Influence of Hydroxytyrosol Acetate Enrichment of an Oil Rich in Omega-6 Groups on the Evolution of Its Oxidation and Oxylipin Formation When Subjected to Accelerated Storage. A Global Study by Proton Nuclear Magnetic Resonance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Samples of Study
2.2. Storage Conditions
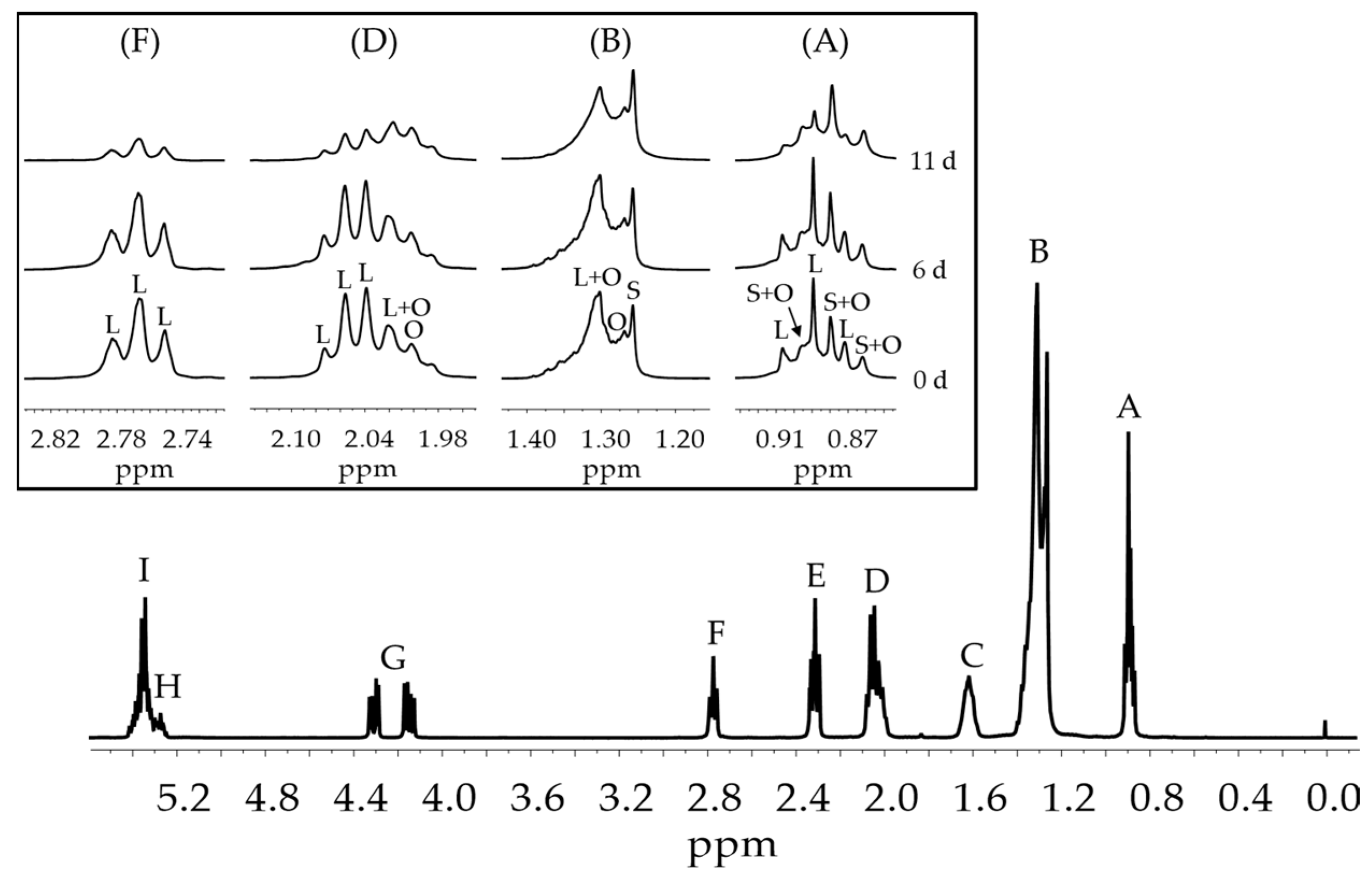
2.3. Study of the Samples Evolution by 1H NMR Spectroscopy
2.3.1. H NMR Spectroscopy Operating Conditions
2.3.2. Identification of Components
2.3.3. Quantification of the Components
2.4. Statistical Analysis
3. Results and Discussion
3.1. Degradation of Sunflower Oil Main Components and HTy-Ac Added in the Different Samples Submitted to Accelerated Storage
3.1.1. Evolution of the Concentration of Sunflower Oil Linoleic Acyl Group throughout the Accelerated Storage
3.1.2. Evolution of the HTy-Ac Concentration over the Storage Time in the Different Samples
3.2. Influence of Sunflower Oil Enrichment in HTy-Ac on Oxylipins Formation and Evolution of Their Concentration throughout the Accelerated Storage
3.2.1. Effect of the Sunflower Oil Enrichment in HTy-Ac on the Formation of Long Chain Oxylipins with Origin in the Peroxidation of the Linoleic Acyl Group, and Evolution of Their Concentration over the Storage Time
- (A)
- Monohydroperoxy conjugated dienes (mHPO-c-dEs).
