Anti-Inflammatory and Antioxidant Chinese Herbal Medicines: Links between Traditional Characters and the Skin Lipoperoxidation “Western” Model
Abstract
:1. Introduction
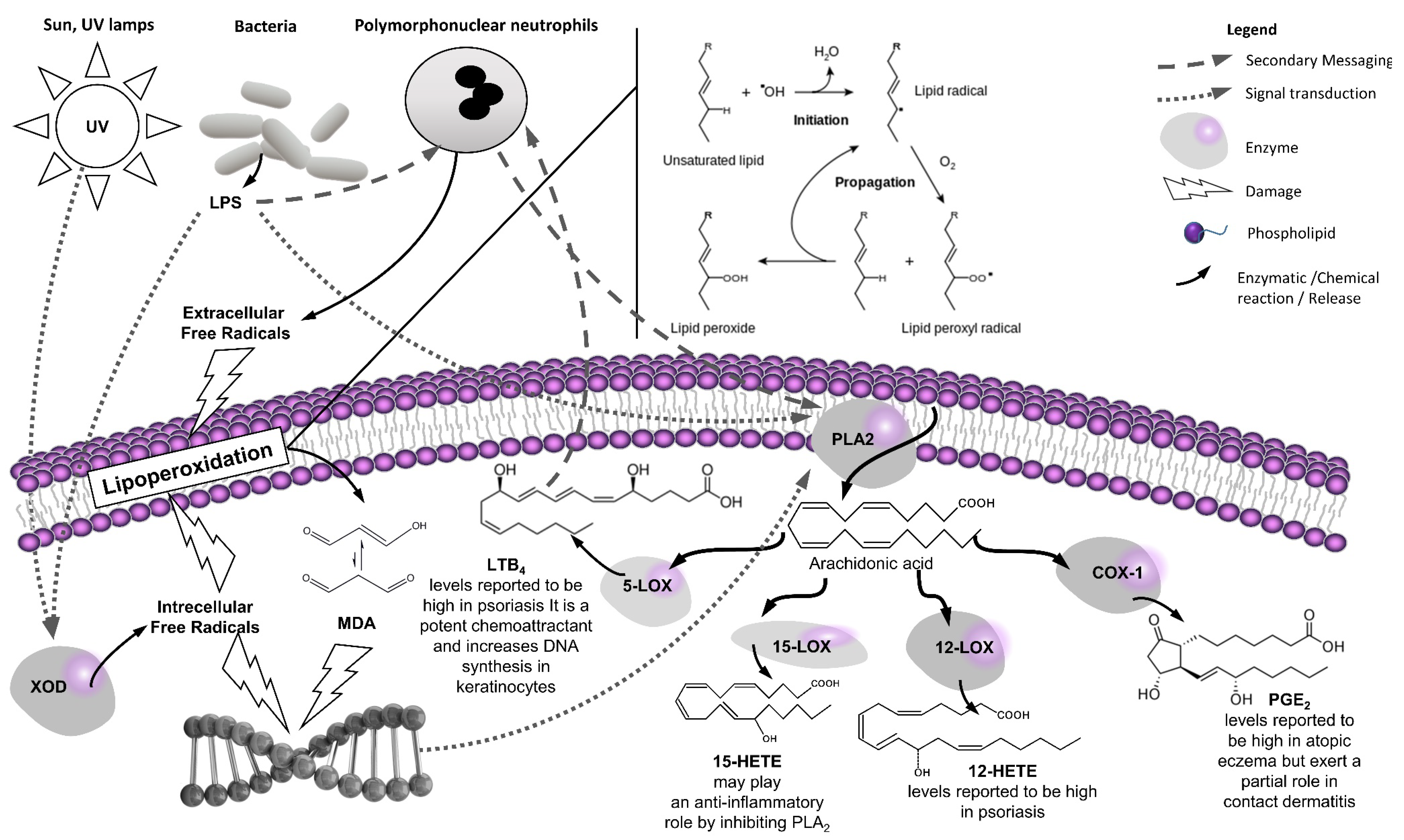
1.1. Introduction to the “Western” Skin Lipoperoxidation Model
1.2. Introduction to the “Eastern” Skin Inflammation Model
1.3. Therapeutic Opportunities at the Western–Eastern Interface
2. A Focused Review on the Anti-Inflammatory (Eicosanoid Inhibition) and Antioxidant (Lipoperoxidation) Properties of the Selected Medicinal Plants
2.1. Methods
2.2. Angelica dahurica
2.3. Angelica pubescens
2.4. Angelica sinensis
2.5. Astragalus membranaceus
2.6. Atratylodes macrocephala
2.7. Codonopsis pilosula
2.8. Coptis chinensis
2.9. Curcuma aromatica
2.10. Forsythia suspensa
2.11. Lentinus edodes
2.12. Paeonia lactiflora
2.13. Phellodendron amurense
2.14. Poria cocos
2.15. Rehmannia glutinosa
2.16. Scutellaria baicalensis
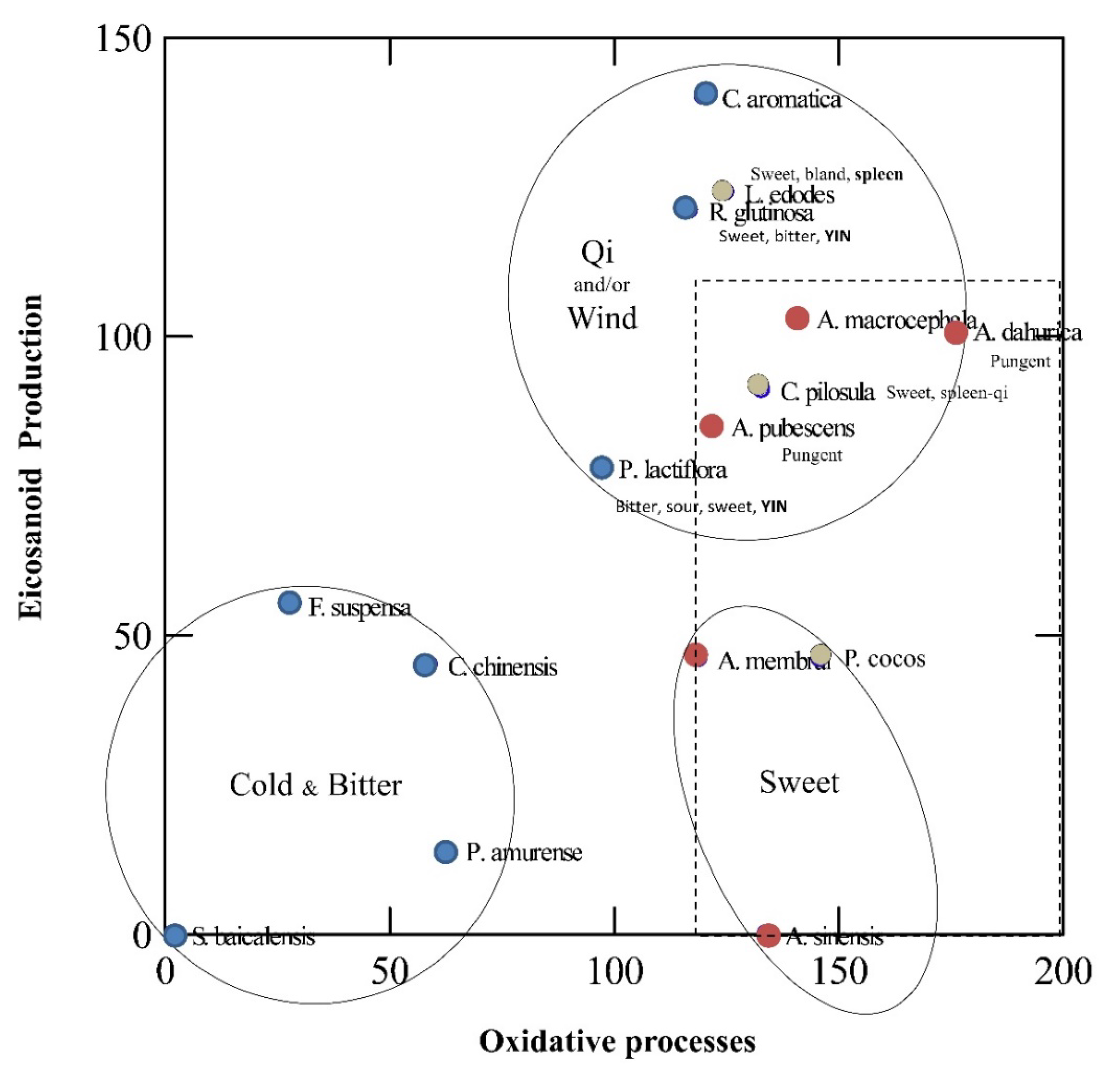
3. Unveiling Links between Traditional Chinese Plant Characters and Quantitative Antioxidant/Eicosanoid Inhibitory Activities of the Extracts
3.1. Data Sourcing
3.2. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simpson, E.L. Atopic dermatitis: A review of topical treatment options. Curr. Med. Res. Opin. 2010, 26, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speeckaert, R.; Dugardin, J.; Lambert, J.; Lapeere, H.; Verhaeghe, E.; Speeckaert, M.M.; van Geel, N. Critical appraisal of the oxidative stress pathway in vitiligo: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Antioxidative Phytochemicals Accelerate Epidermal Terminal Differentiation via the AHR-OVOL1 Pathway: Implications for Atopic Dermatitis. Acta Derm. Venereol. 2018, 98, 918–923. [Google Scholar] [CrossRef] [Green Version]
- Umamaheswaran, S.; Dasari, S.K.; Yang, P.; Lutgendorf, S.K.; Sood, A.K. Stress, inflammation, and eicosanoids: An emerging perspective. Cancer Metastasis Rev. 2018, 37, 203–211. [Google Scholar] [CrossRef]
