Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Ultrasound-Assisted Extraction
2.4. Experimental Design and Optimization
2.4.1. Total Phenolic Content
2.4.2. ABTS Radical Scavenging Activity Assay
2.4.3. DPPH Free Radical Scavenging Assay
2.4.4. Ferric Reducing Antioxidant Power Assay
2.5. HPLC-PDA Analysis
2.6. Evaluation of In Vitro Scavenging Capacity of Reactive Oxygen Species
2.6.1. Superoxide Anion Radical Scavenging Assay
2.6.2. Hypochlorous Acid Scavenging Assay
2.7. Evaluation of In Vitro Cell Effects
2.8. Statistical Analysis
3. Results
3.1. Optimization of UAE
3.2. Response Surface Analysis
3.3. Characterization of the Optimal Extract
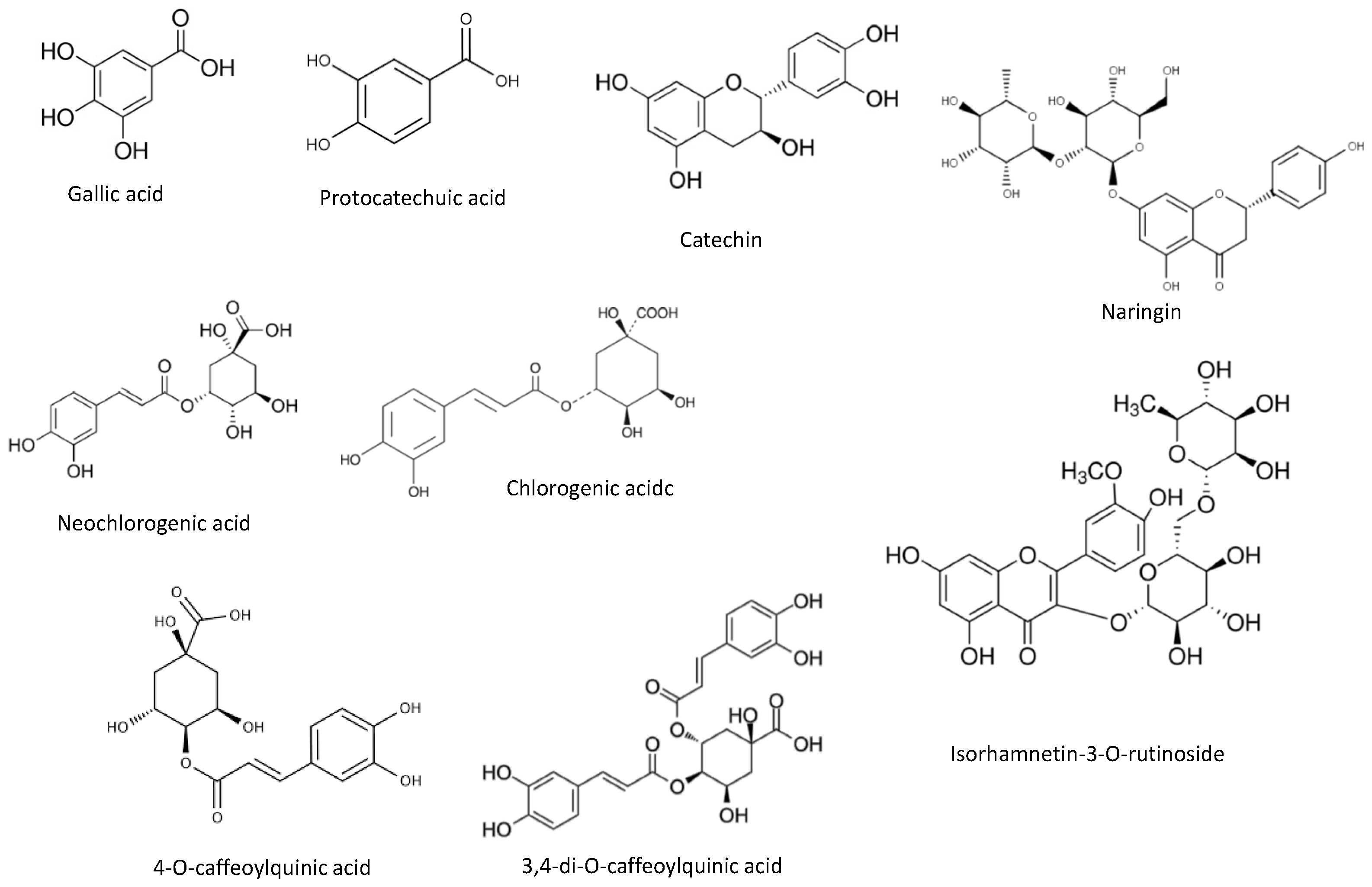
3.3.1. Phenolic Profile Identification and Quantification by HPLC-PDA
3.3.2. In Vitro Scavenging Capacity of ROS
3.3.3. In Vitro Cell Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Orgazination of the United Nations (FAO). Food Wastage Footprint: Impacts on Natural Resources; FAO: Rome, Italy, 2013. [Google Scholar]
- World Health Organization (WHO). Fruit and vegetable promotion initiative. In Fruit and Vegetable Promotion Initiative; WHO: Geneva, Switzerland, 2003; p. 32. [Google Scholar]
- Mateos-Aparicio, I. Plant-based by-products. In Food Waste Recovery-Processing Technologies, Industrial Techniques, and Applications, 2nd ed.; Galankis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 367–397. [Google Scholar]
- Comunian, T.A.; Silva, M.P.; Souza, C.J. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Cosmetics—Food waste recovery. In Food Waste Recovery-Processing Technologies, Industrial Techniques, and Applications, 2nd ed.; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 503–528. [Google Scholar]
- Chamorro, F.; Carpena, M.; Nuñez-Estevez, B.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi by-products for the recovery of bioactive compounds: Circular economy model. Proceedings 2021, 70, 9. [Google Scholar]