- (B)
- Dihydroperoxy non conjugated dienes (dHPO-nc(E,E)-dEs).
- (C)
- Non vicinal monohydroperoxy monoepoxy E-monoenes (non vicinal mHPO-mEPO-E-mEs).
- (D)
- Monohydroxy conjugated Z,E-dienes (mHO-c(Z,E)-dEs).
- (E)
- Non vicinal monohydroxy monoepoxy E-monoenes (non vicinal mHO-mEPO-E-mEs).
- (F)
- Monoketo conjugated dienes (mKO-c-dEs).
- (G)
- Non vicinal monoketo monoepoxy monoenes (non vicinal mKO-mEPO-mEs).
3.2.2. Effect of the Oil Enrichment in HTy-Ac on the Formation of Oxylipins Coming from the Cleavage of Long Chain Oxidation Derivatives, and Evolution of Their Concentration throughout the Storage Time
- (A)
- 4-Hydroperoxy-2E-alquenals (4HPO-2E-alkenals), 4-hydroxy-2E-alkenals, (4HO-2E-alkenals), n-alkanals and 2E-alkenals.
- (B)
- Other aldehydes. 2E,4E-alkadienals, 4,5-epoxy-2E-alkenals (4,5-EPO-2E-alkenals), 4-oxo-2E-alkenals (4-KO-2E-alkenals) and 2Z-alkenals.
- (C)
- Other oxidation compounds with origin in the cleavage of long chain oxylipins. 5-alkyl-(5H)-furan-2-ones, 5-alkyl-furans and formic acid.
3.2.3. Effect of the Oil Enrichment in HTy-Ac on the Formation of Oxylipins Derived from Epoxidation of Linoleic Acyl Group Evolution of Their Concentration throughout the Storage Time
- (A)
- Monoepoxy monoenes (mEPO-mEs).
- (B)
- Oxylipins derived from oxirane ring opening.
3.3. Lipolysis Extent and 1,2-Diglycerides Formation throughout the Storage Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57 (Suppl. 5), 779S–786S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckl, P.M.; Bresgen, N. Genotoxicity of lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 244–252. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive oil, the mediterranean diet, and arterial blood pressure: The greek european prospective investigation into cancer and nutrition (EPIC) study. Am. J. Clin. Nutr. 2004, 80, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467). EFSA J. 2011, 9, 2033. [Google Scholar]
- Pérez-Rodrigo, C.; Aranceta, J. Olive Oil: Its Role in the Diet. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 158–166. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Rios, J.J.; Garrido, A. Phenolic compounds in spanish olive oils. J. Agric. Food Chem. 1999, 47, 3535–3540. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Medina, E.; De Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Cárdeno, A.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary hydroxytyrosol and hydroxytyrosyl acetate supplementation prevent pristane-induced systemic lupus erythematous in mice. J. Funct. Foods 2017, 29, 84–92. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Villegas, I.; Aparicio-Soto, M.; Cárdeno, A.; Rosillo, M.T.; González-Benjumea, A.; Alarcón de la Lastra, C. Effects of dietary virgin olive oil polyphenols: Hydroxytyrosyl acetate and 3, 4-dihydroxyphenylglycol on DSS-induced acute colitis in mice. J. Nutr. Biochem. 2015, 26, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Sánchez-Hidalgo, M.; Castejón, M.L.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Extra-virgin olive oil phenols hydroxytyrosol and hydroxytyrosol acetate, down-regulate the production of mediators involved in joint erosion in human synovial cells. J. Funct. Foods 2017, 36, 27–33. [Google Scholar] [CrossRef]
- Ghalandari, M.; Naghmachi, M.; Oliverio, M.; Nardi, M.; Shirazi, H.R.G.; Eilami, O. Antimicrobial effect of hydroxytyrosol, hydroxytyrosol acetate and hydroxytyrosol oleate on staphylococcus aureus and staphylococcus epidermidis. Electron. J. Gen. Med. 2018, 15, em46. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Pei, D.; Qu, L.; Li, Y.; Chen, J.; Gao, K. Antibacterial activity of hydroxytyrosol acetate from olive leaves (olea europaea L.). Nat. Prod. Res. 2018, 32, 1967–1970. [Google Scholar] [CrossRef]