- Tretter, V.; Hochreiter, B.; Zach, M.L.; Krenn, K.; Klein, K.U. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int. J. Mol. Sci. 2021, 23, 106. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- De Luca, C.; Valacchi, G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediat. Inflamm. 2010, 2010, 321494. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef]
- Niki, E. Lipid oxidation in the skin. Free Radic. Res. 2015, 49, 827–834. [Google Scholar] [CrossRef]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, A. Eicosanoids in skin inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [Green Version]
- Coras, R.; Kavanaugh, A.; Boyd, T.; Huynh, Q.; Pedersen, B.; Armando, A.M.; Dahlberg-Wright, S.; Marsal, S.; Jain, M.; Paravar, T.; et al. Pro- and anti-inflammatory eicosanoids in psoriatic arthritis. Metabolomics 2019, 15, 65. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Gong, L.; Zhou, F.; Long, Y.; Li, Z.; Xiao, Z.; Ouyang, B.; Liu, M. Anti-inflammatory effect of traditional Chinese medicine preparation Penyanling on pelvic inflammatory disease. J. Ethnopharmacol. 2021, 266, 113405. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Bensky, D. Chinese Herbal Medicine: Materia Medica, 3rd ed.; Eastland Press: Seattle, WA, USA, 2004. [Google Scholar]
- Li, S. Compendium of Materia Medica: Bencao Gangmu, 1st ed.; Foreign Languages Press: Beijing, China, 2003. [Google Scholar]
- Koo, J.; Desai, R. Traditional Chinese medicine in dermatology. Dermatol. Ther. 2003, 16, 98–105. [Google Scholar] [CrossRef]
- Amenta, R.; Camarda, L.; Di Stefano, V.; Lentini, F.; Venza, F. Traditional medicine as a source of new therapeutic agents against psoriasis. Fitoterapia 2000, 71 (Suppl. 1), S13–S20. [Google Scholar] [CrossRef]
- Tang, W.C.; Eisenbrand, G. Chinese Drugs of Plant Origin, Subtitle: Chemistry, Pharmacology and Use in Traditional and Modern Medicine; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Pacific College of Medicine. Acupuncture for Eczema & Skin Disorders. 2022. Available online: https://www.pacificcollege.edu/news/blog/2015/01/16/acupuncture-eczema-skin-disorders (accessed on 8 March 2022).