- Marangi, F.; Pinto, D.; de Francisco, L.; Alves, R.C.; Puga, H.; Sut, S.; Dall’Acqua, S.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwi leaves extracted by multi-frequency multimode modulated technology: A sustainable and promising by-product for industry. Food Res. Int. 2018, 112, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Luís, A.S.; Moreira, M.M.; Ferraz, R.; Brezo-Borjan, T.; Švarc-Gajić, J.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Influence of temperature on the subcritical water extraction of Actinidia arguta leaves: A screening of pro-healthy compounds. Sustain. Chem. Pharm. 2022, 25, 100593. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Fernandes, I.; Freitas, V.d.; Cádiz-Gurrea, M.d.l.L.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. An insight into kiwiberry leaf valorization: Phenolic composition, bioactivity and health benefits. Molecules 2021, 26, 2314. [Google Scholar] [CrossRef]
- Henriques, J.; Ribeiro, M.J.; Falé, P.L.; Pacheco, R.; Ascensão, L.; Florêncio, M.H.; Serralheiro, M.L.M. Valorization of kiwifruit production: Leaves of the pruning branches of Actinidia deliciosa as a promising source of polyphenols. Eur. Food Res. Technol. 2017, 243, 1343–1353. [Google Scholar] [CrossRef]
- Dias, M.; Caleja, C.; Pereira, C.; Calhelha, R.C.; Kostic, M.; Sokovic, M.; Tavares, D.; Baraldi, I.J.; Barros, L.; Ferreira, I.C. Chemical composition and bioactive properties of byproducts from two different kiwi varieties. Food Res. Int. 2020, 127, 108753. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wen, I.; Wibisono, R.; Melton, L.D.; Wadhwa, S. Evaluation of the extraction efficiency for polyphenol extracts from by-products of green kiwifruit juicing. Int. J. Food Sci. Technol. 2009, 44, 2644–2652. [Google Scholar] [CrossRef]