- González Correa, J.A.; López-Villodres, J.A.; Asensi, R.; Espartero, J.L.; Rodríguez-Gutiérez, G.; De La Cruz, J.P. Virgin olive oil polyphenol hydroxytyrosol acetate inhibits in vitro platelet aggregation in human whole blood: Comparison with hydroxytyrosol and acetylsalicylic acid. Br. J. Nutr. 2009, 101, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- González-Correa, J.A.; Navas, M.D.; Muñoz-Marín, J.; Trujillo, M.; Fernández-Bolaños, J.; De La Cruz, J.P. Effects of hydroxytyrosol and hydroxytyrosol acetate administration to rats on platelet function compared to acetylsalicylic acid. J. Agric. Food Chem. 2008, 56, 7872–7876. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Navas, M.D.; Lopez-Villodres, J.A.; Trujillo, M.; Espartero, J.L.; De La Cruz, J.P. Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia-reoxygenation. Neurosci. Lett. 2008, 446, 143–146. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Lubberts, E.; Alarcón-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally occurring hydroxytyrosol derivatives: Hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol modulate inflammatory response in murine peritoneal macrophages. Potential utility as new dietary supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.H.; Paiva-Martins, F.; Almeida, M. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J. Agric. Food Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Lois, S.; Alcantara, D.; Lucas, R.; Morales, J.C. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 9773–9779. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, S.; Giusti, A.M.; Masci, A.; Mosca, L.; Saso, L.; Bovicelli, P. Antioxidant properties of hydroxytyrosyl acetate compared with hydroxytyrosol and their protective capacity against oxidative stress in human neuroblastoma cells. J. Sci. Ind. Res. 2011, 70, 929–937. [Google Scholar]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New lipophilic tyrosyl esters. comparative antioxidant evaluation with hydroxytyrosyl esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Domínguez, M.M.; Espartero, J.L.; Cert, A. Antioxidant effect of phenolic compounds, α-tocopherol, and other minor components in virgin olive oil. J. Agric. Food Chem. 2003, 51, 7170–7175. [Google Scholar] [CrossRef]
- Trujillo, M.; Mateos, R.; De Teran, L.C.; Espartero, J.L.; Cert, R.; Jover, M.; Parrado, J. Lipophilic hydroxytyrosyl esters. Antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef]
- Cao, J.; Li, H.; Xia, X.; Zou, X.G.; Li, J.; Zhu, X.M.; Deng, Z.Y. Effect of Fatty Acid and Tocopherol on Oxidative Stability of Vegetable Oils with Limited Air. Int. J. Food Prop. 2015, 18, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Monitoring of minor compounds in corn oil oxidation by direct immersion-solid phase microextraction-gas chromatography/mass spectrometry. New oil oxidation markers. Food Chem. 2019, 290, 286–294. [Google Scholar] [CrossRef]
- Caño-Ochoa, S.; Ruiz-Aracama, A.; Guillén, M.D. Alpha-Tocopherol, a Powerful Molecule, Leads to the Formation of Oxylipins in Polyunsaturated Oils Differently to the Temperature Increase: A Detailed Study by Proton Nuclear Magnetic Resonance of Walnut Oil Oxidation. Antioxidants 2022, 11, 604. [Google Scholar] [CrossRef]
- Pokorný, J.; Yanishlieva, N.; Gordon, M.H. Antioxidants in Food: Practical Applications; Woodhead Publishing: Sawston, UK, 2001. [Google Scholar]
- Farhoosh, R. Initiation and propagation kinetics of inhibited lipid peroxidation. Sci. Rep. 2021, 11, 6864. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. In search of better methods to evaluate natural antioxidants and oxidative stability in food lipids. Trends Food Sci. Technol. 1993, 4, 220–225. [Google Scholar] [CrossRef]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring the oxidation of unsaturated oils and formation of oxygenated aldehydes by proton NMR. Eur. J. Lipid Sci. Technol. 2005, 107, 36–47. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Study of the oxidative stability of salted and unsalted salmon fillets by 1H nuclear magnetic resonance. Food Chem. 2004, 86, 297–304. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Contribution to further understanding of the evolution of sunflower oil submitted to frying temperature in a domestic fryer: Study by 1H nuclear magnetic resonance. J. Agric. Food Chem. 2009, 57, 7790–7799. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N.; Ibargoitia, M.L.; Ruiz, A. Study of both sunflower oil and its headspace throughout the oxidation process. Occurrence in the headspace of toxic oxygenated aldehydes. J. Agric. Food Chem. 2005, 53, 1093–1101. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. A thorough insight into the complex effect of gamma-tocopherol on the oxidation process of soybean oil by means of 1H nuclear magnetic resonance. Comparison with alpha-tocopherol. Food Res. Int. 2018, 114, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W.; Kleiman, R.; Weisleder, D. Homolytic decomposition of linoleic acid hydroperoxide: Identification of fatty acid products. Lipids 1974, 9, 696–706. [Google Scholar] [CrossRef]
