Abstract
Among gynaecologic malignancies, ovarian cancer is one of the most dangerous, with a high fatality rate and relapse due to the occurrence of chemoresistance. Many researchers demonstrated that oxidative stress is involved in tumour occurrence, growth and development. Nuclear factor erythroid 2-related factor 2 (NRF2) is an important transcription factor, playing an important role in protecting against oxidative damage. Increased levels of Reactive Oxygen Species (ROS) activate NRF2 signalling, inducing the expression of antioxidant enzymes, such as haem oxygenase (HO-1), catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD), that protect cells against oxidative stress. However, NRF2 activation in cancer cells is responsible for the development of chemoresistance, inactivating drug-mediated oxidative stress that normally leads to cancer cells’ death. In this review, we report evidence from the literature describing the effect of NRF2 on ovarian cancer, with a focus on its function in drug resistance, NRF2 natural and synthetic modulators and its protective function in normal ovarian preservation.
1. Introduction
Among gynaecologic malignancies, ovarian cancer is a leading cause of death because it is often diagnosed at an advanced stage of disease. Although the majority of ovarian cancers are epithelial ovarian cancer (EOC), different histological subtypes of EOC have been identified, including serous (the most common), endometrioid, clear cell and mucinous cancers. The current treatment for advanced ovarian cancer is surgery, followed by platinum/taxane chemotherapy, but many patients relapse within 18 months due to chemoresistance onset. This explains the high mortality rates of this type of cancer [1].
Oxidative stress can affect all phases of oncogenesis, i.e., cancer initiation, promotion, and progression, activating many transcription factors, such as nuclear factor (NF)-κB, peroxisome proliferator-activated receptor γ (PPARγ), p53, hypoxia inducible factor 1α (HIF-1α) and Nuclear Factor Erythroid 2-Related Factor 2 (NFE2L2 or NRF2). These factors can induce the expression of numerous genes involved in many cellular processes, including inflammatory responses, apoptosis, cell proliferation and differentiation [2].
Cell metabolism, infection, exposure to carcinogens and environmental toxicants are the main producers of endogenous and exogenous Reactive Oxygen Species (ROS), highly reactive molecules, such as hydroxyl radical (OH−), hydrogen peroxide (H2O2) and superoxide (O2−), as well as reactive nitrogen species that can damage cellular DNA, proteins and lipids, if not eliminated [3,4,5]. Therefore, cells developed different mechanisms to tolerate ROS presence. In fact, cells can tolerate low ROS levels acting as second messengers and modulating different intracellular pathways involved in many cellular processes, including cell proliferation, differentiation and migration [3,6]. However, high levels of ROS cause cell death because of cellular components oxidation as membrane lipids, proteins and DNA [3]. ROS can be neutralized by glutathione (GSH), coenzyme Q, lipoic acid or antioxidant enzymes, such as superoxide dismutase (SOD), catalases (CATs), thioredoxins (Trxs), peroxiredoxins (Prxs), reductases and peroxidases [3,7].
Moreover, ROS scavenging enzymes, carrying antioxidant response elements (AREs) in their promoter regions [8], represent an intrinsic defence mechanism to avoid cell damage caused by ROS. A key regulator of AREs is NRF2, a basic leucine zipper transcription factor that binds ARE regions present in the promoter of many antioxidant enzyme-activating genes [8].
Normally, NRF2 is directly bound to a negative regulator Kelch-like ECH-Associated Protein 1 (KEAP1) that is further bound to Cullin 3 (CUL3) and RING-box protein 1 (RBX1)/E3-ubiquitin ligase, forming the KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex that targets NRF2 for proteasomal degradation. In the presence of oxidant stimuli, the binding between ROS and cysteine residues of KEAP1 leads to a conformation change in KEAP1. The latter causes the inhibition of NRF2 ubiquitination and its translocation into the nucleus (active form), with consequent binding to the ARE regions in the promoter of antioxidant genes, inducing their expression (Figure 1) [9]. The NRF2 pathway also plays a key role in carcinogenesis because it inhibits apoptosis, promoting cell proliferation and chemoresistance. Thus, the NRF2 pathway is emerging as a chemotherapeutic target in many types of cancer [10].
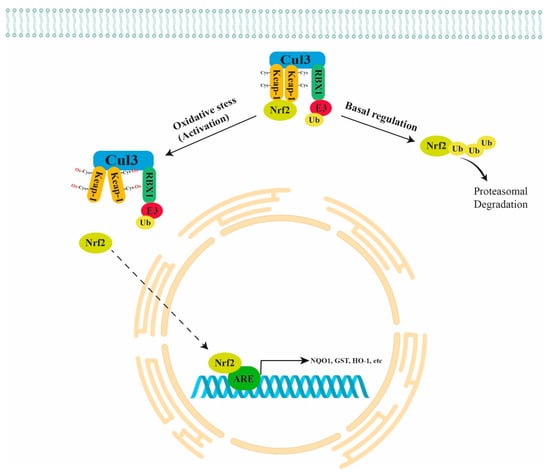
Figure 1.
Schematic representation of NRF2 regulation. Normally, NRF2 is directly bound to the KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex that targets NRF2 for proteasomal degradation. Under oxidant stimuli, ROS oxidate the cysteine residues of KEAP1 leading to a conformation change in KEAP1 that causes the inhibition of NRF2 ubiquitination and its translocation into the nucleus with consequent binding to the ARE regions of antioxidant genes (NQO1, GST, HO-1, etc.). KEAP1 = Kelch-like ECH Associated Protein 1; CUL3 = Cullin 3; RBX1 = RING-box protein 1.
To date, platinum drugs (cisplatin, carboplatin, and oxaliplatin), alone or in combination with other drugs, are the most used clinical agents in chemotherapy against ovarian cancer, and among them, cisplatin (cis-diamminedichloroplatinum II, CDDP) is one of the most efficient. Cisplatin forms both mono-adducts, covalently interacting with N7-guanine in DNA, and intra- and/or inter-strand crosslinks [11,12,13]. Cell apoptosis, due to the block of DNA synthesis and transcription, is the consequence of these alterations due to cisplatin [12,14]. Unfortunately, cisplatin resistance is one of the major problems occurring in cisplatin-based chemotherapy. Interestingly, it has been shown that the cisplatin-resistant human ovarian cancer SKOV3 cell line, which retains high levels of GSH, could be sensitized to cisplatin treatment by inhibiting NRF2. In fact, NRF2 inhibition led to GSH depletion, increasing cisplatin cytotoxicity and proving that the NRF2 pathway plays a key role in cisplatin resistance [15]. Interestingly, this cell line has high basal ROS levels that, enabling NRF2 nuclear translocation, favour NRF2 antioxidant activity and allow a better cell resistance to endogenous oxidant agents. Additionally, NRF2 and KEAP1 basal levels are cell line specific and positively correlate both to cell growth rates and basal ROS, in the following ovarian cancer cell lines: PEO1, PEO4, PEO6, SKOV3, OVCAR3 and OVCAR4. In particular, basal ROS levels and cell growth are higher in SKOV3, OVCAR3 and PEO1 compared to the other cell lines. The high basal ROS levels favour NRF2 stability and antioxidant activity, allowing a better cell resistance to the exogenous oxidant agents [16]. Similar findings were found in Doxorubicin (DR) resistance, an oxidative chemotherapeutic drug used in the treatment of many solid tumours, including ovarian cancer [17,18].