- Latocha, P.; Vereecke, D.; Debersaques, F. Kiwiberry commercial production-what stage are we at? In Proceedings of the IX International Symposium on Kiwifruit Porto, Porto, Portugal, 6–9 September 2017; pp. 559–564. [Google Scholar]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Silva, A.M.; Costa, P.C.; Delerue-Matos, C.; Latocha, P.; Rodrigues, F. Extraordinary composition of Actinidia arguta by-products as skin ingredients: A new challenge for cosmetic and medical skincare industries. Trends Food Sci. Technol. 2021, 116, 842–853. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Xu, E.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies–A review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, J.; Wu, G.; Tong, J.; Xie, G.; Duan, J.-A.; Qin, M. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (RSM) for rapid analysis of bioactive compounds in the flower head of Chrysanthemum morifolium Ramat. Ind. Crops Prod. 2015, 74, 192–199. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Pinto, D.; Cádiz-Gurrea, M.d.L.L.; Sut, S.; Ferreira, A.S.; Leyva-Jimenez, F.J.; Dall’acqua, S.; Segura-Carretero, A.; Delerue-Matos, C.; Rodrigues, F. Valorisation of underexploited Castanea sativa shells bioactive compounds recovered by supercritical fluid extraction with CO2: A response surface methodology approach. J. CO2 Util. 2020, 40, 101194. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999, 299, 15–27. [Google Scholar]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorg. Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of ultrasound-assisted extraction. In Water Extraction of Bioactive Compounds; González, H.D., Muñoz, M.J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 11, pp. 291–316. [Google Scholar]