- Gardner, H.W.; Kleiman, R. Degradation of linoleic acid hydroperoxides by a cysteine FeCl3 catalyst as a model for similar biochemical reactions. II. Specificity in formation of fatty acid epoxides. Biochim. Biophys. Acta Lipids Lipid Metab. 1981, 665, 113–125. [Google Scholar] [CrossRef]
- Gardner, H.W.; Crawford, C.G. Degradation of linoleic acid hydroperoxides by a cysteine FeCl3 catalyst as a model for similar biochemical reactions. III. A novel product, trans-12,13-epoxy-11-oxo-trans-9-octadecenoic acid, from 13-l (S)-hydroperoxy-cis-9,trans-11-octadecadienoic acid. Biochim. Biophys. Acta Lipids Lipid Metab. 1981, 665, 126–133. [Google Scholar] [CrossRef]
- Schieberle, P.; Trebert, Y.; Firl, J.; Grosch, W. Photolysis of unsaturated fatty acid hydroperoxides 4. Fatty acid products from the aerobic decomposition of methyl 13(S)-hydroperoxy-9(Z),11(E)-octadecadienoate dissolved in cyclohexane. Chem. Phys. Lipids 1988, 48, 281–288. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1H nuclear magnetic resonance. Food Chem. 2018, 245, 312–323. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Guillén, M.D. Enrichment of sunflower oil with γ-tocopherol. Study by 1H NMR of its effect under accelerated storage conditions. Eur. J. Lipid Sci. Technol. 2019, 121, 1800457. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Nakamura, T. The effects of linoleate hydroperoxide on respiration and oxidative phosphorylation of rat liver mitochondria. J. Biochem. 1979, 86, 1041–1047. [Google Scholar] [CrossRef]
- Shiotani, A.; Watanabe, T.; Matsuoka, I.; Nakamura, T. Comparative studies on the effects of linoleate and methyl linoleate and their hydroperoxides on the respiration and reactivities of rat heart mitochondria. J. Biochem. 1980, 88, 677–683. [Google Scholar] [CrossRef]
- Imagawa, T.; Kasai, S.; Matsui, K.; Nakamura, T. Methyl hydroperoxy-epoxy-octadecenoate as an autoxidation product of methyl linoleate: A new inhibitor-uncoupler of mitochondrial respiration. J. Biochem. 1982, 92, 1109–1121. [Google Scholar] [CrossRef]
- Evans, M.V.; Turton, H.E.; Grant, C.M.; Dawes, I.W. Toxicity of linoleic acid hydroperoxide to saccharomyces cerevisiae: Involvement of a respiration-related process for maximal sensitivity and adaptive response. J. Bacteriol. 1998, 180, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corteselli, E.M.; Gibbs-Flournoy, E.; Simmons, S.O.; Bromberg, P.; Gold, A.; Samet, J.M. Long chain lipid hydroperoxides increase the glutathione redox potential through glutathione peroxidase 4. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 950–959. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, G.R.Y.; Bult, H.; Verbeuren, T.J.; Herman, A.G. The role of endothelial cells in the relaxations induced by 13-hydroxy- and 13-hydroperoxylinoleic acid in canine arteries. Br. J. Pharmacol. 1992, 107, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr, M.; Narasimhulu, C.A.; Keewan, E.; Hamid, S.; Parthasarathy, S. The dietary peroxidized lipid, 13-HPODE, promotes intestinal inflammation by mediating granzyme B secretion from natural killer cells. Food Funct. 2020, 11, 9526–9534. [Google Scholar] [CrossRef] [PubMed]
- Neff, W.E.; Frankel, E.N.; Selke, E.; Weisleder, D. Photosensitized oxidation of methyl linoleate monohydroperoxides: Hydroperoxy cyclic peroxides, dihydroperoxides, keto esters and volatile thermal decomposition products. Lipids 1983, 18, 868–876. [Google Scholar] [CrossRef]
- Schneider, C.; Tallman, K.A.; Porter, N.A.; Brash, A.R. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of non enzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001, 276, 20831–20838. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Boeglin, W.E.; Yin, H.; Stec, D.F.; Hachey, D.L.; Porter, N.A.; Brash, A.R. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal. Lipids 2005, 40, 1155–1162. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Salomon, R.G. Preparative singlet oxygenation of linoleate provides doubly allylic dihydroperoxides: Putative intermediates in the generation of biologically active aldehydes in vivo. J. Org. Chem. 2006, 71, 5607–5615. [Google Scholar] [CrossRef]
- Zhang, W. Synthesis and Fragmentation Reactions of Linoleic Acid-Derived Hydroperoxides. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2008. [Google Scholar]