NRF2/ARE binding and NRF2 target genes expression are lower in the A2780 cell line, which is highly sensitive to DR, compared to resistant ovarian carcinoma SKOV3 and OV90 cell lines. Contrarily, doxorubicin-resistant A2780DR cells show increased NRF2 activity, enhanced GSH1 and GSH contents, suggesting that NRF2 might be a key factor in DR sensitivity [19]. Thus, an adaptive activation of the NRF2 pathway may participate in the development of DR-acquired resistance. NRF2 silencing in OV90 cells lead to a reduction in GSH levels and retard cell growth sensibilizing OV90 cells to DR treatment. This causes increased apoptosis, suggesting NRF2 as a molecular target to restore DR sensitivity and repress tumour growth [20].
E26 transformation-specific (ETS) proteins are a family of 28 transcription factors, containing a highly conserved ETS domain for DNA binding [21]. They are considered important onco-drivers and play a pivotal role in the progression of many types of cancer [21,22,23]. Interestingly, H2O2 upregulates Ets-1, a member of ETS proteins family, expression in both OV2008 and C13 ovarian carcinoma cell lines. Moreover, H2O2 treatment increases Ets-1 expression by NRF2 binding to ARE in the Ets-1 promoter, suggesting that Ets-1 is clearly modulated by ROS in cancer cells via NRF2 signalling [24].
The aim of this review is to provide an overview of the current literature, regarding the role of NRF2 in ovarian cancer and normal ovarian preservation, with a focus on its cellular modulators and targets.
2. NRF2 in Ovarian Cancer Tissues
Nicotinamide adenine dinucleotide phosphate (NAD-P) H/quinone oxidoreductase 1 (NQO1) is a metabolizing enzyme, capable of generating antioxidant forms of ubiquinone and vitamin E after free radical exposure. It has an important role in cellular defence mechanisms against oxidative stress, acting as antioxidant enzyme. Interestingly, both NQO1 and NRF2 are highly expressed in ovarian carcinoma compared to normal and precancerous lesions, showing a positive correlation in the different lesions. In addition, NRF2 is expressed in both the nucleus and cytoplasm of ovarian cells (90 ovarian carcinomas of different grades and 10 normal ovarian tissues) and it significantly increases as ovarian carcinoma stage advances [25]. Therefore, the authors suggest that NQO1 and NRF2 may be considered therapeutic targets for ovarian cancer care and possible early diagnostic biomarkers. Similarly, Liew and colleagues reported that serous carcinoma has higher KEAP1 cytoplasmic, NRF2 nuclear and lower E-cadherin membrane positivity than mucinous, endometrioid and clear cell cancers, studying 108 cases (47 serous, 23 mucinous, 13 endometrioid and 25 clear cell). Moreover, KEAP1 expression is further increased in serous carcinoma from elderly patients. Multivariate analysis identified International Federation of Gynecology and Obstetrics (FIGO) staging and NRF2 expression as prognostic factors. Thus, NRF2 and Keap1 can be considered key players in serous carcinoma [26].
These results are partially in agreement with another study on NRF2 and KEAP1 evaluation as prognostic indicators. In particular, low KEAP1 expression is associated with both disease recurrence and death, while high KEAP1 expression is predictive of better overall and disease-free survival, suggesting KEAP1 as an independent prognostic factor, not linked to NRF2. Contrarily, high NRF2 expression shows a better overall and disease-free survival, but the results are not statistically significant, and no significant association is detected among chemoresistance, NRF2 and KEAP1 expression [27].
Others reported that nuclear NRF2 expression is low in serous, clear cell, and endometrioid ovarian carcinomas, while it is high in mucinous subtype. Moreover, low nuclear NRF2 expression in those carcinomas is associated with aging. No significant difference was detected in oestrogen receptor α (ERα) expression, comparing all ovarian cancer subtypes, but low-grade carcinomas showed a significantly higher ERα expression compared to high-grade ones. Interestingly, NRF2 cytoplasmic expression was positively correlated with ERα expression in serous ovarian carcinoma and their expressions have been associated with longer overall survival. This suggests that the inhibition of NRF2 may represent an effective therapeutic strategy for OEC treatment [28]. In addition, cytoplasmic NRF2 expression is significantly correlated with both progesterone receptor A and B (PRA and PRB) expressions and associated with increased overall survival [29].
Looking at the studies discussed in this section (and summarized in Table 1), it is possible to see that many of them reach contradictory conclusions. This can be due to the small cohorts’ study and to the limitations of the technique (immunohistochemistry).

Table 1.
NRF2 expression and correlation with ovarian cancer subtypes.
3. NRF2 Cellular Modulators in Ovarian Cancer
MicroRNAs have been associated with many pathological processes, including human pregnancy complication [30,31,32], cancer progression [33,34,35,36] and chemo- or radiotherapeutic resistance [37,38]. MiR-181d is highly expressed in ovarian tissues of DDP-(cisplatin) resistant patients and in the cisplatin-resistant A2780/DDP cell line. Moreover, ectopic expression of miR-181d increases DDP resistance in A2780 non-resistant cells. miR-181d negatively regulates O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) expression by targeting its mRNA in 3′UTR. OGT is an essential enzyme for KEAP1 glycosylation and stabilization that implies NRF2 inactivation [39], resulting in increased DDP resistance in an A2780 cell model [40]. This study highlighted a key role of miR-181d in modulating DDP resistance in ovarian cancer through the OGT/KEAP1/NRF2 axis.
In addition to microRNAs, there are an increasing number of studies showing an important role of long non-coding RNAs (lncRNAs) in carcinogenesis [41,42,43]. In particular, LncRNA H19 is encoded by the H19 gene and is one of the first discovered lncRNAs [44]. LncRNA H19 plays a pivotal role in tumorigenesis and malignant progression, promoting many cell processes, including cell growth, invasion, migration and epithelial-mesenchymal transition [45]. Interestingly, lncRNA H19 levels are significantly increased in cisplatin-resistant A2780/CDDP cells. Moreover, H19 levels are higher in patients with high-grade serous ovarian cancer (HGSC) and correlate with cancer recurrence. Furthermore, A2780/CDDP cells with lncRNA H19 knockdown result in the recovery of cisplatin sensitivity and reduce the expression of six NRF2-modulated proteins, such as NQO1, Glutathione-Disulfide Reductase (GSR), Glucose-6-phosphate Dehydrogenase (G6PD), Glutamate-Cysteine Ligase catalytic subunit (GCLC), Glutamate-cysteine ligase regulatory subunit (GCLM) and Glutathione S-Transferase Pi 1 (GSTP1), involved in oxidative stress control. Additionally, these cells have low glutathione levels and are significantly more sensitive to H2O2 treatment, suggesting that lncRNA H19 may play a key role in cisplatin-resistance-modulating NRF2 signalling [46]. Moreover, mutations of common oncogenes, such as KRAS, BRAF, and MYC, increase NRF2 transcription and activity in malignant cells, protecting tumour cells from ROS cytotoxic effects induced by chemotherapeutic drugs, such as cisplatin, and play a key role in cisplatin resistance [47,48].
Protein p62/SQSTM1 (sequestosome 1) is an antiapoptotic mediator acting as a cargo protein that identifies (via a ubiquitin-binding domain) and delivers specific organelles and protein aggregates to autophagosomes for degradation (a process called selective autophagy) [49]. In this way, autophagy can allow tumour cell survival and growth, facilitating the supply of metabolic demands during tumour progression [50,51]. Autophagy is also involved in processes inducing cisplatin resistance of human ovarian cancer cells [52], and it is higher in cisplatin-resistant SKOV3/CDDP cells than cisplatin-sensitive SKOV3 cells. In particular, p62 interacts with the KEAP1/NRF2/ARE pathway i.e., after p62 phosphorylation, it physically interacts with KEAP1 and, subsequently, NRF2 transfers to the nucleus, activating the expression of antioxidant genes in SKOV3/DDP cells. Therefore, p62 can prevent ROS-induced apoptosis activating the KEAP1/NRF2/ARE signalling [53].