- Darby, H.; Garcia, J.M.P. Development of an HPLC Method for the Quality Control of Chinese Herbal Medicinal Formulation: Three Yellow Cleanser (San Huang Xi Ji). Asian Basic Appl. Res. J. 2020, 2, 50–60. [Google Scholar]
- Keji, C.; Hao, X.U. The integration of traditional Chinese medicine and Western medicine. Eur. Rev. 2003, 11, 225–235. [Google Scholar] [CrossRef]
- Tang, W. Chinese Drugs of Plant Origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine; Springer: Berlin, Germany; New York, NY, USA, 1992. [Google Scholar]
- Cuéllar, M.; Giner, R.; Recio, M.; Just, M.; Máñez, S.; Cerdá, S.; Rios, J.-L. Screening of antiinflammatory medicinal plants used in traditional medicine against skin diseases. Phytother. Res. 1998, 12, 18–23. [Google Scholar] [CrossRef]
- Cuellar, M.J.; Giner, R.M.; Recio, M.C.; Just, M.J.; Mañez, S.; Rios, J.L. Effect of the basidiomycete Poria cocos on experimental dermatitis and other inflammatory conditions. Chem. Pharm. Bull. 1997, 45, 492–494. [Google Scholar] [CrossRef] [Green Version]
- Cuéllar, M.J.; Giner, R.M.; Recio, M.C.; Máñez, S.; Ríos, J.L. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia 2001, 72, 221–229. [Google Scholar] [CrossRef]
- Prieto, J.M.; Recio, M.C.; Giner, R.M.; Manez, S.; Giner-Larza, E.M.; Rios, J.L. Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions. J. Pharm. Pharmacol. 2003, 55, 1275–1282. [Google Scholar] [CrossRef]
- Recio, M.C.; Giner, R.M.; Máñez, S.; Ríos, J.L. Structural considerations on the iridoids as anti-inflammatory agents. Planta Med. 1994, 60, 232–234. [Google Scholar] [CrossRef]
- Rios, J.L.; Waterman, P.G. A review of the pharmacology and toxicology of Astragalus. Phytother. Res. 1997, 11, 411–418. [Google Scholar] [CrossRef]
- Schinella, G.R.; Tournier, H.A.; Prieto, J.M.; Mordujovich de Buschiazzo, P.; Rios, J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci 2002, 70, 1023–1033. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H.; Nishibe, S.; Arichi, S. Effects of caffeoylglycosides on arachidonate metabolism in leukocytes. Planta Med. 1987, 53, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Lee, G.H.; Choi, H.J.; Park, P.S.; Chae, H.S.; Jeong, S.I.; Kim, Y.C.; Sohn, D.H.; Park, H.; Lee, J.H.; et al. Ethyl acetate extract from Angelica Dahuricae Radix inhibits lipopolysaccharide-induced production of nitric oxide, prostaglandin E2 and tumor necrosis factor-alphavia mitogen-activated protein kinases and nuclear factor-kappaB in macrophages. Pharmacol. Res. 2007, 55, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ban, H.S.; Lim, S.S.; Suzuki, K.; Jung, S.H.; Lee, S.; Lee, Y.S.; Shin, K.H.; Ohuchi, K. Inhibitory effects of furanocoumarins isolated from the roots of Angelica dahurica on prostaglandin E2 production. Planta Med. 2003, 69, 408–412. [Google Scholar] [PubMed]
- Lin, C.H.; Chang, C.W.; Wang, C.C.; Chang, M.S.; Yang, L.L. Byakangelicol, isolated from Angelica dahurica, inhibits both the activity and induction of cyclooxygenase-2 in human pulmonary epithelial cells. J. Pharm. Pharmacol. 2002, 54, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Tsai, H.Y.; Wu, T.S. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995, 61, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.N.; Wu, T.S.; Liou, M.J.; Huang, T.F.; Teng, C.M. Inhibition of platelet thromboxane formation and phosphoinositides breakdown by osthole from Angelica pubescens. Thromb. Haemost. 1989, 62, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Zschocke, S.; Reininger, E.; Bauer, R. Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase. Planta Med. 1998, 64, 525–529. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, J.; Xiao, C.; Harqin, C.; Ma, M.; Long, T.; Li, Z.; Yang, Y.; Liu, J.; Zhao, L. Structural characterization of a low-molecular-weight polysaccharide from Angelica pubescens Maxim. f. biserrata Shan et Yuan root and evaluation of its antioxidant activity. Carbohydr. Polym. 2020, 236, 116047. [Google Scholar] [CrossRef]
- Zhang, C.; Hsu, A.C.; Pan, H.; Gu, Y.; Zuo, X.; Dong, B.; Wang, Z.; Zheng, J.; Lu, J.; Zheng, R.; et al. Columbianadin Suppresses Lipopolysaccharide (LPS)-Induced Inflammation and Apoptosis through the NOD1 Pathway. Molecules 2019, 24, 549. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Wang, C.P.; Li, J.; Ma, W.F.; Bai, Y.; Ma, L.; Gao, X.M.; Zhang, B.L.; Chang, Y.X. The pharmacokinetics and oral bioavailability studies of columbianetin in rats after oral and intravenous administration. J. Ethnopharmacol. 2013, 150, 175–180. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Li, W.; Yang, X.W. Biotransformation of columbianadin by rat hepatic microsomes and inhibition of biotransformation products on NO production in RAW 264.7 cells in vitro. Phytochemistry 2012, 81, 109–116. [Google Scholar] [CrossRef]
- Singh, G.; Bhatti, R.; Mannan, R.; Singh, D.; Kesavan, A.; Singh, P. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacology 2019, 27, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Dong, W.P.; Bi, S.H.; Pan, Z.G.; Yu, H.; Wang, X.W.; Ma, T.; Wang, J.; Zhang, W.D. Protective effects of osthole against myocardial ischemia/reperfusion injury in rats. Int. J. Mol. Med. 2013, 32, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Kuo, Y.-H.; Li, W.-C.; Lin, B.-F. The production of nitric oxide and prostaglandin E2 in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morus alba ethyl acetate fractions. J. Ethnopharmacol. 2009, 122, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Lim, J.H.; Sung, M.S.; Lee, E.G.; Oh, Y.J.; Yoo, W.H. Ethyl acetate fraction from Angelica sinensis inhibits IL-1β-induced rheumatoid synovial fibroblast proliferation and COX-2, PGE2, and MMPs production. Biol. Res. 2014, 47, 41. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.R.; Guo, Z.Q.; Liao, J.Z. Experimental study on effects of 18 kinds of Chinese herbal medicine for synthesis of thromboxane A2 and PGI2. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 1993, 13, 167–170. [Google Scholar]