- Gardner, H.W.; Weisleder, D.; Kleiman, R. Formation of trans-12,13-epoxy-9-hydroperoxy-trans-10-octadecenoic acid from 13-L-hydroperoxy-cis-9, trans-11-octadecadienoic acid catalyzed by either a soybean extract or cysteine-FeC13. Lipids 1978, 13, 246–252. [Google Scholar] [CrossRef]
- Gardner, H.W.; Selke, E. Volatiles from thermal decomposition of isomeric methyl (12 S, 13 S)-(E)-12,13-epoxy-9-hydroperoxy-10-octadecenoates. Lipids 1984, 19, 375–380. [Google Scholar] [CrossRef]
- Pryor, W.A.; Porter, N.A. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic. Biol. Med. 1990, 8, 541–543. [Google Scholar] [CrossRef]
- Gu, X.; Salomon, R.G. Fragmentation of a linoleate-derived γ-hydroperoxy-α,β- unsaturated epoxide to γ-hydroxy- and γ-oxo-alkenals involves a unique pseudo-symmetrical diepoxycarbinyl radical. Free Radic. Biol. Med. 2012, 52, 601–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imagawa, T.; Kasai, S.; Matsui, K.; Nakamura, T. Detrimental effects of methyl hydroperoxy-epoxy-octadecenoate on mitochondrial respiration: Detoxication by rat liver mitochondria. J. Biochem. 1983, 94, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kuklev, D.V.; Christie, W.W.; Durand, T.; Rossi, J.C.; Vidal, J.P.; Kasyanov, S.P.; Akulin, V.N.; Bezuglov, V.V. Synthesis of keto- and hydroxydienoic compounds from linoleic acid. Chem. Phys. Lipids 1997, 85, 125–134. [Google Scholar] [CrossRef]
- Schneider, C.; Porter, N.A.; Brash, A.R. Autoxidative transformation of chiral ω6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal. Chem. Res. Toxicol. 2004, 17, 937–941. [Google Scholar] [CrossRef]
- Buchanan, M.R.; Haas, T.A.; Lagarde, M.; Guichardant, M. 13-hydroxyoctadecadienoic acid is the vessel wall chemorepellant factor, LOX. J. Biol. Chem. 1985, 260, 16056–16059. [Google Scholar] [CrossRef]
- Tloti, M.A.; Moon, D.G.; Weston, L.K.; Kaplan, J.E. Effect of 13-hydroxyoctadeca-9,11-dienoic acid (13-HODE) on thrombin induced platelet adherence to endothelial cells in vitro. Thromb. Res. 1991, 62, 305–317. [Google Scholar] [CrossRef]
- Honn, K.V.; Nelson, K.K.; Renaud, C.; Bazaz, R.; Diglio, C.A.; Timar, J. Fatty acid modulation of tumor cell adhesion to microvessel endothelium and experimental metastasis. Prostaglandins 1992, 44, 413–429. [Google Scholar] [CrossRef]
- Murthy, S.; Born, E.; Mathur, S.; Jeffrey Field, F. 13-hydroxy octadecadienoic acid (13-HODE) inhibits triacylglycerol-rich lipoprotein secretion by CaCo-2 cells. J. Lipid Res. 1998, 39, 1254–1262. [Google Scholar] [CrossRef]
- Hampel, J.K.A.; Brownrigg, L.M.; Vignarajah, D.; Croft, K.D.; Dharmarajan, A.M.; Bentel, J.M.; Yeap, B.B. Differential modulation of cell cycle, apoptosis and PPARγ2 gene expression by PPARγ agonists ciglitazone and 9-hydroxyoctadecadienoic acid in monocytic cells. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 283–293. [Google Scholar] [CrossRef]
- Hattori, T.; Obinata, H.; Ogawa, A.; Kishi, M.; Tatei, K.; Ishikawa, O.; Izumi, T. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J. Investig. Dermatol. 2008, 128, 1123–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niculescu, L.S.; Sanda, G.M.; Sima, A.V. HDL inhibit endoplasmic reticulum stress by stimulating apoE and CETP secretion from lipid-loaded macrophages. Biochem. Biophys. Res. Commun. 2013, 434, 173–178. [Google Scholar] [CrossRef]
- Patwardhan, A.M.; Scotland, P.E.; Akopian, A.N.; Hargreaves, K.M. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc. Natl. Acad. Sci. USA 2009, 106, 18820–18824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patwardhan, A.M.; Akopian, A.N.; Ruparel, N.B.; Diogenes, A.; Weintraub, S.T.; Uhlson, C.; Hargreaves, K.M. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J. Clin. Investig. 2010, 120, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Doolen, S.; Keyes, G.S.; Ramsden, C.E. Hydroxy-epoxide and keto-epoxide derivatives of linoleic acid activate trigeminal neurons. Neurobiol. Pain 2020, 7, 100046. [Google Scholar] [CrossRef]