Another protein, Sirtuin 5 (SIRT5), belonging to the Sirtuin family of proteins (Sirt1–7), is involved in the regulation of multiple cellular processes, including glycolysis, fatty acid oxidation, nitrogen metabolism and drug resistance in cancer cells [54,55]. Interestingly, SIRT5, a mitochondrial NAD-dependent deacetylase, is increased in ovarian cancer tissues, predicting a poor response to chemotherapy. Moreover, SIRT5 levels are higher in cisplatin-resistant SKOV-3 and CAOV-3 ovarian cancer cells than in cisplatin-sensitive A2780 cells. SIRT5 overexpression facilitates ovarian cancer cell growth and cisplatin-resistance in an in vitro A2780 cell model, because SIRT5 suppresses cisplatin-induced DNA damage by increasing NRF2 and Haem Oxygenase 1 (HO-1) expression [56].
The KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex is a key inhibitor of NRF2 levels [57]. It is known that the NRF2 pathway is a critical starting pathway for oxidative stress response and this pathway is constitutively active in serous ovarian carcinomas (OVCA) [58,59]. In fact, it has been reported that almost 90% of OVCA cases exhibit loss-of-function alterations in any components of the above inhibitory complex. Copy-number loss (CNL) is the most prominent disruption mechanism and most frequently observed in the RBX1 component. Consequent reduced mRNA complex expression enhances NRF2 target gene expression, suggesting that a remarkably high frequency of DNA and mRNA alterations of the KEAP1/CUL3/RBX1 complex leads to high levels of NRF2, found in OVCA [60].
The p53 upregulated modulator of apoptosis (PUMA) is a member of the Bcl-2 family, localized in the mitochondria and involved in mitochondrial-dysfunction-mediated apoptosis [61]. PUMA is an antagonist of both BCL-XL and MCL-1 antiapoptotic Bcl-2 family members, then acting as a proapoptotic factor [62]. Although the mechanism of action of PUMA remains unclear, it is possible that it could play a key role in inhibiting tumour growth [63]. PUMA, mainly located into the mitochondria, induces apoptosis by ROS production and increases both NRF2 and HO-1 expression in transfected A2780 and SKOV3 cells. Thus, PUMA induces ROS generation, damaging DNA and leading to cell apoptosis, but it enhances NRF2/HO-1 expression, providing an antioxidant response to ROS-mediated oxidative stress [64].
The same research group showed NRF2 nuclear (active form) localization in the majority of EOC specimens analysed with more frequency in clear cell EOC subtype and an upregulation of NRF2 target genes. In fact, 29% of clear cell carcinoma samples shows genetic mutations of the KEAP1 sequence. Importantly, patients with an active NRF2 pathway show resistance to platinum-based therapy and lower overall survival compared with those patients where the NRF2 pathway is inactivated [58]. Others showed that KEAP1 mutations have a key role in NRF2 activation and in platinum-based therapy resistance onset in EOC. In this study, the heterodimer, between the wild-type KEAP1 and the mutant KEAP1 subunits, is inactive and is unable to repress NRF2 in tumours with KEAP1 mutations [65]. Pylväs-Eerola and colleagues showed an increased expression of KEAP1 after Platinum-based neoadjuvant chemotherapy, suggesting a role of KEAP1 in degrading NRF2, promoting chemotherapy response [66]. Studies discussed in this chapter has been summarized in Table 2.

Table 2.
NRF2 cellular modulators in ovarian cancer.
4. NRF2 Cellular Targets in Ovarian Cancer
Oestrogen Receptor α (ERα) mediates the effects of female steroid hormones and can be considered a key regulator of apoptosis and cell proliferation in EOC [67,68,69]. In addition, Progesterone Receptor (PGR) expression has been associated with improved overall survival (OS) and progression-free survival (PFS), probably due to its anti-proliferative effect [70,71]. Interestingly, ERα is reduced in all ovarian cancer cell lines (OVCAR3, ES2, UWB1.289, and TOV112D) compared to the benign cell line HOSEpiC. Contrarily, NRF2 is highly expressed in ovarian cancer cell lines compared with the benign HOSEpiC cell line. Interestingly, NRF2 silencing induces an increase in ERα and PGR mRNA expressions in OVCAR3 cell lines [28,29], confirming a role of NRF2 in regulating ERα and PGR expression in ovarian cancer cell lines.
Another molecule involved in many cellular processes, including apoptosis, cell proliferation and differentiation, is CD99, a transmembrane glycoprotein coded by the MIC2 (MHC class I related antigen 2) gene [72] that is considered a prognostic marker of ovarian cancer [73]. Wu and colleagues demonstrated that CD99 is highly expressed in cisplatin-resistant ovarian cancer cells, using in vitro models (A2780/CDDP and COC1/CDDP) and ovarian tissues. Contrarily, CD99 is poorly expressed in cisplatin-sensitive ovarian cancer cells (A2780 and COC1) and ovarian tissues. Thus, CD99 overexpression results in cisplatin resistance, while CD99 knockdown sensitizes ovarian cancer cells to cisplatin. In addition, NRF2 overexpression increases CD99 expression and cell viability after cisplatin treatment in cisplatin-sensitive cells. Conversely, NRF2 knockdown decreases CD99 expression and cell viability after cisplatin treatment in cisplatin-resistant cells. We can summarize that simultaneous CD99 overexpression and reactivated cisplatin resistance in ovarian cancer cells suggest that CD99 can be an NRF2 downstream gene and that NRF2 can modulate cisplatin resistance by CD99 up or downregulation [74].
Another study demonstrated that NRF2 silencing represses NRF2 signalling in SKOV3 cells and causes cell growth G0/G1 phase arrest. Moreover, NRF2 silencing induces tumour growth retardation in mouse xenografts and a significant decrease in ErbB2 expression, a member of the human epidermal growth factor (EGF) receptor family that plays an essential role in cell proliferation and differentiation [75]. At the same time, ErbB2 downregulation leads to a pAKT decrease and p27 increase, enhancing the effect of NRF2 knockdown in SKOV3 growth [76]. Furthermore, NRF2 inhibition increases the sensitivity to docetaxel cytotoxicity and apoptosis, providing important findings on its role in docetaxel-based chemotherapy.
Aldo-keto reductases comprise AKR1C1–AKR1C4, four enzymes that catalyse NADPH-dependent reductions [77] but are also involved in chemoresistance [78]. Interestingly, NRF2 knockdown leads to decreased AKR1C1, AKR1C2 and AKR1C3 expression and increased ROS production after cisplatin treatment in SKOV3 cells. Moreover, a significant activation of the pJNK/p38 pathway and decreased phosphorylation of Activating transcription factor-2 (ATF2), a member of the leucine zipper family of DNA-binding proteins, implicated as a tumour suppressor, was observed in NRF2 knockdown cells, suggesting that NRF2 markedly modulates cisplatin resistance, regulating the AKR family members via the activation of the pJNK/p38 pathway [79].