- Wu, H.; Kong, L.; Wu, M.; Xi, P. Effects of different processed products of radix Angelica sinensis on clearing out oxygen free radicals and anti-lipid peroxidation. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 1996, 21, 599–601. [Google Scholar]
- Mo, Z.Z.; Lin, Z.X.; Su, Z.R.; Zheng, L.; Li, H.L.; Xie, J.H.; Xian, Y.F.; Yi, T.G.; Huang, S.Q.; Chen, J.P. Angelica sinensis Supercritical Fluid CO(2) Extract Attenuates D-Galactose-Induced Liver and Kidney Impairment in Mice by Suppressing Oxidative Stress and Inflammation. J. Med. Food 2018, 21, 887–898. [Google Scholar] [CrossRef]
- Li, J.; Hua, Y.; Ji, P.; Yao, W.; Zhao, H.; Zhong, L.; Wei, Y. Effects of volatile oils of Angelica sinensis on an acute inflammation rat model. Pharm. Biol. 2016, 54, 1881–1890. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.C.; Garrard, I.J.; Cho, C.W.; Annie Bligh, S.W.; Lu, G.H.; Fan, T.P.; Fisher, D. Bioactivity-guided fractionation of the volatile oil of Angelica sinensis radix designed to preserve the synergistic effects of the mixture followed by identification of the active principles. J. Chromatogr. A 2012, 1236, 132–138. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Zhang, Y.; Wang, C.; Duan, D.; Wang, Z. Chinese Angelica Polysaccharide (CAP) Alleviates LPS-Induced Inflammation and Apoptosis by Down-Regulating COX-1 in PC12 Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 49, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yin, M.; Lan, P.; Wang, H.; Nie, H.; Ji, X. Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: Extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agric. 2021, 8, 13. [Google Scholar] [CrossRef]
- Lv, J.L.; Zhang, L.B.; Guo, L.M. Phthalide dimers from Angelica sinensis and their COX-2 inhibition activity. Fitoterapia 2018, 129, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Bain, D. Pharmacological and Biochemical Action of Angelica sinensis (Dong Quai): Natural Product with Therapeutic Potential. Int. J. Recent Res. Life Sci. 2015, 2, 8–23. [Google Scholar]
- Jia, Z.; He, J. Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp. Ther. Med. 2016, 11, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Hirata, A.; Murakami, Y.; Atsumi, T.; Shoji, M.; Ogiwara, T.; Shibuya, K.; Ito, S.; Yokoe, I.; Fujisawa, S. Ferulic Acid Dimer Inhibits Lipopolysaccharide-stimulated Cyclooxygenase-2 Expression in Macrophages. In Vivo 2005, 19, 849–853. [Google Scholar]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-κB Activation and Nrf2 Response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xu, L.; Sang, R.; Yu, Y.; Ge, B.; Zhang, X. Immunomodulatory and anti-inflammatory effects of total flavonoids of Astragalus by regulating NF-ΚB and MAPK signalling pathways in RAW 264.7 macrophages. Pharmazie 2018, 73, 589–593. [Google Scholar]
- Zhang, B.; Hao, Z.; Zhou, W.; Zhang, S.; Sun, M.; Li, H.; Hou, N.; Jing, C.; Zhao, M. Formononetin protects against ox-LDL-induced endothelial dysfunction by activating PPAR-γ signaling based on network pharmacology and experimental validation. Bioengineered 2021, 12, 4887–4898. [Google Scholar] [CrossRef]
- Wang, J.; Ke, J.; Wu, X.; Yan, Y. Astragaloside prevents UV-induced keratinocyte injury by regulating TLR4/NF-κB pathway. J. Cosmet. Dermatol. 2022, 21, 1163–1170. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.Y.; Wang, Z.; Luo, X.H.; Sun, W.C.; Wang, H.B. Phenolic derivatives from Radix Astragali and their anti-inflammatory activities. Nat. Prod. Commun. 2014, 9, 1577–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiso, Y.; Tohkin, M.; Hikino, H. Antihepatotoxic Principles of Atractylodes Rhizomes. J. Nat. Prod. 1983, 46, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Dong, G.Z.; Lee, H.J.; Ryu, J.H. Anti-Inflammatory Compounds from Atractylodes macrocephala. Molecules 2019, 24, 1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.X.; Lu, W.W.; Geng, Y.C.; Yu, C.H.; Sun, H.J.; Kim, Y.J.; Zhang, G.; Kim, T. Antioxidant, Antimicrobial and Anti-Inflammatory Activities of Essential Oil Derived from the Wild Rhizome of Atractylodes macrocephala. Chem. Biodivers. 2020, 17, e2000268. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, G. Effects of Codonopsis pilosulae on the synthesis of thromboxane A2 and prostacyclin. Zhong Xi Yi Jie He Za Zhi = Chin. J. Mod. Dev. Tradit. Med. 1990, 10, 391–394. [Google Scholar]
- Yoon, I.S.; Cho, S.S. Effects of lobetyolin on xanthine oxidase activity in vitro and in vivo: Weak and mixed inhibition. Nat. Prod. Res. 2021, 35, 1667–1670. [Google Scholar] [CrossRef]
- Zou, Y.F.; Zhang, Y.Y.; Paulsen, B.S.; Rise, F.; Chen, Z.L.; Jia, R.Y.; Li, L.X.; Song, X.; Feng, B.; Tang, H.Q.; et al. New pectic polysaccharides from Codonopsis pilosula and Codonopsis tangshen: Structural characterization and cellular antioxidant activities. J. Sci. Food Agric. 2021, 101, 6043–6052. [Google Scholar] [CrossRef]