- Keyes, G.S.; Maiden, K.; Ramsden, C.E. Stable analogs of 13-hydroxy-9,10-trans-epoxy-(11E)-octadecenoate (13,9-HEL), an oxidized derivative of linoleic acid implicated in the epidermal skin barrier. Prostaglandins Leukot. Essent. Fatty Acids 2021, 174, 102357. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R.; Vioque, E. Syntheses and reactions of methyl (Z)-9,10-epoxy-13-oxo-(E)-11-octadecenoate and methyl (E)-9,10-epoxy-13-oxo-(E)-11-octadecenoate. Chem. Phys. Lipids 1992, 60, 225–233. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.A.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Altmann, R.; Hausmann, M.; Spöttl, T.; Gruber, M.; Bull, A.W.; Menzel, K.; Rogler, G. 13-oxo-ODE is an endogenous ligand for PPARγ in human colonic epithelial cells. Biochem. Pharmacol. 2007, 74, 612–622. [Google Scholar] [CrossRef]
- Armstrong, M.M.; Diaz, G.; Kenyon, V.; Holman, T.R. Inhibitory and mechanistic investigations of oxo-lipids with human lipoxygenase isozymes. Bioorg. Med. Chem. 2014, 22, 4293–4297. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Sakurai, Y.; Shibata, K.; Kikkawa, F.; Tomoda, Y.; Mizukami, H. Cytotoxic fatty acid ketodienes from eggplants. Jpn. J. Food Chem. Saf. 2014, 21, 42–47. [Google Scholar]
- Burstyn, P.; Horrobin, D. Possible mechanism of action for aldosterone-induced hypertension. Lancet 1970, 295, 973–976. [Google Scholar] [CrossRef]
- Goodfriend, T.L.; Ball, D.L.; Gardner, H.W. An oxidized derivative of linoleic acid affects aldosterone secretion by adrenal cells in vitro. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodfriend, T.L.; Ball, D.L.; Egan, B.M.; Campbell, W.B.; Nithipatikom, K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension 2004, 43, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Ramsden, C.E.; Domenichiello, A.F.; Yuan, Z.X.; Sapio, M.R.; Keyes, G.S.; Mishra, S.K.; Iadarola, M.J. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci. Signal. 2017, 10, eaal5241. [Google Scholar] [CrossRef] [Green Version]
- Guillen, M.D.; Goicoechea, E. Formation of oxygenated α,β-unsaturated aldehydes and other toxic compounds in sunflower oil oxidation at room temperature in closed receptacles. Food Chem. 2008, 111, 157–164. [Google Scholar] [CrossRef]
- Guillén, M.D.; Carton, I.; Salmeron, J.; Casas, C. Headspace composition of cod liver oil and its evolution in storage after opening. First evidence of the presence of toxic aldehydes. Food Chem. 2009, 114, 1291–1300. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α,β unsaturated aldehydes. Food Chem. 2012, 131, 915–926. [Google Scholar] [CrossRef]
- Spickett, C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol. 2013, 1, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Gassenmeier, K.; Schieberle, P. Formation of the intense flavor compound trans-4,5-epoxy-(E)-2-decenal in thermally treated fats. J. Am. Oil Chem. Soc. 1994, 71, 1315–1319. [Google Scholar] [CrossRef]
- Adams, A.; Bouckaert, C.; Van Lancker, F.; De Meulenaer, B.; De Kimpe, N. Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes. J. Agric. Food Chem. 2011, 59, 11058–11062. [Google Scholar] [CrossRef] [PubMed]
- Wanjala, G.W.; Onyango, A.N.; Abuga, D.; Onyango, C.; Makayoto, M. Does lysine drive the conversion of fatty acid hydroperoxides to aldehydes and alkyl-furans? Sci. Afr. 2021, 12, e00797. [Google Scholar] [CrossRef]
- Loury, M. Possible mechanisms of autoxidative rancidity. Lipids 1972, 7, 671–675. [Google Scholar] [CrossRef]
- deMan, J.M.; Tie, F.; deMan, L. Formation of short chain volatile organic acids in the automated AOM method. J. Am. Oil Chem. Soc. 1987, 64, 993–996. [Google Scholar] [CrossRef]
- Lee, S.H.; Oe, T.; Blair, I.A. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science 2001, 292, 2083–2086. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Blair, I.A. Lipid hydroperoxide-mediated DNA damage. Exp. Gerontol. 2001, 36, 1473–1481. [Google Scholar] [CrossRef]
- Zarkovic, N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003, 24, 281–291. [Google Scholar] [CrossRef]
- Alary, J.; Guéraud, F.; Cravedi, J.P. Fate of 4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol. Asp. Med. 2003, 24, 177–187. [Google Scholar] [CrossRef]
- Uchida, K. 4-hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Signorini, C.; De Felice, C.; Durand, T.; Oger, C.; Galano, J.M.; Leoncini, S.; Hayek, J. Isoprostanes and 4-hydroxy-2-nonenal: Markers or mediators of disease? Focus on rett syndrome as a model of autism spectrum disorder. Oxid. Med. Cell. Longev. 2013, 2013, 343824. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Rossin, D.; Poli, G.; Biasi, F. Lipid oxidation products in the pathogenesis of inflammation-related gut diseases. Curr. Med. Chem. 2018, 25, 1311–1326. [Google Scholar] [CrossRef]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid oxidation derived aldehydes and oxysterols between health and disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1700490. [Google Scholar] [CrossRef] [Green Version]