Hepatocyte Growth Factor Receptor (HGFR/c-MET) and Epidermal Growth Factor Receptor (EGFR) are cell surface receptor tyrosine kinases (RTK), primarily expressed by epithelial cells that modulate different cell functions, including cell proliferation, survival and motility. Additionally, they can induce chemotherapy resistance [80]. Interestingly, NRF2 silencing increases miR-206 expression and reduces c-MET and EGFR levels through the direct binding to the 3′-untranslated region of the c-MET and EGFR genes in SKOV3 cells. The increased miR-206 levels have repressed c-MET/EGFR expression, inhibiting cell proliferation. In addition, miR-206 inhibits BCRP/ABCG2 expression, the human breast cancer resistance protein (BCRP, gene symbol ABCG2) that is an ATP-binding cassette (ABC) efflux transporter, and increases doxorubicin sensitivity, suggesting NRF2 as a modulator of BCRP/ABCG2 expression via miR-206 regulation [81].
ABC transporters are a superfamily of thirteen transporter (ABCA3, ABCB1 (MDR1), ABCB6, ABCB8, ABCB10, ABCB11, ABCC1 (MRP1), ABCC4, ABCC9, ABCD3, ABCD4, ABCE1, and ABCF2) proteins that transport various molecules across membranes, utilizing ATP as an energy source [82]. It has been reported that many of the ABC transporters are involved in chemoresistance occurrence, including ABCF2 that is involved in cisplatin resistance [83]. Interestingly, it has been reported that ABCF2 has a functional antioxidant response element (ARE) in its promoter region, suggesting ABCF2 as an NRF2 target gene. In fact, A2780 cells, overexpressing NRF2, contain high levels of ABCF2 and are more resistant to cisplatin-induced apoptosis, while the NRF2-knockdown A2780 cell line contains low ABCF2 levels and is more sensitive to cisplatin treatments. Furthermore, ABCF2 overexpression decreases apoptosis and increases cell viability following cisplatin treatment, indicating ABCF2 as a novel NRF2 target gene, playing a critical role in cisplatin resistance in ovarian cancer [84].
Solute carrier family 40 member 1 (SLC40A1) is an iron exporter, essential for iron metabolism homeostasis [85]. Interestingly, cisplatin-resistant ovarian cancer cells (A2780CP, COC1/DDP, PEO4) increase NRF2 levels and reduce SLC40A1 levels compared with cisplatin-sensitive cells (A2780, COC1, PEO1). Moreover, NRF2 knockdown leads to increased expression of SLC40A1, while NRF2 overexpression decreases SLC40A1 expression, showing that SLC40A1 expression is controlled by NRF2. In conclusion, SLC40A1 overexpression could reverse cisplatin resistance induced by NRF2, while SLC40A1 knockdown could restore cisplatin resistance and increase iron concentration. [86]. Studies discussed in this section are summarized in Table 3.

Table 3.
NRF2 cellular targets in ovarian cancer.
5. NRF2 in Ovarian Function Preservation
Ovarian cancer is an important worldwide public health problem and thousands of young women are exposed to cytotoxic chemotherapy, causing DNA damage and apoptosis of oocytes, leading to infertility and ovarian aging [87,88]. Thus, preserving ovarian function and fertility in young women exposed to these therapies is important to guarantee a major quality-of-life factor in these patients. Currently, cryopreservation of gametes and ovarian tissues is an important tool to preserve fertility in young women, but it cannot reverse menopause or restore the ovarian function [89,90]. Thus, finding natural or synthetic compounds able to preserve ovarian function during chemotherapy may be a more useful way than cryopreservation, in maintaining fertility in these patients. In addition, cryopreservation of ovarian tissues induces ROS generation and oxidative stress that damage follicles, affecting the recovery efficiency and pregnancy rate.
Doxorubicin (DOX) is one of the most used antitumour drugs that causes gonad toxicity, inducing oxidative stress and leading to ovarian dysfunction [87]. Interestingly, Niringiyumukiza and colleagues found that DOX intraperitoneal injection in ICR mice increases NRF2, HO-1 and catalase (CAT), while reducing Glutathione peroxidase (GSH-Px) and SOD-1 expressions. DOX administration with SB216763, a potent Glycogen synthase kinase 3 (GSK-3) inhibitor, enhances NRF2 expression, restoring GSH-Px and SOD-1 expression levels. Moreover, DOX significantly decreases the number of primordial, primary, preantral and antral follicles, while it increases the number of atretic follicles, but these effects were reversed by SB216763 administration. Furthermore, SB216763 and DOX combined administration reduces the mature oocyte abnormalities, suggesting that GSK-3 and NRF2 crosstalk may reduce DOX-induced ovarian damages [91]. Thus, the use of naturals or synthetic GSK-3 inhibitors [92] could be useful tools for fertility preserving in young women undergoing DOX chemotherapy treatments.
Natural compounds (also called phytochemicals or phytonutrients) are biological substances present in plants (e.g., carotenoids, flavonoids, anthocyanins and polyphenols) that are normally used to protect themselves from external influences or against predators [93,94]. Although the precise mechanism of action of many natural compounds is unknown, these are normally used as worldwide supplements, showing beneficial effects in many diseases [93,95,96,97,98]. Despite remarkable toxicities, Busulfan, Cyclophosphamide and Melphan (Bu/Cy/Mel) is one of the most frequent drug combinations used in the treatment of patients with haematologic malignancy [99,100]. Interestingly, Wu and colleagues found that Resveratrol (3,5,4′-trihydroxy-trans-stilbene, RES), a natural phenol with antioxidant properties, derived from plants, relieves oogonial stem cells loss, attenuating the busulfan/cyclophosphamide (Bu/Cy)-induced oxidative apoptosis in mouse ovaries. RES could exert antioxidant function, activating NRF2, and it could be used combined to chemotherapeutics to prevent chemotherapy-induced ovarian aging [101].
Melatonin (N-acetyl-5-methoxytryptamine) is mainly synthesized and secreted by the pineal organ, acting as scavenger for many free radicals, and as an antioxidant, upregulating the expression of antioxidant proteins [102]. Interestingly, it has been shown that melatonin could protect follicular integrity, preventing cell apoptosis, decreasing ROS production, malondialdehyde (MDA) and nitric oxide (NO) levels. Moreover, melatonin could increase the activities of glutathione peroxidases (GSH-Px), GSH, CAT, and SOD in cryopreserved ovarian tissues. These effects may be related to NRF2 pathway activation, since the authors found increased NRF2 downstream genes, such as haem oxygenase-1 (HO-1), glutathione S-transferase M1 (GSTM1), SOD, and CAT. In summary, melatonin plays an important role in protecting follicular integrity during cryopreservation, acting not only as a direct ROS scavenger, but also inducing antioxidative enzymes activation, probably modulating the NRF2 pathway [103].
Cyclophosphamide (CTX) is a common drug used to treat female cancers but induces ROS production, damaging DNA and inducing follicular apoptosis, leading to early menopause and infertility [104]. For this reason, preserving female fertility during CTX treatment is very important. Interestingly, female mice exposed to CTX and treated with Epigallocatechin gallate (EGCG) and theaflavins (TFs), two natural compounds derived from green tea or black tea, improved the ovarian endocrine function and reproductivity. These natural compounds reduce DNA follicular damage due to oxidation induced by activating the NRF2/HO-1 and SOD2 pathways and reducing the apoptosis in growing follicles [105].
The organic solvent, 1-Bromopropane (1-BP), also known as n-propyl bromide, is widely used in industrial and commercial applications. However, it has been reported that exposure to 1-BP can cause toxic effects on the nervous system [106] and induce ovarian dysfunction [107]. Yang and colleagues showed that 1-BP treatments led to an increase in both ROS and MDA production and decreased SOD activity in OVCAR-3 cells. Moreover, they found that 1-BP activates NRF2, increases HO-1 expression and apoptosis. Interestingly, vitamin C alleviates 1-BP-induced apoptosis, activating the NRF2 pathway. Therefore, it can be deduced that 1-BP induces oxidative stress and apoptosis by inactivating NRF2 signalling in OVCAR-3 cells [108].
