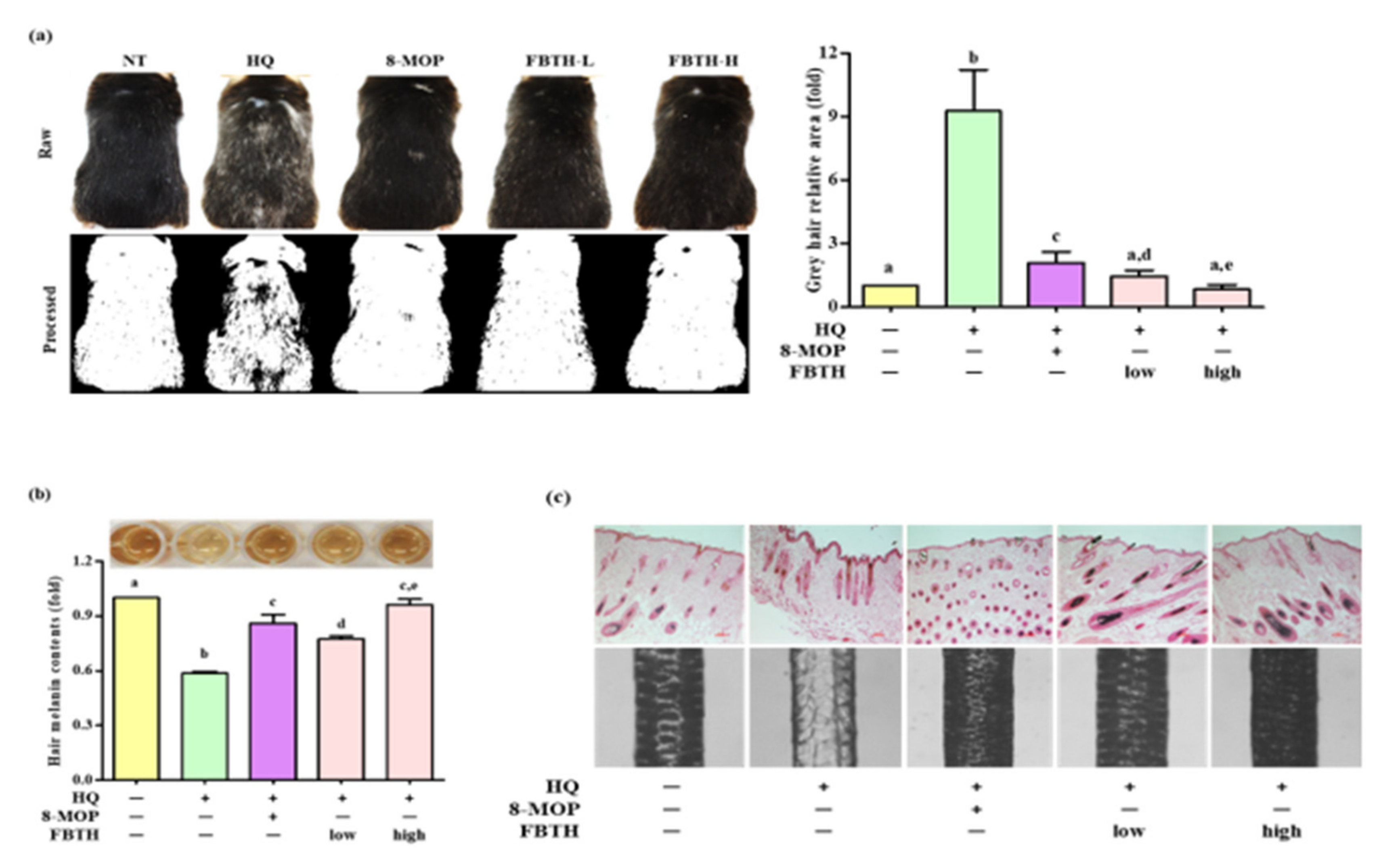
3.7. Topical Application of FBTH Improves Hydroquinone-Induced Hair Graying in C57BL/6 Mice
3.8. Effects of FBTH on Antioxidant Enzyme Expression in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acer, E.; Kaya Erdoğan, H.; İğrek, A.; Parlak, H.; Saraçoğlu, Z.N.; Bilgin, M. Relationship between diet, atopy, family history, and premature hair graying. J. Cosmet. Dermatol. 2019, 18, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, S.; Rachmin, I.; He, M.; Baral, P.; Choi, S.; Gonçalves, W.A.; Shwartz, Y.; Fast, E.M.; Su, Y.; et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 2020, 577, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, D.J. Aging of the hair follicle pigmentation system. Int. J. Trichol. 2009, 1, 83–93. [Google Scholar] [CrossRef]
- Arck, P.C.; Overall, R.; Spatz, K.; Liezman, C.; Handjiski, B.; Klapp, B.F.; Birch-Machin, M.A.; Peters, E.M. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006, 20, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.K.; Granter, S.R.; Fisher, D.E. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science 2005, 307, 720–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslam, I.S.; Jadkauskaite, L.; Szabó, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.L.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.; Kim, M.M. H2O2 promotes the aging process of melanogenesis through modulation of MITF and Nrf2. Mol. Biol. Rep. 2019, 46, 2461–2471. [Google Scholar] [CrossRef]
- Mo, H.; Zhu, Y.; Chen, Z. Microbial fermented tea—A potential source of natural food preservatives. Trends Food Sci. Technol. 2008, 19, 124–130. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Z.; Liu, Z.; Huang, J.; Wen, Q.; Zhou, X.; Zhu, S. Discussion on the mechanism of quality and flavor formation of Fuzhuan brick tea. J. Tea Sci. 1991, 11, 49–55. [Google Scholar]
- Zhu, M.-Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.-M.; Xu, W.; Li, J.; Lin, H.-Y.; Zhang, Z.; Xiao, J.-B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Alam, M.B.; Lee, S.H. Protection of UVB-Induced Photoaging by Fuzhuan-Brick Tea Aqueous Extract via MAPKs/Nrf2-Mediated Down-Regulation of MMP-1. Nutrients 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Zhonghua, L. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef]
- Xiang, X.; Xiang, Y.; Jin, S.; Wang, Z.; Xu, Y.; Su, C.; Shi, Q.; Chen, C.; Yu, Q.; Song, C. The hypoglycemic effect of extract/fractions from Fuzhuan Brick-Tea in streptozotocin-induced diabetic mice and their active components characterized by LC-QTOF-MS/MS. J. Food Sci. 2020, 85, 2933–2942. [Google Scholar] [CrossRef]