- Sonowal, H.; Ramana, K.V. 4-hydroxy-trans-2-nonenal in the regulation of anti-oxidative and pro-inflammatory signaling pathways. Oxid. Med. Cell. Longev. 2019, 2019, 5937326. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.D.; Goicoechea, E. Toxic oxygenated α,β-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Photolyse von 13(S)-Hydroperoxy-9(Z),11(E)-octadecadiensäuremethylester in gegenwart von sauerstoff—analyse der niedermolekularen reaktionsprodukte. Fette Seifen Anstrichm. 1985, 87, 76–80. [Google Scholar] [CrossRef]
- Shahidi, F.; Oh, W.Y. Lipid-derived flavor and off-flavor of traditional and functional foods: An overview. J. Food Bioact. 2020, 10, 20–31. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Oxidation process of oils with high content of linoleic acyl groups and formation of toxic hydroperoxy- and hydroxyalkenals. A study by 1H nuclear magnetic resonance. J. Sci. Food Agric. 2005, 85, 2413–2420. [Google Scholar] [CrossRef]
- Moumtaz, S.; Percival, B.C.; Parmar, D.; Grootveld, K.L.; Jansson, P.; Grootveld, M. Toxic aldehyde generation in and food uptake from culinary oils during frying practices: Peroxidative resistance of a monounsaturate-rich algae oil. Sci. Rep. 2019, 9, 4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Oxylipins associated to current diseases detected for the first time in the oxidation of corn oil as a model system of oils rich in omega-6 polyunsaturated groups. A global, broad and in-depth study by 1H NMR spectroscopy. Antioxidants 2020, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984, 23, 197–221. [Google Scholar] [CrossRef]
- Giuffrida, F.; Destaillats, F.; Robert, F.; Skibsted, L.H.; Dionisi, F. Formation and hydrolysis of triacylglycerol and sterols epoxides: Role of unsaturated triacylglycerol peroxyl radicals. Free Radic. Biol. Med. 2004, 37, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- La Scala, J.; Wool, R.P. Effect of FA composition on epoxidation kinetics of TAG. J. Am. Oil Chem. Soc. 2002, 79, 373–378. [Google Scholar] [CrossRef]
- Anuar, S.T.; Zhao, Y.Y.; Mugo, S.M.; Curtis, J.M. Monitoring the epoxidation of canola oil by non-aqueous reversed phase liquid chromatography/mass spectrometry for process optimization and control. J. Am. Oil Chem. Soc. 2012, 89, 1951–1960. [Google Scholar] [CrossRef]
- Chen, J.; De Liedekerke Beaufort, M.; Gyurik, L.; Dorresteijn, J.; Otte, M.; Klein Gebbink, R.J.M. Highly efficient epoxidation of vegetable oils catalyzed by a manganese complex with hydrogen peroxide and acetic acid. Green Chem. 2019, 21, 2436–2447. [Google Scholar] [CrossRef] [Green Version]
- Harry-O’Kuru, R.E.; Carriere, C.J. Synthesis, rheological characterization, and constitutive modeling of polyhydroxy triglycerides derived from milkweed oil. J. Agric. Food Chem. 2002, 50, 3214–3221. [Google Scholar] [CrossRef]
- Yang, J.; Morton, M.D.; Hill, D.W.; Grant, D.F. NMR and HPLC-MS/MS analysis of synthetically prepared linoleic acid diol glucuronides. Chem. Phys. Lipids 2006, 140, 75–87. [Google Scholar] [CrossRef]
- Monteavaro, L.L.; Da Silva, E.O.; Costa, A.P.O.; Samios, D.; Gerbase, A.E.; Petzhold, C.L. Polyurethane networks from formiated soy polyols: Synthesis and mechanical characterization. J. Am. Oil Chem. Soc. 2005, 82, 365–371. [Google Scholar] [CrossRef]
- Lopes, R.D.V.V.; Zamian, J.R.; Resck, I.S.; Sales, M.J.A.; Dos Santos, M.L.; Da Cunha, F.R. Physicochemical and rheological properties of passion fruit oil and its polyol. Eur. J. Lipid Sci. Technol. 2010, 112, 1253–1262. [Google Scholar] [CrossRef]
- Caillol, S.; Desroches, M.; Boutevin, G.; Loubat, C.; Auvergne, R.; Boutevin, B. Synthesis of new polyester polyols from epoxidized vegetable oils and biobased acids. Eur. J. Lipid Sci. Technol. 2012, 114, 1447–1459. [Google Scholar] [CrossRef]
- De Souza, V.H.R.; Silva, S.A.; Ramos, L.P.; Zawadzki, S.F. Synthesis and characterization of polyols derived from corn oil by epoxidation and ozonolysis. J. Am. Oil Chem. Soc. 2012, 89, 1723–1731. [Google Scholar] [CrossRef]