Cigarette smoke contains thousands of harmful components that have been reported to be dangerous on female reproductive organs, including ovaries, causing ovarian dysfunction. In fact, the use of Cigarette Smoke Extract (CSE), commonly used to simulate smoking effects in in vitro studies, showed that it can impair ovulation, oocyte morphology and causes apoptosis [109]. CSE treatments reduce cell proliferation by reducing Cyclins B1 and D1 expression, and induce apoptosis, modulating the Bcl-2 signalling in SKOV3 and OVCAR3 ovarian cancer cell lines. Additionally, CSE induces oxidative stress, increasing ROS levels and decreasing NRF2 expression by increasing KEAP1. This causes ovarian function damage, inducing the inhibition of cell proliferation and oxidative stress, probably by NRF2 activity [110].
6. Conclusions and Further Remarks
Recent studies show that the NRF2/KEAP1/ARE pathway plays a key role in many processes involved in the regulation of ovarian cancer progression, proliferation and chemoresistance. It is clear that NQO1 and NRF2 are highly expressed in ovarian carcinoma compared with normal tissues and that NRF2 expression increases with ovarian carcinoma stage advancing. Moreover, low KEAP1 expression is associated with disease recurrence and death, while high KEAP1 expression is predictive of better overall and disease-free survival. Interestingly, it has been reported that both low nuclear NRF2 expression and high KEAP1 expression depend on the age of patients, suggesting that efficiency in countering ROS decreases with aging and it is associated to an increased risk of carcinogenesis. Moreover, NRF2 can regulate ERα and PGR expressions in ovarian cancer cells, playing a pivotal role in cell response to oestrogen and progesterone (see Table 1 and Table 3).
Several lines of research have demonstrated that NRF2 signalling can be indirectly modulated by non-coding RNA, such as miR-181d and Lin-H19, and that these can modulate drug response in ovarian cancer [40,46]. Moreover, p62 can activate NRF2, protecting cancer cells from chemotherapeutic agents inducing autophagy [53,111]. Moreover, it is known a direct action of NRF2 on proteins involved in chemoresistance such as AKR1C1-3, ABCF2, SLC40A1, as well as on the modulation of important growth factor receptors, such as c-MET, ErbB2 and EGFR regulating tumour growth [76,81].
Unfortunately, chemotherapy causes cytotoxic effects, both in cancer and normal cells. Every year, thousands of young women are exposed to chemotherapy, with serious consequences on fertility and ovarian tissue [87]. To date, the ovarian tissues and gametes cryopreservation is the only way to give a chance to these women to become pregnant [89]. It has been reported that GSK-3 inhibitors, resveratrol, melatonin, epigallocatechin gallate, theaflavins can protect ovarian tissues exposed to chemotherapeutic agents or cryopreservation, activating NRF2 signalling to preserve female fertility (see Table 4).

Table 4.
Modulators of NRF2 in ovarian preservation.
In conclusion, NRF2 has many important functions, modulating different enzymes, receptors and miRNAs (see Figure 2). Thus, the NRF2/KEAP1/ARE pathway can protect cells from oxidative stress and regulate cancerous cells response to chemotherapeutic agents. Therefore, NRF2 can be considered a promising target for future research on ovarian cancer progression and treatment and could have a significant clinical impact in developing new therapies. Moreover, the use of natural or synthetic compounds activating NRF2 can play a pivotal role in ovarian function preservation in patients undergoing chemotherapy treatments.
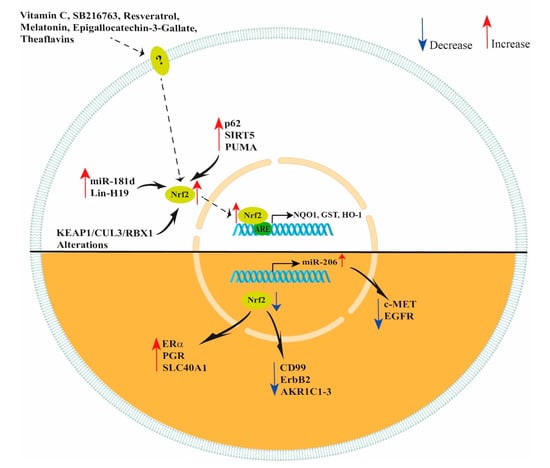
Figure 2.
Schematic representation of NRF2 modulation. Vitamin C, SB216763, resveratrol, melatonin, epigallocatechin-3-gallate and theaflavins increase NRF2 expression in ovarian cells improving their response to oxidant agents. In ovarian cancer cells, increased levels of miR-181d, Lin-H19, p62, SIRT5, PUMA and KEAP1/CUL3/RBX1 alterations lead to an increase in NRF2 expression. However, decreased levels of NRF2 lead to an increased expression of ERα, PGR, SLC40A1, miR-206 and decreased expression of CD99, ErbB2 and AKR1C1-3. In addition, NRF2 indirectly decreases c-MET and EGFR expression by increasing miR-206 levels.
Author Contributions
Conceptualization, G.T. and D.M.; methodology, G.T. and S.F.; writing the original draft, G.T.; editing, D.M., E.M., G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Marche Polytechnic University (Contributi Ricerca Scientifica 2022) to G.G.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rojas, V.; Hirshfield, K.M.; Ganesan, S.; Rodriguez-Rodriguez, L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int. J. Mol. Sci. 2016, 17, 2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saed, G.M.; Diamond, M.P.; Fletcher, N.M. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Mateuszuk, L.; Campagna, R.; Kutryb-Zajac, B.; Kus, K.; Slominska, E.M.; Smolenski, R.T.; Chlopicki, S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem. Pharmacol. 2020, 178, 114019. [Google Scholar] [CrossRef]
- Campagna, R.; Mateuszuk, L.; Wojnar-Lason, K.; Kaczara, P.; Tworzydlo, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Hine, C.M.; Mitchell, J.R. NRF2 and the Phase II Response in Acute Stress Resistance Induced by Dietary Restriction. J. Clin. Exp. Pathol. 2012, 4 (Suppl. S4), 7329. [Google Scholar] [CrossRef] [Green Version]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [Green Version]
- Campagna, R.; Bacchetti, T.; Salvolini, E.; Pozzi, V.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Ferretti, G.; Offidani, A.; et al. Paraoxonase-2 Silencing Enhances Sensitivity of A375 Melanoma Cells to Treatment with Cisplatin. Antioxidants 2020, 9, 1238. [Google Scholar] [CrossRef]
- Yang, L.; Xie, H.J.; Li, Y.Y.; Wang, X.; Liu, X.X.; Mai, J. Molecular mechanisms of platinumbased chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 2022, 232, 114205. [Google Scholar] [CrossRef] [PubMed]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.M.; Manandhar, S.; Lee, H.R.; Park, H.M.; Kwak, M.K. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: Implication to cancer cell resistance. Cancer Lett. 2008, 260, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.S.; Goltsov, A.; Langdon, S.P.; Harrison, D.J.; Bown, J.; Deeni, Y. Quantitative analysis of NRF2 pathway reveals key elements of the regulatory circuits underlying antioxidant response and proliferation of ovarian cancer cells. J. Biotechnol. 2015, 202, 12–30. [Google Scholar] [CrossRef]
