Therapeutic Potential of Inducible Endogenous Cytoprotective Heme Oxygenase-1 in Mitigating SARS-CoV-2 Infection and Associated Inflammation
Abstract
1. Introduction
2. HO-1 in Host Defense against Viral Infections
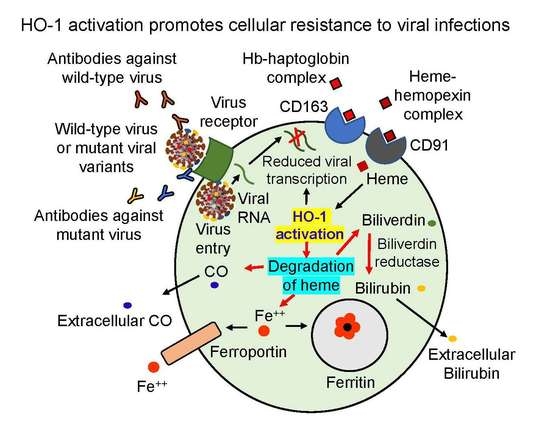
3. Potential of HO-1 in Enhancing Cellular Resistance to SARS-CoV-2
4. Potential of HO-1 in Regulating Innate Immune Function against SARS-CoV-2—Why Is it Critical for Managing COVID-19 Pandemic?
5. Anti-Inflammatory Potential of HO-1 in COVID-19-Associated Clinical Consequences
6. Summary and Perspectives
Funding
Conflicts of Interest
References
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in upper respiratory specimens of infected patients. N. Eng. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Duvigneau, J.C.; Esterbauer, H.; Kozlov, A.V. Role of heme oxygenase as a modulator of heme-mediated pathways. Antioxidants 2019, 8, 475. [Google Scholar] [CrossRef]
- Vogel, M.E.; Zucker, S.D. Bilirubin acts as an endogenous regulator of inflammation by disrupting adhesion molecule-mediated leukocyte migration. Inflamm. Cell Signal. 2016, 3, e1178. [Google Scholar] [CrossRef]
- Devadas, K.; Dhawan, S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J. Immunol. 2006, 176, 4252–4257. [Google Scholar] [CrossRef]
- Schmidt, W.N.; Mathahs, M.M.; Zhu, Z. Heme and HO-1 inhibition of HCV, HBV, and HIV. Front. Pharmacol. 2012, 3, 129. [Google Scholar] [CrossRef]
- Hill-Batorski, L.; Halfmann, P.; Neumann, G.; Kawaoka, Y. The cytoprotective enzyme heme oxygenase-1 suppresses Ebola virus replication. J. Virol. 2013, 87, 13795–13802. [Google Scholar] [CrossRef]
- Huang, H.; Falgout, B.; Takeda, K.; Yamada, K.M.; Dhawan, S. Nrf2-dependent induction of innate host defense via heme oxygenase-1 inhibits Zika virus infection. Virology 2017, 503, 1–5. [Google Scholar] [CrossRef]
- Pamplona, A.; Ferreira, A.; Balla, J.; Jeney, V.; Balla, G.; Epiphanio, S.; Chora, A.; Rodrigues, C.D.; Gregoire, I.P.; Cunha-Rodrigues, M.; et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007, 13, 703–710. [Google Scholar] [CrossRef]
- Hou, W.H.; Rossi, L.; Shan, Y.; Zheng, J.Y.; Lambrecht, R.W.; Bonkovsky, H.L. Iron increases HMOX1 and decreases hepatitis C viral expression in HCV-expressing cells. World. J. Gastroenterol. 2009, 15, 4499–4510. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Dabrazhnetskaya, A.; Pluznik, J.; Zheng, J.; Wu, Y.; Chizhikov, V.; Buehler, P.W.; Yamada, K.M.; Dhawan, S. Hemin activation abrogates Mycoplasma hyorhinis replication in chronically infected prostate cancer cells via heme oxygenase-1 induction. FEBS Open Bio. 2020, 11, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Nat. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2020, 6, 422–428. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-inflammatory actions of heme and other hemoglobin-derived DAMPs. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Wasan, H.; Reeta, K.H. Heme oxygenase-1 modulation: A potential therapeutic target for COVID-19 and associated complications. Free Radic Biol Med. 2020, 161, 263–271. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ahn, H.S.; Go, H.J.; Kim, D.Y.; Kim, J.H.; Lee, J.B.; Park, S.Y.; Song, C.S.; Lee, S.W.; Ha, S.D.; et al. Hemin as a novel candidate for treating COVID-19 via heme oxygenase-1 induction. Sci. Rep. 2021, 11, 21462. [Google Scholar] [CrossRef]
- Toro, A.; Ruiz, M.S.; Lage-Vickers, S.; Sanchis, P.; Sabater, A.; Pascual, G.; Seniuk, R.; Cascardo, F.; Ledesma-Bazan, S.; Vilicich, F.; et al. A journey into clinical relevance of heme oxygenase 1 for human inflammatory disease and viral clearance: Why does it matter on the COVID-19 scene? Antioxidants 2022, 11, 276. [Google Scholar] [CrossRef]