- Alam, M.B.; Bajpai, V.K.; Lee, J.; Zhao, P.; Byeon, J.H.; Ra, J.S.; Majumder, R.; Lee, J.S.; Yoon, J.I.; Rather, I.A.; et al. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-Kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci. Rep. 2017, 7, 45858. [Google Scholar] [CrossRef]
- Zhao, P.; Alam, M.B.; An, H.; Choi, H.J.; Cha, Y.H.; Yoo, C.Y.; Kim, H.H.; Lee, S.H. Antimelanogenic Effect of an Oroxylum indicum Seed Extract by Suppression of MITF Expression through Activation of MAPK Signaling Protein. Int. J. Mol. Sci. 2018, 19, 760. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.B.; Ju, M.K.; Lee, S.H. DNA Protecting Activities of Nymphaea nouchali (Burm. f) Flower Extract Attenuate t-BHP-Induced Oxidative Stress Cell Death through Nrf2-Mediated Induction of Heme Oxygenase-1 Expression by Activating MAP-Kinases. Int. J. Mol. Sci. 2017, 18, 2069. [Google Scholar] [CrossRef] [Green Version]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Song, Z.; Setaluri, V. Oxidative stress and vitiligo: The Nrf2-ARE signaling connection. J. Investig. Dermatol. 2014, 134, 2074–2076. [Google Scholar] [CrossRef] [Green Version]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental pollution, toxicity, and microbial answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Li, S.; Chen, X.; Zhang, W.; Chang, Y.; He, Y.; Zhang, S.; Su, X.; Gao, T.; Li, C.; et al. Berberine protects immortalized line of human melanocytes from H2O2-induced oxidative stress via activation of Nrf2 and Mitf signaling pathway. J. Dermatol. Sci. 2019, 94, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Sextius, P.; Betts, R.; Benkhalifa, I.; Commo, S.; Eilstein, J.; Massironi, M.; Wang, P.; Michelet, J.F.; Qiu, J.; Tan, X.; et al. Polygonum multiflorum Radix extract protects human foreskin melanocytes from oxidative stress in vitro and potentiates hair follicle pigmentation ex vivo. Int. J. Cosmet. Sci. 2017, 39, 419–425. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Zhang, D.D.; Wondrak, G.T. Topical Bixin Confers NRF2-Dependent Protection against Photodamage and Hair Graying in Mouse Skin. Front. Pharmacol. 2018, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, N.; Hata, T.; Kamiya, E.; Homma, T.; Kobayashi, A.; Aoki, H.; Kunisada, T. Eriodictyon angustifolium extract, but not Eriodictyon californicum extract, reduces human hair greying. Int. J. Cosmet. Sci. 2020, 42, 336–345. [Google Scholar] [CrossRef]