- Van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Pignata, S.; Cecere, S.C.; Du Bois, A.; Harter, P.; Heitz, F. Treatment of recurrent ovarian cancer. Ann. Oncol. 2017, 28, viii51–viii56. [Google Scholar] [CrossRef]
- Shim, G.S.; Manandhar, S.; Shin, D.H.; Kim, T.H.; Kwak, M.K. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic. Biol. Med. 2009, 47, 1619–1631. [Google Scholar] [CrossRef]
- Manandhar, S.; Lee, S.; Kwak, M.K. Effect of stable inhibition of NRF2 on doxorubicin sensitivity in human ovarian carcinoma OV90 cells. Arch. Pharm. Res. 2010, 33, 717–726. [Google Scholar] [CrossRef]
- Dittmer, J. The role of the transcription factor Ets1 in carcinoma. Semin. Cancer Biol. 2015, 35, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Hsing, M.; Wang, Y.; Rennie, P.S.; Cox, M.E.; Cherkasov, A. ETS transcription factors as emerging drug targets in cancer. Med. Res. Rev. 2020, 40, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, K.; Viswakarma, N.; Rana, A.; Rana, B. Transcription Factors in Cancer Development and Therapy. Cancers 2020, 12, 2296. [Google Scholar] [CrossRef]
- Wilson, L.A.; Gemin, A.; Espiritu, R.; Singh, G. ets-1 is transcriptionally up-regulated by H2O2 via an antioxidant response element. FASEB J. 2005, 19, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.; Abd El-Maqsoud, N.M.R.; El Gelany, S.A.A. Correlation of NQO1 and Nrf2 in Female Genital Tract Cancer and Their Precancerous Lesions (Cervix, Endometrium and Ovary). World J. Oncol. 2015, 6, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Liew, P.L.; Hsu, C.S.; Liu, W.M.; Lee, Y.C.; Lee, Y.C.; Chen, C.L. Prognostic and predictive values of Nrf2, Keap1, p16 and E-cadherin expression in ovarian epithelial carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 5642–5649. [Google Scholar]
- Cho, H.Y.; Kim, K.; Kim, Y.B.; Kim, H.; No, J.H. Expression Patterns of Nrf2 and Keap1 in Ovarian Cancer Cells and their Prognostic Role in Disease Recurrence and Patient Survival. Int. J. Gynecol. Cancer 2017, 27, 412–419. [Google Scholar] [CrossRef]
- Czogalla, B.; Kahaly, M.; Mayr, D.; Schmoeckel, E.; Niesler, B.; Kolben, T.; Burges, A.; Mahner, S.; Jeschke, U.; Trillsch, F. Interaction of ERalpha and NRF2 Impacts Survival in Ovarian Cancer Patients. Int. J. Mol. Sci. 2018, 20, 112. [Google Scholar] [CrossRef] [Green Version]
- Czogalla, B.; Kahaly, M.; Mayr, D.; Schmoeckel, E.; Niesler, B.; Hester, A.; Zeder-Goss, C.; Kolben, T.; Burges, A.; Mahner, S.; et al. Correlation of NRF2 and progesterone receptor and its effects on ovarian cancer biology. Cancer Manag. Res. 2019, 11, 7673–7684. [Google Scholar] [CrossRef] [Green Version]
- Licini, C.; Avellini, C.; Picchiassi, E.; Mensa, E.; Fantone, S.; Ramini, D.; Tersigni, C.; Tossetta, G.; Castellucci, C.; Tarquini, F.; et al. Pre-eclampsia predictive ability of maternal miR-125b: A clinical and experimental study. Transl. Res. 2021, 228, 13–27. [Google Scholar] [CrossRef]
- Zheng, H.; Yu, Z.; Wang, H.; Liu, H.; Chen, X. MicroRNA-195-5p facilitates endothelial dysfunction by inhibiting vascular endothelial growth factor A in gestational diabetes mellitus. Reprod. Biol. 2022, 22, 100605. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, Y.; Chu, Z. MiR-212-3p promotes proliferation and migration of trophoblast in fetal growth restriction by targeting placental growth factor. Bioengineered 2021, 12, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Avellini, C.; Licini, C.; Lazzarini, R.; Gesuita, R.; Guerra, E.; Tossetta, G.; Castellucci, C.; Giannubilo, S.R.; Procopio, A.; Alberti, S.; et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 2017, 8, 58642–58653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulidankazha, C.; Han, W.; He, T.; Lin, H.; Cheng, K.; Nie, X.; Chen, Q. miR-146a Inhibited Pancreatic Cancer Cell Proliferation by Targeting SOX7. J. Healthc. Eng. 2022, 2022, 2240605. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Li, Z.; Li, S.; Wu, C. MiR-139-5p Inhibits the Development of Gastric Cancer through Targeting TPD52. J. Healthc. Eng. 2022, 2022, 4033373. [Google Scholar] [CrossRef]
- Shi, L.; Su, Y.; Zheng, Z.; Qi, J.; Wang, W.; Wang, C. miR-146b-5p promotes colorectal cancer progression by targeting TRAF6. Exp. Ther. Med. 2022, 23, 231. [Google Scholar] [CrossRef]
- Moghbeli, M. MicroRNAs as the critical regulators of Cisplatin resistance in ovarian cancer cells. J. Ovarian Res. 2021, 14, 127. [Google Scholar] [CrossRef]
- Masadah, R.; Rauf, S.; Pratama, M.Y.; Tiribelli, C.; Pascut, D. The Role of microRNAs in the Cisplatin- and Radio-Resistance of Cervical Cancer. Cancers 2021, 13, 1168. [Google Scholar] [CrossRef]
- Chen, P.H.; Smith, T.J.; Wu, J.; Siesser, P.F.; Bisnett, B.J.; Khan, F.; Hogue, M.; Soderblom, E.; Tang, F.; Marks, J.R.; et al. Glycosylation of KEAP1 links nutrient sensing to redox stress signaling. EMBO J. 2017, 36, 2233–2250. [Google Scholar] [CrossRef]
- Huang, W.; Chen, L.; Zhu, K.; Wang, D. Oncogenic microRNA-181d binding to OGT contributes to resistance of ovarian cancer cells to cisplatin. Cell Death Discov. 2021, 7, 379. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X. Non-coding RNAs in DNA damage response. Am. J. Cancer Res. 2012, 2, 658–675. [Google Scholar] [PubMed]
- Pierce, J.B.; Zhou, H.; Simion, V.; Feinberg, M.W. Long Noncoding RNAs as Therapeutic Targets. Adv. Exp. Med. Biol. 2022, 1363, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Zamaraev, A.V.; Volik, P.I.; Sukhikh, G.T.; Kopeina, G.S.; Zhivotovsky, B. Long non-coding RNAs: A view to kill ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188584. [Google Scholar] [CrossRef] [PubMed]
- Smits, G.; Mungall, A.J.; Griffiths-Jones, S.; Smith, P.; Beury, D.; Matthews, L.; Rogers, J.; Pask, A.J.; Shaw, G.; VandeBerg, J.L.; et al. Conservation of the H19 noncoding RNA and H19-IGF2 imprinting mechanism in therians. Nat. Genet. 2008, 40, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qi, M.; Fei, X.; Wang, X.; Wang, K. LncRNA H19: A novel oncogene in multiple cancers. Int. J. Biol. Sci. 2021, 17, 3188–3208. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Xu, H.; Suo, S.S.; Xu, X.L.; Ni, M.W.; Gu, L.H.; Chen, W.; Wang, L.Y.; Zhao, Y.; Tian, B.; et al. The Essential Role of H19 Contributing to Cisplatin Resistance by Regulating Glutathione Metabolism in High-Grade Serous Ovarian Cancer. Sci. Rep. 2016, 6, 26093. [Google Scholar] [CrossRef] [Green Version]