- Zou, Y.F.; Zhang, Y.Y.; Zhu, Z.K.; Fu, Y.P.; Paulsen, B.S.; Huang, C.; Feng, B.; Li, L.X.; Chen, X.F.; Jia, R.Y.; et al. Characterization of inulin-type fructans from two species of Radix Codonopsis and their oxidative defense activation and prebiotic activities. J. Sci. Food Agric. 2021, 101, 2491–2499. [Google Scholar] [CrossRef]
- Qin, T.; Ren, Z.; Liu, X.; Luo, Y.; Long, Y.; Peng, S.; Chen, S.; Zhang, J.; Ma, Y.; Li, J.; et al. Study of the selenizing Codonopsis pilosula polysaccharides protects RAW264.7 cells from hydrogen peroxide-induced injury. Int. J. Biol. Macromol. 2019, 125, 534–543. [Google Scholar] [CrossRef]
- Qin, T.; Ren, Z.; Lin, D.; Song, Y.; Li, J.; Ma, Y.; Hou, X.; Huang, Y. Effects of Selenizing Codonopsis pilosula Polysaccharide on Macrophage Modulatory Activities. J. Microbiol. Biotechnol. 2016, 26, 1358–1366. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.M.; Yan, J.; Xu, J. Clinical and experimental study on inhibitory effect of sanhuang mixture on platelet aggregation. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 1995, 15, 465–467. [Google Scholar]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Koshiji, M.; Akao, S.; Fujiwara, H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J. Ethnopharmacol. 1999, 66, 227–233. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T.B. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000, 66, 725–735. [Google Scholar] [CrossRef]
- Meng, X.; Tang, G.Y.; Liu, P.H.; Zhao, C.J.; Liu, Q.; Li, H.B. Antioxidant activity and hepatoprotective effect of 10 medicinal herbs on CCl(4)-induced liver injury in mice. World J. Gastroenterol. 2020, 26, 5629–5645. [Google Scholar] [CrossRef]
- Wang, S.; Lee, D.Y.; Shang, Y.; Liao, J.; Cao, X.; Xie, L.; Zhang, T.; Liu, J.; Dai, R. The bioactive alkaloids identified from Cortex Phellodendri ameliorate benign prostatic hyperplasia via LOX-5/COX-2 pathways. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 93, 153813. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J.; Park, W. Berberine modulates hyper-inflammation in mouse macrophages stimulated with polyinosinic-polycytidylic acid via calcium-CHOP/STAT pathway. Sci. Rep. 2021, 11, 11298. [Google Scholar] [CrossRef]
- Pongkittiphan, V.; Chavasiri, W.; Supabphol, R. Antioxidant Effect of Berberine and its Phenolic Derivatives Against Human Fibrosarcoma Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5371–5376. [Google Scholar] [CrossRef] [Green Version]
- Rajasekhar, K.; Samanta, S.; Bagoband, V.; Murugan, N.A.; Govindaraju, T. Antioxidant Berberine-Derivative Inhibits Multifaceted Amyloid Toxicity. iScience 2020, 23, 101005. [Google Scholar] [CrossRef]
- Misík, V.; Bezáková, L.; Máleková, L.; Kostálová, D. Lipoxygenase inhibition and antioxidant properties of protoberberine and aporphine alkaloids isolated from Mahonia aquifolium. Planta Med. 1995, 61, 372–373. [Google Scholar] [CrossRef]
- Guo, Y.; Li, F.; Ma, X.; Cheng, X.; Zhou, H.; Klaassen, C.D. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica Fate Foreign Compd. Biol. Syst. 2011, 41, 996–1005. [Google Scholar] [CrossRef]
- Ammon, H.P.; Safayhi, H.; Mack, T.; Sabieraj, J. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J. Ethnopharmacol. 1993, 38, 113–119. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phytother. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Pari, L.; Tewas, D.; Eckel, J. Role of curcumin in health and disease. Arch. Physiol. Biochem. 2008, 114, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Rao, C.V. Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol. 2007, 595, 213–226. [Google Scholar]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Umar, N.M.; Parumasivam, T.; Aminu, N.; Toh, S.M. Phytochemical and pharmacological properties of Curcuma aromatica Salisb (wild turmeric). J. Appl. Pharm. Sci. 2020, 10, 180–194. [Google Scholar]
- Tohda, C.; Nakayama, N.; Hatanaka, F.; Komatsu, K. Comparison of Anti-inflammatory Activities of Six Curcuma Rhizomes: A Possible Curcuminoid-independent Pathway Mediated by Curcuma phaeocaulis Extract. Evid.-Based Complementary Altern. Med. 2006, 3, 785620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Liang, X.; Chi, L.; Chen, Y.; Liang, L.; Zhao, J.; Luo, Y.; Zhang, W.; Cai, Q.; Wu, X.; et al. Ethanol extracts from twelve Curcuma species rhizomes in China: Antimicrobial, antioxidative and anti-inflammatory activities. S. Afr. J. Bot. 2021, 140, 167–172. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Yang, Z.; Chen, F.; Zheng, X.; Liu, X. Chemical compositions, antioxidative, antimicrobial, anti-inflammatory and antitumor activities of Curcuma aromatica Salisb. essential oils. Ind. Crops Prod. 2017, 108, 6–16. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Xi, L.; Yang, Y.; Wang, X.; Lei, D.; Zheng, X.; Liu, X. Phytochemical profiles and bioactivities of essential oils extracted from seven Curcuma herbs. Ind. Crops Prod. 2018, 111, 298–305. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Chen, D.; Huang, Z.; Li, Y.; Lan, X.; Su, P.; Pan, W.; Zhou, W.; Zheng, X.; et al. Variation on Composition and Bioactivity of Essential Oils of Four Common Curcuma Herbs. Chem. Biodivers. 2017, 14, e1700280. [Google Scholar] [CrossRef]
- Lee, S.K.; Hong, C.H.; Huh, S.K.; Kim, S.S.; Oh, O.J.; Min, H.Y.; Park, K.K.; Chung, W.Y.; Hwang, J.K. Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 2002, 21, 141–148. [Google Scholar] [CrossRef]