- Emerit, I.; Filipe, P.; Freitas, J.; Vassy, J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem. Photobiol. 2004, 80, 579–582. [Google Scholar] [CrossRef]
- Seiberg, M. Age-induced hair greying—The multiple effects of oxidative stress. Int. J. Cosmet. Sci. 2013, 35, 532–538. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Y.; Deng, J.; Chen, M.; Lu, Y.; Wang, Y.; Yao, H.; Zhou, L.; Liu, Z.; Lai, L.; et al. CRISPR/Cas9-mediated mutation of tyrosinase (Tyr) 3’ UTR induce graying in rabbit. Sci. Rep. 2017, 7, 1569. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, T.; Silversides, D.W.; Waymire, K.G.; Kwon, B.S.; Takeuchi, T.; Overbeek, P.A. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 1990, 18, 7293–7298. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Yue, Y.; Deng, X.Y.; Huang, B.; Guo, Z.M.; Ma, Y.; Lin, Y.L.; Hong, X.; Tang, H.; Xu, K.; et al. Rescue of the albino phenotype by introducing a functional tyrosinase minigene into Kunming albino mice. World J. Gastroenterol. 2007, 13, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Jackson, I.J. Light is a dominant mouse mutation resulting in premature cell death. Nat. Genet. 1992, 1, 226–229. [Google Scholar] [CrossRef]
- Guyonneau, L.; Murisier, F.; Rossier, A.; Moulin, A.; Beermann, F. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Mol. Cell. Biol. 2004, 24, 3396–3403. [Google Scholar] [CrossRef] [Green Version]
- Michard, Q.; Commo, S.; Belaidi, J.P.; Alleaume, A.M.; Michelet, J.F.; Daronnat, E.; Eilstein, J.; Duche, D.; Marrot, L.; Bernard, B.A. TRP-2 specifically decreases WM35 cell sensitivity to oxidative stress. Free Radic. Biol. Med. 2008, 44, 1023–1031. [Google Scholar] [CrossRef]
- Harris, M.L.; Fufa, T.D.; Palmer, J.W.; Joshi, S.S.; Larson, D.M.; Incao, A.; Gildea, D.E.; Trivedi, N.S.; Lee, A.N.; Day, C.P.; et al. A direct link between MITF, innate immunity, and hair graying. PLoS Biol. 2018, 16, e2003648. [Google Scholar] [CrossRef]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim. Biophys. Acta 1991, 1073, 85–90. [Google Scholar] [CrossRef]
- Huo, S.X.; Wang, Q.; Liu, X.M.; Ge, C.H.; Gao, L.; Peng, X.M.; Yan, M. The Effect of Butin on the Vitiligo Mouse Model Induced by Hydroquinone. Phytother. Res. PTR 2017, 31, 740–746. [Google Scholar] [CrossRef]
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar]
- Yamaoka, Y.; Ohguchi, K.; Itoh, T.; Nozawa, Y.; Akao, Y. Effects of theaflavins on melanin biosynthesis in mouse b16 melanoma cells. Biosci. Biotechnol. Biochem. 2009, 73, 1429–1431. [Google Scholar] [CrossRef] [Green Version]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Yoshiyuki Osamura, R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004, 17, 66–73. [Google Scholar] [CrossRef]