- Staurengo-Ferrari, L.; Badaro-Garcia, S.; Hohmann, M.S.N.; Manchope, M.F.; Zaninelli, T.H.; Casagrande, R.; Verri, W.A., Jr. Contribution of Nrf2 Modulation to the Mechanism of Action of Analgesic and Anti-inflammatory Drugs in Pre-clinical and Clinical Stages. Front. Pharmacol. 2018, 9, 1536. [Google Scholar] [CrossRef] [Green Version]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Jeong, S.J.; Zhang, X.; Rodriguez-Velez, A.; Evans, T.D.; Razani, B. p62/SQSTM1 and Selective Autophagy in Cardiometabolic Diseases. Antioxid. Redox Signal. 2019, 31, 458–471. [Google Scholar] [CrossRef]
- Cerda-Troncoso, C.; Varas-Godoy, M.; Burgos, P.V. Pro-Tumoral Functions of Autophagy Receptors in the Modulation of Cancer Progression. Front. Oncol. 2020, 10, 619727. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; White, E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019, 33, 610–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Su, J.; Xu, Y.; Kang, J.; Li, H.; Zhang, L.; Yi, H.; Xiang, X.; Liu, F.; Sun, L. p62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. Eur. J. Cancer 2011, 47, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Yu, H.; Gu, S.; Xu, Y.; Su, J.; Li, H.; Kang, J.; Cui, M. p62/SQSTM1 is involved in cisplatin resistance in human ovarian cancer cells via the Keap1-Nrf2-ARE system. Int. J. Oncol. 2014, 45, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zuo, Y.; Feng, Y.; Zhang, M. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour Biol. 2014, 35, 10699–10705. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, X.; Chen, T.; Gao, W.; Wu, Z.; Hu, Z.; Wei, D.; Gao, C.; Li, Q. Targeting a Sirt5-Positive Subpopulation Overcomes Multidrug Resistance in Wild-Type Kras Colorectal Carcinomas. Cell Rep. 2018, 22, 2677–2689. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, S.; Gai, J.; Guan, J.; Li, J.; Li, Y.; Zhao, J.; Zhao, C.; Fu, L.; Li, Q. SIRT5 Promotes Cisplatin Resistance in Ovarian Cancer by Suppressing DNA Damage in a ROS-Dependent Manner via Regulation of the Nrf2/HO-1 Pathway. Front. Oncol. 2019, 9, 754. [Google Scholar] [CrossRef] [Green Version]
- Martinez, V.D.; Vucic, E.A.; Thu, K.L.; Pikor, L.A.; Lam, S.; Lam, W.L. Disruption of KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex components by multiple genetic mechanisms: Association with poor prognosis in head and neck cancer. Head Neck 2015, 37, 727–734. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Spentzos, D.; Fountzilas, E.; Francoeur, N.; Sanisetty, S.; Grammatikos, A.P.; Hecht, J.L.; Cannistra, S.A. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011, 71, 5081–5089. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Zhou, Q.; Zhang, Z.; Wang, Q.; Sun, Y.; Yi, X.; Feng, Y. NRF2 is overexpressed in ovarian epithelial carcinoma and is regulated by gonadotrophin and sex-steroid hormones. Oncol. Rep. 2012, 27, 1918–1924. [Google Scholar] [CrossRef] [Green Version]
- Martinez, V.D.; Vucic, E.A.; Thu, K.L.; Pikor, L.A.; Hubaux, R.; Lam, W.L. Unique pattern of component gene disruption in the NRF2 inhibitor KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex in serous ovarian cancer. Biomed. Res. Int. 2014, 2014, 159459. [Google Scholar] [CrossRef]
- Li, M. The role of P53 up-regulated modulator of apoptosis (PUMA) in ovarian development, cardiovascular and neurodegenerative diseases. Apoptosis 2021, 26, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Vavrova, J.; Rezacova, M. Importance of proapoptotic protein PUMA in cell radioresistance. Folia Biol (Praha) 2014, 60, 53–56. [Google Scholar] [PubMed]
- Yu, J.; Zhang, L. PUMA, a potent killer with or without p53. Oncogene 2008, 27 (Suppl. S1), S71–S83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhao, X.; Tang, M.; Li, L.; Lei, Y.; Cheng, P.; Guo, W.; Zheng, Y.; Wang, W.; Luo, N.; et al. The role of ROS and subsequent DNA-damage response in PUMA-induced apoptosis of ovarian cancer cells. Oncotarget 2017, 8, 23492–23506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, T.; Iijima, K.; Miyamoto, M.; Nakahara, I.; Tanaka, H.; Ohtsuji, M.; Suzuki, T.; Kobayashi, A.; Yokota, J.; Sakiyama, T.; et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008, 68, 1303–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pylvas-Eerola, M.; Liakka, A.; Puistola, U.; Koivunen, J.; Karihtala, P. Cancer Stem Cell Properties as Factors Predictive of Chemoresistance in Neoadjuvantly-treated Patients with Ovarian Cancer. Anticancer Res. 2016, 36, 3425–3431. [Google Scholar]
- Cunat, S.; Hoffmann, P.; Pujol, P. Estrogens and epithelial ovarian cancer. Gynecol. Oncol. 2004, 94, 25–32. [Google Scholar] [CrossRef]
- Galtier-Dereure, F.; Capony, F.; Maudelonde, T.; Rochefort, H. Estradiol stimulates cell growth and secretion of procathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. J. Clin. Endocrinol. Metab. 1992, 75, 1497–1502. [Google Scholar] [CrossRef]
- Langdon, S.P.; Hirst, G.L.; Miller, E.P.; Hawkins, R.A.; Tesdale, A.L.; Smyth, J.F.; Miller, W.R. The regulation of growth and protein expression by estrogen in vitro: A study of 8 human ovarian carcinoma cell lines. J. Steroid Biochem. Mol. Biol. 1994, 50, 131–135. [Google Scholar] [CrossRef]
- Luo, H.; Li, S.; Zhao, M.; Sheng, B.; Zhu, H.; Zhu, X. Prognostic value of progesterone receptor expression in ovarian cancer: A meta-analysis. Oncotarget 2017, 8, 36845–36856. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Lee, M.; Shin, S.; Park, J. Apoptosis induced by progesterone in human ovarian cancer cell line SNU-840. J. Cell. Biochem. 2001, 82, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Pasello, M.; Manara, M.C.; Scotlandi, K. CD99 at the crossroads of physiology and pathology. J. Cell Commun. Signal. 2018, 12, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.L.; Kim, H.S.; Ahn, G. Immunoexpression of inhibin alpha subunit, inhibin/activin betaA subunit and CD99 in ovarian tumors. Arch. Pathol. Lab. Med. 2000, 124, 563–569. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Li, H.; Wu, S.; Liu, Z. Nrf2 induced cisplatin resistance in ovarian cancer by promoting CD99 expression. Biochem. Biophys. Res. Commun. 2019, 518, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Choi, B.H.; Jung, K.A.; Ryoo, I.G.; Song, M.; Kang, S.J.; Choi, H.G.; Kim, J.A.; Park, P.H.; Kwak, M.K. NRF2 inhibition represses ErbB2 signaling in ovarian carcinoma cells: Implications for tumor growth retardation and docetaxel sensitivity. Free Radic. Biol. Med. 2012, 52, 1773–1785. [Google Scholar] [CrossRef]