- Oh, O.J.; Min, H.Y.; Lee, S.K. Inhibition of inducible prostaglandin E2 production and cyclooxygenase-2 expression by curdione from Curcuma zedoaria. Arch. Pharmacal. Res. 2007, 30, 1236–1239. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J. Cell. Biochem. 2012, 113, 3653–3662. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Jiao, J.; Jiao, L. Ar-Turmerone Exerts Anti-proliferative and Anti-inflammatory Activities in HaCaT Keratinocytes by Inactivating Hedgehog Pathway. Inflammation 2020, 43, 478–486. [Google Scholar] [CrossRef]
- Lee, H.S. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour. Technol. 2006, 97, 1372–1376. [Google Scholar] [CrossRef]
- Panich, U.; Kongtaphan, K.; Onkoksoong, T.; Jaemsak, K.; Phadungrakwittaya, R.; Thaworn, A.; Akarasereenont, P.; Wongkajornsilp, A. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biol. Toxicol. 2010, 26, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-P.; Lin, L.-G.; Wang, Y.-T. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. Chin. Med. 2015, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Xia, Q.; Liu, X.; Liu, W.; Huang, W.; Mei, X.; Luo, J.; Shan, M.; Lin, R.; Zou, D.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, C.; Fu, K.; Gong, L.-H.; Zhang, Y.-F.; Zhou, H.-L.; Li, Y.-X. Phillygenin Attenuates Carbon Tetrachloride-Induced Liver Fibrosis via Modulating Inflammation and Gut Microbiota. Front. Pharmacol. 2021, 12, 756924. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, P. Phillygenin inhibits the inflammation and apoptosis of pulmonary epithelial cells by activating PPARγ signaling via downregulation of MMP8. Mol. Med. Rep. 2021, 24, 775. [Google Scholar] [CrossRef]
- Zhou, S.; Wen, H.; Han, X.; Li, H. Phillygenin protects against osteoarthritis by repressing inflammation via PI3K/Akt/NF-κB signaling: In vitro and vivo studies. J. Funct. Foods 2021, 80, 104456. [Google Scholar] [CrossRef]
- Sia, G.M.; Candlish, J.K. Effects of shiitake (Lentinus edodes) extract on human neutrophils and the U937 monocytic cell line. Phytother. Res. 1999, 13, 133–137. [Google Scholar] [CrossRef]
- Attarat, J.; Phermthai, T. Bioactive Compounds in Three Edible Lentinus Mushrooms. Walailak J. Sci. Technol. 2014, 12, 491–504. [Google Scholar]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Zi, Y.; Zhang, B.; Jiang, B.; Yang, X.; Liang, Z.; Liu, W.; He, C.; Liu, L. Antioxidant action and protective and reparative effects of lentinan on oxidative damage in HaCaT cells. J. Cosmet. Dermatol. 2018, 17, 1108–1114. [Google Scholar] [CrossRef]
- Zhang, Z.; Zha, Z.; Zhao, Z.; Liu, W.; Li, W. Lentinan Inhibits AGE-Induced Inflammation and the Expression of Matrix-Degrading Enzymes in Human Chondrocytes. Drug Des. Dev. Ther. 2020, 14, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Wang, Y.; Li, Y.; Zheng, H.; Ma, F.; Ma, C.; Zhang, X.; Lu, B.; Xie, Z.; et al. The effect of probiotics and polysaccharides on the gut microbiota composition and function of weaned rats. Food Funct. 2018, 9, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Chen, T.; Guo, Z.P.; Jiao, X.Y.; Jia, R.Z.; Zhang, Y.H.; Li, J.Y.; Huang, X.L.; Liu, H.J. Peoniflorin suppresses tumor necrosis factor-α induced chemokine production in human dermal microvascular endothelial cells by blocking nuclear factor-κB and ERK pathway. Arch. Dermatol. Res. 2011, 303, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.S.; Jiang, Y.; Yuan, J.P.; Jiang, S.B.; Yang, Y.; Zhu, P.Y.; Sun, Y.Z.; Qi, R.Q.; Liu, T.; Wang, H.X.; et al. UVA Induced Oxidative Stress Was Inhibited by Paeoniflorin/Nrf2 Signaling or PLIN2. Front Pharm. 2020, 11, 736. [Google Scholar] [CrossRef]
- You, O.H.; Shin, E.A.; Lee, H.; Kim, J.H.; Sim, D.Y.; Kim, J.H.; Kim, Y.; Khil, J.H.; Baek, N.I.; Kim, S.H. Apoptotic Effect of Astragalin in Melanoma Skin Cancers via Activation of Caspases and Inhibition of Sry-related HMg-Box Gene 10. Phytother. Res. 2017, 31, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Ziereis, K. The antipsoriatic Mahonia aquifolium and its active constituents; I. Pro- and antioxidant properties and inhibition of 5-lipoxygenase. Planta Med. 1994, 60, 421–424. [Google Scholar] [CrossRef]
- Giner-Larza, E.M.; Máñez, S.; Giner-Pons, R.M.; Carmen Recio, M.; Ríos, J.L. On the anti-inflammatory and anti-phospholipase A(2) activity of extracts from lanostane-rich species. J. Ethnopharmacol. 2000, 73, 61–69. [Google Scholar] [CrossRef]
- Fang, C.L.; Paul, C.R.; Day, C.H.; Chang, R.L.; Kuo, C.H.; Ho, T.J.; Hsieh, D.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Poria cocos (Fuling) targets TGFβ/Smad7 associated collagen accumulation and enhances Nrf2-antioxidant mechanism to exert anti-skin aging effects in human dermal fibroblasts. Environ. Toxicol. 2021, 36, 729–736. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, S.; Moon, E.; Park, H.J.; Park, H.B.; Kim, K.H. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorganic Chem. 2017, 70, 94–99. [Google Scholar] [CrossRef]
- Jiang, G.P.; Liao, Y.J.; Huang, L.L.; Zeng, X.J.; Liao, X.H. Effects and molecular mechanism of pachymic acid on ferroptosis in renal ischemia reperfusion injury. Mol. Med. Rep. 2021, 23, 63. [Google Scholar] [CrossRef]
- Choi, E.; Kang, Y.G.; Hwang, S.H.; Kim, J.K.; Hong, Y.D.; Park, W.S.; Kim, D.; Kim, E.; Cho, J.Y. In vitro Effects of Dehydrotrametenolic Acid on Skin Barrier Function. Molecules 2019, 24, 4583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhang, Y.; Gapter, L.A.; Ling, H.; Agarwal, R.; Ng, K.Y. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem. Pharm. Bull. 2008, 56, 1459–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Zhang, Y.; Wang, X.; Huang, X.; Fei, Y.; Yu, Y.; Shou, D. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016, 83, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, Z.; Pu, Y.; Bao, Y. Immunomodulatory effects exerted by Poria cocos polysaccharides via TLR4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed. Pharmacother. 2019, 112, 108709. [Google Scholar] [CrossRef]