- Takekoshi, S.; Matsuzaki, K.; Kitatani, K. Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J. Exp. Clin. Med. 2013, 38, 129–134. [Google Scholar]
- Park, W.S.; Kwon, O.; Yoon, T.J.; Chung, J.H. Anti-graying effect of the extract of Pueraria thunbergiana via upregulation of cAMP/MITF-M signaling pathway. J. Dermatol. Sci. 2014, 75, 153–155. [Google Scholar] [CrossRef]
- Saha, B.; Singh, S.K.; Mallick, S.; Bera, R.; Datta, P.K.; Mandal, M.; Roy, S.; Bhadra, R. Sphingolipid-mediated restoration of Mitf expression and repigmentation in vivo in a mouse model of hair graying. Pigment Cell Melanoma Res. 2009, 22, 205–218. [Google Scholar] [CrossRef]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 2006, 19, 595–605. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Chu, J.H.; Chou, G.X.; Yu, Z.L. Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp. Dermatol. 2011, 20, 755–757. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jin, S.H.; Kang, H.Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch. Dermatol. Res. Arch. Dermatol. Forsch. 2008, 300, 325–329. [Google Scholar] [CrossRef]






| Groups | Reagent | Treatments |
|---|---|---|
| A (NT) | Vehicle | / |
| B (Control) | HQ, 200 mg/kg/day | / |
| C (Positive control) | HQ, 200 mg/kg/day | 8-MOP, 4.5 mg/kg/day |
| D (Sample low) | HQ, 200 mg/kg/day | FBTH-L, 50 mg/kg/day |
| E (Sample high) | HQ, 200 mg/kg/day | FBTH-H, 100 mg/kg/day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Park, N.H.; Alam, M.B.; Lee, S.-H. Fuzhuan Brick Tea Boosts Melanogenesis and Prevents Hair Graying through Reduction of Oxidative Stress via NRF2-HO-1 Signaling. Antioxidants 2022, 11, 599. https://doi.org/10.3390/antiox11030599
Zhao P, Park NH, Alam MB, Lee S-H. Fuzhuan Brick Tea Boosts Melanogenesis and Prevents Hair Graying through Reduction of Oxidative Stress via NRF2-HO-1 Signaling. Antioxidants. 2022; 11(3):599. https://doi.org/10.3390/antiox11030599
Chicago/Turabian StyleZhao, Peijun, Na Hyun Park, Md Badrul Alam, and Sang-Han Lee. 2022. "Fuzhuan Brick Tea Boosts Melanogenesis and Prevents Hair Graying through Reduction of Oxidative Stress via NRF2-HO-1 Signaling" Antioxidants 11, no. 3: 599. https://doi.org/10.3390/antiox11030599
APA StyleZhao, P., Park, N. H., Alam, M. B., & Lee, S.-H. (2022). Fuzhuan Brick Tea Boosts Melanogenesis and Prevents Hair Graying through Reduction of Oxidative Stress via NRF2-HO-1 Signaling. Antioxidants, 11(3), 599. https://doi.org/10.3390/antiox11030599