- Maestro, S.; Córdoba, K.M.; Olague, C.; Argemi, J.; Ávila, M.A.; González-Aseguinolaza, G.; Smerdou, C.; Fontanellas, A. Heme oxygenase-1 inducer hemin does not inhibit SARS-CoV-2 virus infection. Biomed. Pharmacother. 2021, 137, 111384. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, K.; Yuan, C.; Xing, R.; Ni, J.; Hu, G.; Chen, F.; Wang, X. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017, 39, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S. Simple and economical multifunctional therapeutic swab device system for treating upper respiratory infections, including SARS-CoV-2, on a mass-scale. Am. Pharma. Rev. 2022, 25, 20–24. [Google Scholar]
- Taefehshokr, N.; Taefehshokr, S.; Hemmat, N.; Heit, B. COVID-19: Perspectives on innate immune evasion. Front. Immunol. 2020, 11, 580641. [Google Scholar] [CrossRef]
- Rettig, T.A.; Harbin, J.N.; Harrington, A.; Dohmen, L.; Fleming, S.D. Evasion and interactions of the humoral innate immune response in pathogen evasion, autoimmune disease, and cancer. Clin. Immunol. 2015, 160, 244–254. [Google Scholar] [CrossRef][Green Version]
- Wagener, F.A.D.T.G.; Pickkers, P.; Peterson, S.; Immenschuh, S.; Abraham, N.G. Targeting the heme-heme oxygenase system to prevent severe complications following COVID-19 infections. Antioxidants 2020, 9, 540. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Dhawan, S. Innate cellular immunity for suppressing viral infections. Curr. Trends Immunol. 2021, 22, 19–21. [Google Scholar]
- Dhawan, S. Necessity for the evaluation of stimulated cellular immunity against SARS-CoV-2 infection. Curr. Trends Immunol. 2021, 22, 43–47. [Google Scholar]
- Jentho, E.; Novakovic, B.; Ruiz-Moreno, C.; Kourtzelis, I.; Martins, R.; Chavakis, T.; Soares, M.P.; Kalafati, L.; Guerra, J.; Roestel, F.; et al. Heme induces innate immune memory. bioRxiv 2019. [Google Scholar] [CrossRef]
- Thorne, L.G.; Bouhaddou, M.; Reuschl, A.K.; Zuliani-Alvarez, L.; Polacco, B.; Pelin, A.; Batra, J.; Whelan, M.V.; Hosmillo, M.; Fossati, A.; et al. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature 2022, 602, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, S.; Bessis, S.; Annane, D.; de la Grandmaison, G.L.; Cramer–Bordé, E.; Prideaux, B.; Eugenin, E.A.; Bomsel, M. COVID-19 Lung pathogenesis in SARS-CoV-2 autopsy cases. Front. Immunol. 2021, 12, 735922. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J. The multisystem inflammatory syndrome in adults with SARS-CoV-2 infection-another piece of an expanding puzzle. JAMA Netw. Open. 2021, 4, e2110344. [Google Scholar] [CrossRef] [PubMed]
- Gustine, J.N.; Jones, D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Sacco, K.; Castagnoli, R.; Vakkilainen, S.; Liu, C.; Delmonte, O.M.; Oguz, C.; Kaplan, I.M.; Alehashemi, S.; Burbelo, P.D.; Bhuyan, F.; et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 2022, 1–3. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Fernandez, P.; Guillen, M.I. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharm. Design. 2003, 9, 2541–2551. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhawan, S. Therapeutic Potential of Inducible Endogenous Cytoprotective Heme Oxygenase-1 in Mitigating SARS-CoV-2 Infection and Associated Inflammation. Antioxidants 2022, 11, 662. https://doi.org/10.3390/antiox11040662
Dhawan S. Therapeutic Potential of Inducible Endogenous Cytoprotective Heme Oxygenase-1 in Mitigating SARS-CoV-2 Infection and Associated Inflammation. Antioxidants. 2022; 11(4):662. https://doi.org/10.3390/antiox11040662
Chicago/Turabian StyleDhawan, Subhash. 2022. "Therapeutic Potential of Inducible Endogenous Cytoprotective Heme Oxygenase-1 in Mitigating SARS-CoV-2 Infection and Associated Inflammation" Antioxidants 11, no. 4: 662. https://doi.org/10.3390/antiox11040662
APA StyleDhawan, S. (2022). Therapeutic Potential of Inducible Endogenous Cytoprotective Heme Oxygenase-1 in Mitigating SARS-CoV-2 Infection and Associated Inflammation. Antioxidants, 11(4), 662. https://doi.org/10.3390/antiox11040662