- Chao, C.L.; Huang, H.W.; Su, M.H.; Lin, H.C.; Wu, W.M. The Lanostane Triterpenoids in Poria cocos Play Beneficial Roles in Immunoregulatory Activity. Life 2021, 11, 111. [Google Scholar] [CrossRef]
- Kubo, M.; Asano, T.; Shiomoto, H.; Matsuda, H. Studies on rehmanniae radix. I. Effect of 50% ethanolic extract from steamed and dried rehmanniae radix on hemorheology in arthritic and thrombosic rats. Biol. Pharm. Bull. 1994, 17, 1282–1286. [Google Scholar] [CrossRef] [Green Version]
- Tomoda, M.; Miyamoto, H.; Shimizu, N. Structural features and anti-complementary activity of rehmannan SA, a polysaccharide from the root of Rehmannia glutinosa. Chem. Pharm. Bull. 1994, 42, 1666–1668. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S. Catalpol reduces the production of inflammatory mediators via PPAR-γ activation in human intestinal Caco-2 cells. J. Nat. Med. 2016, 70, 620–626. [Google Scholar] [CrossRef]
- Si, N.; Kanazawa, H.; Okuyama, K.; Imada, K.; Wang, H.; Yang, J.; Zhao, H.; Bian, B.; Ito, A.; Sato, T. Involvement of Catechols in Acteoside in the Activation of Promatrix Metalloproteinase-2 and Membrane Type-1-Matrix Metalloproteinase Expression via a Phosphatidylinositol-3-Kinase Pathway in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2018, 41, 1530–1536. [Google Scholar] [CrossRef] [Green Version]
- Butenko, I.G.; Gladtchenko, S.V.; Galushko, S.V. Anti-inflammatory properties and inhibition of leukotriene C4 biosynthesis in vitro by flavonoid baicalein from Scutellaria baicalensis georgy roots. Agents Actions 1993, 39, C49–C51. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Jong, H.G.; Kim, H.P. Inhibition of cyclooxygenase/lipoxygenase from human platelets by polyhydroxylated/methoxylated flavonoids isolated from medicinal plants. Arch. Pharmacal Res. 1999, 22, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Lee, Y.J.; Lu, F.J.; Chiang, H.C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993, 13, 2165–2170. [Google Scholar] [PubMed]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Sanz, M.J.; Ferrandiz, M.L.; Cejudo, M.; Terencio, M.C.; Gil, B.; Bustos, G.; Ubeda, A.; Gunasegaran, R.; Alcaraz, M.J. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica Fate Foreign Compd. Biol. Syst. 1994, 24, 689–699. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Zhu, L.; Guo, S.; Yi, X.; Cui, T.; He, Y.; Chang, Y.; Liu, B.; Li, C.; et al. Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic. Biol. Med. 2018, 129, 492–503. [Google Scholar] [CrossRef]
- Yoon, J.J.; Jeong, J.W.; Choi, E.O.; Kim, M.J.; Hwang-Bo, H.; Kim, H.J.; Hong, S.H.; Park, C.; Lee, D.H.; Choi, Y.H. Protective effects of Scutellaria baicalensis Georgi against hydrogen peroxide-induced DNA damage and apoptosis in HaCaT human skin keratinocytes. EXCLI J. 2017, 16, 426–438. [Google Scholar]
- Chang, W.S.; Lin, E.Y.; Hsu, S.W.; Hu, P.S.; Chuang, C.L.; Liao, C.H.; Fu, C.K.; Su, C.H.; Gong, C.L.; Hsiao, C.L.; et al. Baicalin Scavenged Reactive Oxygen Species and Protected Human Keratinocytes Against UVB-induced Cytotoxicity. Vivo 2016, 30, 605–610. [Google Scholar]
- Wang, S.C.; Chen, S.F.; Lee, Y.M.; Chuang, C.L.; Bau, D.T.; Lin, S.S. Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity. In Vivo 2013, 27, 707–714. [Google Scholar]
- Seok, J.K.; Kwak, J.Y.; Choi, G.W.; An, S.M.; Kwak, J.H.; Seo, H.H.; Suh, H.J.; Boo, Y.C. Scutellaria radix Extract as a Natural UV Protectant for Human Skin. Phytother. Res. 2016, 30, 374–379. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur. J. Pharmacol. 2011, 661, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, B.H.; Kim, H.J.; Cho, H.J.; Shin, H.C.; Im, K.S.; Choi, S.H.; Shin, T.J.; Lee, S.M.; Nam, S.W.; et al. Suppression of metastasis of intravenously-inoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: Involvement of autotaxin inhibition. Int. J. Oncol. 2013, 42, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Im, K.R.; Yoon, K.-S. Anti-inflammatory effects of YeongyoSeungma-tang. J. Ethnopharmacol. 2009, 126, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Jiang, S.; Volshonok, H.; Wu, J.; Zhang, D.Y. Molecular Mechanism of Anti-Prostate Cancer Activity of Scutellaria baicalensis Extract. Nutr. Cancer 2007, 57, 100–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, Y.-F.; Zhang, L.-L.; Wang, L.; He, L.-J.; Wang, C.; Chai, Z.-Z.; Fan, R.-Y.; Li, S.; Wang, Y.-H. Finding new sources from “using different plants as the same herb”: A case study of Huang-lian in Northwest Yunnan, China. J. Ethnopharmacol. 2015, 169, 413–425. [Google Scholar] [CrossRef]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, Y.; Katoh, H.; Kobayashi, K.; Yamamoto, S.; Morisawa, S. Protection of liver cells against experimental damage by extract of cultured Lentinus edodes mycelia (LEM). Gastroenterol. Jpn. 1987, 22, 459–464. [Google Scholar] [CrossRef]
- Henke, D.; Danilowicz, R.; Eling, T. Arachidonic acid metabolism by isolated epidermal basal and differentiated keratinocytes from the hairless mouse. Biochim. Biophys. Acta 1986, 876, 271–279. [Google Scholar] [CrossRef]