- Harry-O’kuru, R.E.; Biresaw, G.; Tisserat, B.; Evangelista, R. Synthesis of polyformate esters of vegetable oils: Milkweed, pennycress, and soy. J. Lipids 2016, 2016, 3128604. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, B.M.; Zubairi, S.I.; Huri, H.Z.; Hairunisa, N.; Yousif, E.; Basu, R.C. Polyesters based on linoleic acid for biolubricant basestocks: Low-temperature, tribological and rheological properties. PLoS ONE 2016, 11, e0151603. [Google Scholar] [CrossRef] [Green Version]
- Milchert, E.; Smagowicz, A. The influence of reaction parameters on the epoxidation of rapeseed oil with peracetic acid. J. Am. Oil Chem. Soc. 2009, 86, 1227–1233. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bogdal, D.; Prociak, A. Microwave-assisted synthesis of polyols from rapeseed oil and properties of flexible polyurethane foams. Polymers 2012, 4, 1462–1477. [Google Scholar] [CrossRef] [Green Version]
- Derawi, D. Experimental design using response surface methods for palm olein-based hydroxy-ether systhesis. Sains Malays. 2016, 45, 1149–1154. [Google Scholar]
- Favero, D.; Marcon, V.R.R.; Barcellos, T.; Gómez, C.M.; Sanchis, M.J.; Carsí, M.; Bianchi, O. Renewable polyol obtained by microwave-assisted alcoholysis of epoxidized soybean oil: Preparation, thermal properties and relaxation process. J. Mol. Liq. 2019, 285, 136–145. [Google Scholar] [CrossRef]
- Zheng, J.; Plopper, C.G.; Lakritz, J.; Storms, D.H.; Hammock, B.D. Leukotoxin-diol: A putative toxic mediator involved in acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2001, 25, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Nilewski, C.; Chapelain, C.L.; Wolfrum, S.; Carreira, E.M. Synthesis and biological evaluation of chlorinated analogs of leukotoxin diol. Org. Lett. 2015, 17, 5602–5605. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.H.; Mon, T.; Hendrickson, T.L.; Mitchell, L.A.; Grant, D.F. Defining mechanisms of toxicity for linoleic acid monoepoxides and diols in sf-21 cells. Chem. Res. Toxicol. 2001, 14, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.F.; Grant, D.F.; Cheek, J.M.; Greene, J.F.; Williamson, K.C.; Hammock, B.D. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 1997, 3, 562–566. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. 1H NMR study of the in vitro digestion of highly oxidized soybean oil and the effect of the presence of ovalbumin. Foods 2021, 10, 1573. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Biermann, U.; Metzger, J.O. Synthesis and characterization of polyurethanes from epoxidized methyl oleate based polyether polyols as renewable resources. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 634–645. [Google Scholar] [CrossRef]

















| Samples | First Stage | Second Stage | ||
|---|---|---|---|---|
| Time (Days) | DR1L (mmol/mol TG Day) | Time (Days) | DR2L (mmol/mol TG Day) | |
| S | 0–5 | 22.9 (0.98) | 5–11 | 214.0 (0.96) |
| S025 | 0–9 | 15.4 (0.95) | 9–15 | 204.3 (0.98) |
| S125 | 0–18 | 12.0 (0.94) | 18–26 | 147.1 (0.93) |
| S250 | 0–26 | 12.0 (0.98) | 26–34 | 140.8 (0.98) |
| S750 | 0–43 | 10.9 (0.98) | 43–58 | 65.8 (0.94) |
| Samples | First Stage | Second Stage | ||
|---|---|---|---|---|
| Time (Days) | DR1H (mmol/mol TG Day) | Time (Days) | DR2H (mmol/mol TG Day) | |
| S025 | 0–4 | 0.08 (0.96) | 4–10 | 0.24 (0.96) |
| S125 | 0–8 | 0.11 (0.89) | 8–20 | 0.34 (0.98) |
| S250 | 0–12 | 0.19 (0.90) | 12–28 | 0.52 (0.99) |
| S750 | 0–12 | 0.29 (0.82) | 12–44 | 0.88 (0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caño-Ochoa, S.d.; Ruiz-Aracama, A.; Guillén, M.D. Influence of Hydroxytyrosol Acetate Enrichment of an Oil Rich in Omega-6 Groups on the Evolution of Its Oxidation and Oxylipin Formation When Subjected to Accelerated Storage. A Global Study by Proton Nuclear Magnetic Resonance. Antioxidants 2022, 11, 722. https://doi.org/10.3390/antiox11040722
Caño-Ochoa Sd, Ruiz-Aracama A, Guillén MD. Influence of Hydroxytyrosol Acetate Enrichment of an Oil Rich in Omega-6 Groups on the Evolution of Its Oxidation and Oxylipin Formation When Subjected to Accelerated Storage. A Global Study by Proton Nuclear Magnetic Resonance. Antioxidants. 2022; 11(4):722. https://doi.org/10.3390/antiox11040722
Chicago/Turabian StyleCaño-Ochoa, Sofía del, Ainhoa Ruiz-Aracama, and María D. Guillén. 2022. "Influence of Hydroxytyrosol Acetate Enrichment of an Oil Rich in Omega-6 Groups on the Evolution of Its Oxidation and Oxylipin Formation When Subjected to Accelerated Storage. A Global Study by Proton Nuclear Magnetic Resonance" Antioxidants 11, no. 4: 722. https://doi.org/10.3390/antiox11040722