- Mindnich, R.D.; Penning, T.M. Aldo-keto reductase (AKR) superfamily: Genomics and annotation. Hum. Genom. 2009, 3, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M.; Jonnalagadda, S.; Trippier, P.C.; Rizner, T.L. Aldo-Keto Reductases and Cancer Drug Resistance. Pharmacol. Rev. 2021, 73, 1150–1171. [Google Scholar] [CrossRef]
- Chen, J.; Solomides, C.; Simpkins, F.; Simpkins, H. The role of Nrf2 and ATF2 in resistance to platinum-based chemotherapy. Cancer Chemother. Pharmacol. 2017, 79, 369–380. [Google Scholar] [CrossRef]
- Wood, G.E.; Hockings, H.; Hilton, D.M.; Kermorgant, S. The role of MET in chemotherapy resistance. Oncogene 2021, 40, 1927–1941. [Google Scholar] [CrossRef]
- Choi, B.H.; Ryu, D.Y.; Ryoo, I.G.; Kwak, M.K. NFE2L2/NRF2 silencing-inducible miR-206 targets c-MET/EGFR and suppresses BCRP/ABCG2 in cancer cells. Oncotarget 2017, 8, 107188–107205. [Google Scholar] [CrossRef] [Green Version]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Khalil, H.S.; Langdon, S.P.; Goltsov, A.; Soininen, T.; Harrison, D.J.; Bown, J.; Deeni, Y.Y. A novel mechanism of action of HER2 targeted immunotherapy is explained by inhibition of NRF2 function in ovarian cancer cells. Oncotarget 2016, 7, 75874–75901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, L.; Wu, J.; Dodson, M.; Rojo de la Vega, E.M.; Ning, Y.; Zhang, Z.; Yao, M.; Zhang, D.D.; Xu, C.; Yi, X. ABCF2, an Nrf2 target gene, contributes to cisplatin resistance in ovarian cancer cells. Mol. Carcinog. 2017, 56, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gogele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2014, 5, 4926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Bao, L.; Zhang, Z.; Yi, X. Nrf2 induces cisplatin resistance via suppressing the iron export related gene SLC40A1 in ovarian cancer cells. Oncotarget 2017, 8, 93502–93515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Aharon, I.; Bar-Joseph, H.; Tzarfaty, G.; Kuchinsky, L.; Rizel, S.; Stemmer, S.M.; Shalgi, R. Doxorubicin-induced ovarian toxicity. Reprod. Biol. Endocrinol. 2010, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Meirow, D. Reproduction post-chemotherapy in young cancer patients. Mol. Cell. Endocrinol. 2000, 169, 123–131. [Google Scholar] [CrossRef]
- Linkeviciute, A.; Boniolo, G.; Chiavari, L.; Peccatori, F.A. Fertility preservation in cancer patients: The global framework. Cancer Treat. Rev. 2014, 40, 1019–1027. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fertility Preservation in Women. N. Engl. J. Med. 2018, 378, 400–401. [Google Scholar] [CrossRef]
- Niringiyumukiza, J.D.; Cai, H.; Chen, L.; Li, Y.; Wang, L.; Zhang, M.; Xu, X.; Xiang, W. Protective properties of glycogen synthase kinase-3 inhibition against doxorubicin-induced oxidative damage to mouse ovarian reserve. Biomed. Pharmacother. 2019, 116, 108963. [Google Scholar] [CrossRef] [PubMed]
- Eldar-Finkelman, H.; Martinez, A. GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS. Front. Mol. Neurosci. 2011, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marzioni, D. The Multifaced Actions of Curcumin in Pregnancy Outcome. Antioxidants 2021, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Rejhova, A.; Opattova, A.; Cumova, A.; Sliva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Wozniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Licini, C.; Marzioni, D.; Mattioli-Belmonte, M. The multifaced role of HtrA1 in the development of joint and skeletal disorders. Bone 2022, 157, 116350. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Long, B.; Zhang, X.; Wen, R.; He, Y.; Li, X. A novel intensive conditioning regimen for allogeneic hematopoietic stem cell transplantation in the treatment of relapsed/refractory acute myeloid leukemia. Neoplasma 2021, 68, 1351–1358. [Google Scholar] [CrossRef]
- Phillips, G.L.; Shepherd, J.D.; Barnett, M.J.; Lansdorp, P.M.; Klingemann, H.G.; Spinelli, J.J.; Nevill, T.J.; Chan, K.W.; Reece, D.E. Busulfan, cyclophosphamide, and melphalan conditioning for autologous bone marrow transplantation in hematologic malignancy. J. Clin. Oncol. 1991, 9, 1880–1888. [Google Scholar] [CrossRef]
- Wu, M.; Ma, L.; Xue, L.; Ye, W.; Lu, Z.; Li, X.; Jin, Y.; Qin, X.; Chen, D.; Tang, W.; et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging 2019, 11, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Nickkholgh, A.; Yi, X.; Bruns, H.; Gross, M.L.; Hoffmann, K.; Mohr, E.; Zorn, M.; Buchler, M.W.; Schemmer, P. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J. Pineal Res. 2009, 46, 365–372. [Google Scholar] [CrossRef]
- Sun, T.C.; Liu, X.C.; Yang, S.H.; Song, L.L.; Zhou, S.J.; Deng, S.L.; Tian, L.; Cheng, L.Y. Melatonin Inhibits Oxidative Stress and Apoptosis in Cryopreserved Ovarian Tissues via Nrf2/HO-1 Signaling Pathway. Front. Mol. Biosci. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Xu, Z.; Li, X.; Du, D.; Wu, T.; Zhou, S.; Yan, W.; Wu, M.; Jin, Y.; Zhang, J.; et al. Epigallocatechin gallate and theaflavins independently alleviate cyclophosphamide-induced ovarian damage by inhibiting the overactivation of primordial follicles and follicular atresia. Phytomedicine 2021, 92, 153752. [Google Scholar] [CrossRef] [PubMed]
- Samukawa, M.; Ichihara, G.; Oka, N.; Kusunoki, S. A case of severe neurotoxicity associated with exposure to 1-bromopropane, an alternative to ozone-depleting or global-warming solvents. Arch. Intern. Med. 2012, 172, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ichihara, G.; Wang, H.; Yu, X.; Maeda, K.; Tsukamura, H.; Kamijima, M.; Nakajima, T.; Takeuchi, Y. Exposure to 1-bromopropane causes ovarian dysfunction in rats. Toxicol. Sci. 2003, 71, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xiang, Y.; Zhou, W.; Zhong, X.; Zhang, Y.; Lin, D.; Huang, X. 1-Bromopropane-induced apoptosis in OVCAR-3 cells via oxidative stress and inactivation of Nrf2. Toxicol. Ind. Health 2021, 37, 59–67. [Google Scholar] [CrossRef]
- Mai, Z.; Lei, M.; Yu, B.; Du, H.; Liu, J. The effects of cigarette smoke extract on ovulation, oocyte morphology and ovarian gene expression in mice. PLoS ONE 2014, 9, e95945. [Google Scholar] [CrossRef]
- Kim, C.W.; Go, R.E.; Hwang, K.A.; Bae, O.N.; Lee, K.; Choi, K.C. Effects of cigarette smoke extracts on apoptosis and oxidative stress in two models of ovarian cancer in vitro. Toxicol. Vitr. 2018, 52, 161–169. [Google Scholar] [CrossRef]
- Xia, M.H.; Yan, X.Y.; Zhou, L.; Xu, L.; Zhang, L.C.; Yi, H.W.; Su, J. p62 Suppressed VK3-induced Oxidative Damage through Keap1/Nrf2 Pathway in Human Ovarian Cancer Cells. J. Cancer 2020, 11, 1299–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).