| TCM Drug Botanical Species Chinese Name/Other Names | Properties Meridians | Actions |
|---|---|---|
| Radix Angelica dahurica Angelica dahurica Fisch. ex Hoffm. Bai Zhi/Chinese angelica | Pungent and warm. Lung, stomach and large intestine. | Expel wind and release exterior, alleviate pain, relieve stuffy nose, dry dampness, and stop leucorrhoea. |
| Radix Angelica pubescens Angelica pubescens Franch. Du Huo/Shishiudo | Pungent, bitter, slightly warm. Liver, kidney, and lung. | Dispel wind-damp, alleviate pain, release exterior. |
| Radix Angelica sinensis Angelica sinensis (Oliv.) Diels Dang Gui/Female ginseng | Sweet, pungent, warm. Heart and liver. | Tonify blood, activate blood, alleviate pain, regulate menstruation, and moisten intestines. |
| Radix Astragali Astragalus membranaceus (Fisch.) Bunge (Now A. propinquus Schischkin) Huang Qi/Mongolian milkvetch | Sweet, warm. Lung and spleen. | Tonify qi, raise yang, tonify defensive aspect to secure superficial, relieve edema through diuretic, dispel toxin to promote skin generation, nourish blood. |
| Atractylodis macrocephalae rhizome Atractylodes macrocephala Koidz. Bai Zhu | Sweet, bitter, warm. spleen and stomach. | Tonify spleen qi, dry dampness, induce diuresis, arrest sweating and prevent abortion. |
| Radix Codonopsis Codonopsis pilosula Franch. Dang Shen | Sweet, neutral. Lung and spleen. | Invigorate lung-qi and spleen-qi, nourish blood, and promote the generation of body fluid. |
| Rhizoma Coptidis Coptis chinensis Franch. Huang Lian | Bitter, cold. Heart, stomach, Large intestine and liver. | Clear heat and dry dampness, purge fire and relieve toxicity. |
| Radix Curcumae Curcuma aromatica Salisb. Yu Jin/Turmeric | Pungent, bitter, cold. Liver, gallbladder and heart. | Activate blood and alleviate pain, move qi and relieve depression, clear heat and cool blood, promote excretion or bile and remove jaundice. |
| Fructus Forsythiae Forsythia suspensa (Thunb.) Vahl Lian Qiao/Weeping forsythia | Bitter, slightly pungent, cold. Lung, heart and small intestine. | Clear heat and remove toxicity, disperse wind-heat, clear heart-heat. |
| Lentinus edodes Lentinus edodes (Berk.) Pegler Xianggu/Oakwood mushroom | Sweet, neutral. liver and stomach. | Tonify deficiency, strengthen the spleen, stimulate the appetite, expel wind, and promote eruption, resolve phlegm and regulate the flow of qi, remove toxicity and treat cancer. |
| Radix Paeoniae Alba Paeonia lactiflora Pall. Bai Shao (Chi Shao)/Chinese peony | Bitter, sour, sweet, slightly cold. Spleen and liver. | Tonify blood, astringe yin to check sweating, emolliate liver to alleviate pain, calm and suppress liver yang. |
| Phellodendri Amurensis Cortex Phellodendron amurense Rupr. Huang Bo/Amur cork tree | Bitter, cold. Liver, gallbladder, Large intestine, kidney and bladder. | Clear heat and dry dampness, purge fire and remove toxicity, subdue deficiency heat. |
| Poria Poria cocos F.A.Wolf Fu Ling/Poria | Sweet, bland, neutral. Heart, spleen, and kidney. | Induce diuresis and drain dampness, invigorate spleen, and induce tranquilization. |
| Radix Rehmanniae Rehmannia glutinosa (Gaertn.) DC. Di Huang/Chinese Foxglove | Sweet, bitter, cold. Heart, liver, stomach and kidney. | Clear heat and cool blood, stop bleeding, nourish yin |
| Radix Scutellariae Scutellaria baicalensis Georgi Huang Qin/Skullcap | Bitter, cold. Lung, stomach, gallbladder, large intestine or bladder. | Clear heat and dry dampness, purge fire and relieve toxicity, cool blood, and stop bleeding. |
| TCM Drug | Lipoperoxidation [35] | Eicosanoids [32] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LNE | LE | XO | α | 5LO | COX1 | β | 12LO | χ | ||
| 1. | A. dahurica | 94 | 149 | 74 | 191 | 98 | 25 a | 101 | - | - |
| 2. | A. pubescens | 88 | 85 | 73 | 122 | 85 | 5 | 85 | 83 | 119 |
| 3. | A. sinensis | 95 | 94 | 90 | 133 | 0 | 0 b | 0 | - | - |
| 4. | A. membranaceus | 89 | 79 | 116 | 119 | 37 | 28 | 46 | 101 | 111 |
| 5. | A. macrocephala | 91 | 108 | 89 | 141 | 86 | 56 | 103 | 110 | 150 |
| 6. | C. pilosula | 94 | 94 | 99 | 132 | 62 | 66 | 91 | - | - |
| 7. | C. chinensis | 57 | 17 | 79 | 60 | 44 | 11 | 45 | 30 | 54 |
| 8. | C. aromatica | 92 | 77 | 54 | 120 | 86 | 111 | 140 | 106 | 176 |
| 9. | F. suspensa | 3 | 28 | 20 | 28 | 24 | 50 c | 55 | 119 | 131 |
| 10. | L. edodes | 92 | 85 | 88 | 125 | 104 | 67 | 124 | 91 | 154 |
| 11. | P. lactiflora | 74 | 63 | 56 | 97 | 72 | 30 | 78 | 96 | 124 |
| 12. | P. amurense | 60 | 20 | 85 | 63 | 0 | 14 | 14 | 35 | 38 |
| 13. | P. cocos | 94 | 112 | 75 | 146 | 39 | 25 | 46 | 44 | 64 |
| 14. | R. glutinosa | 92 | 73 | 79 | 117 | 104 | 61 | 121 | 120 | 170 |
| 15. | S. baicalensis | 2 | 1 | - | 2 | 0 d | 0 e | 0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, J.M.; Schinella, G.R. Anti-Inflammatory and Antioxidant Chinese Herbal Medicines: Links between Traditional Characters and the Skin Lipoperoxidation “Western” Model. Antioxidants 2022, 11, 611. https://doi.org/10.3390/antiox11040611
Prieto JM, Schinella GR. Anti-Inflammatory and Antioxidant Chinese Herbal Medicines: Links between Traditional Characters and the Skin Lipoperoxidation “Western” Model. Antioxidants. 2022; 11(4):611. https://doi.org/10.3390/antiox11040611
Chicago/Turabian StylePrieto, Jose M., and Guillermo R. Schinella. 2022. "Anti-Inflammatory and Antioxidant Chinese Herbal Medicines: Links between Traditional Characters and the Skin Lipoperoxidation “Western” Model" Antioxidants 11, no. 4: 611. https://doi.org/10.3390/antiox11040611