- Dorosh, O.; Moreira, M.M.; Rodrigues, F.; Peixoto, A.F.; Freire, C.; Morais, S.; Delerue-Matos, C. Vine-canes valorisation: Ultrasound-assisted extraction from lab to pilot scale. Molecules 2020, 25, 1739. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, H.J.; Na, C.; Jang, D.S.; Shin, Y.-K.; Lee, S.H. The protective effect of Adenocaulon himalaicum Edgew. And its bioactive compound neochlorogenic acid against uvb-induced skin damage in human dermal fibroblasts and epidermal keratinocytes. Plants 2021, 10, 1669. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, J.K.; Kim, J.; Jung, S.-H.; Lee, K. Characterization of caffeoylquinic acids from lepisorus thunbergianus and their melanogenesis inhibitory activity. ACS Omega 2020, 5, 30946–30955. [Google Scholar] [CrossRef]
- Tada, T.; Tezuka, Y.; Shimomura, K.; Ito, S.; Hattori, H.; Kadota, S. Effect of depigmentation for 3, 4-di-O-caffeoylquinic acid guided by tyrosinase inhibitory activity from Conyza filaginoides. J. Oleo Sci. 2001, 50, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, S.; Mitoma, T.; Tsuruta, K.; Todo, H.; Sugibayashi, K. Effect of emulsification on the skin permeation and UV protection of catechin. Pharm. Dev. Technol. 2014, 19, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Eddine, L.S.; Segni, L.; Redha, O.M.; Noureddine, G. Free radical scavenging activity of leaf extract of Rumex vesicarius L. obtained by different methods. Int. J. Toxicol. Pharm. Res. 2015, 7, 140–146. [Google Scholar]
- Kitagawa, S.; Yoshii, K.; Morita, S.-y.; Teraoka, R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chem. Pharm. Bull. 2011, 59, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Aslantürk, Ö.S. In vitro cytotoxicity and cell viability assays: Principles, advantages, and disadvantages. In Genotoxicity: A Predictable Risk to Our Actual World; Larramendy, M., Soloneski, S., Eds.; InTech: Houston, TX, USA, 2018; Volume 1. [Google Scholar]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
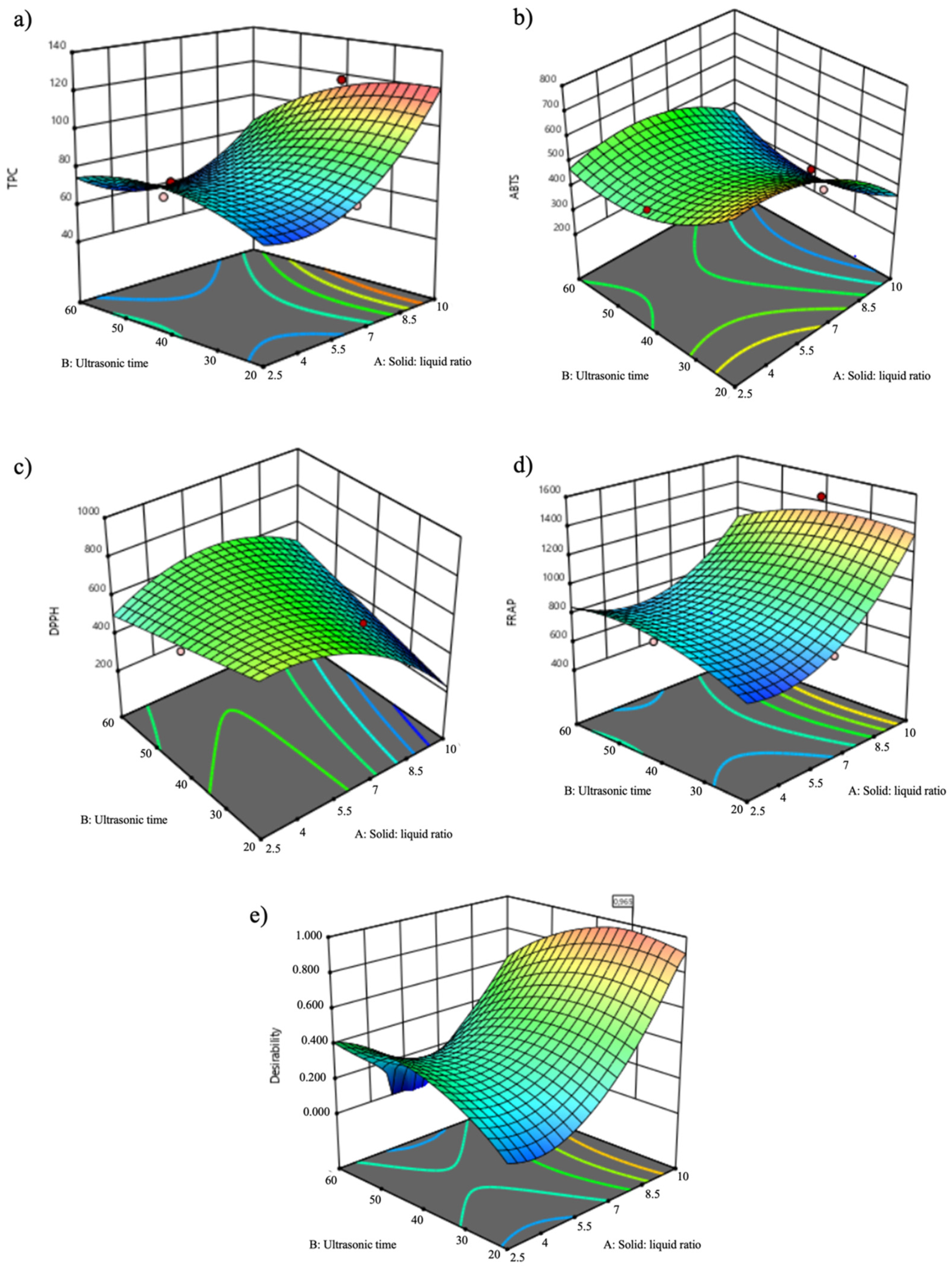
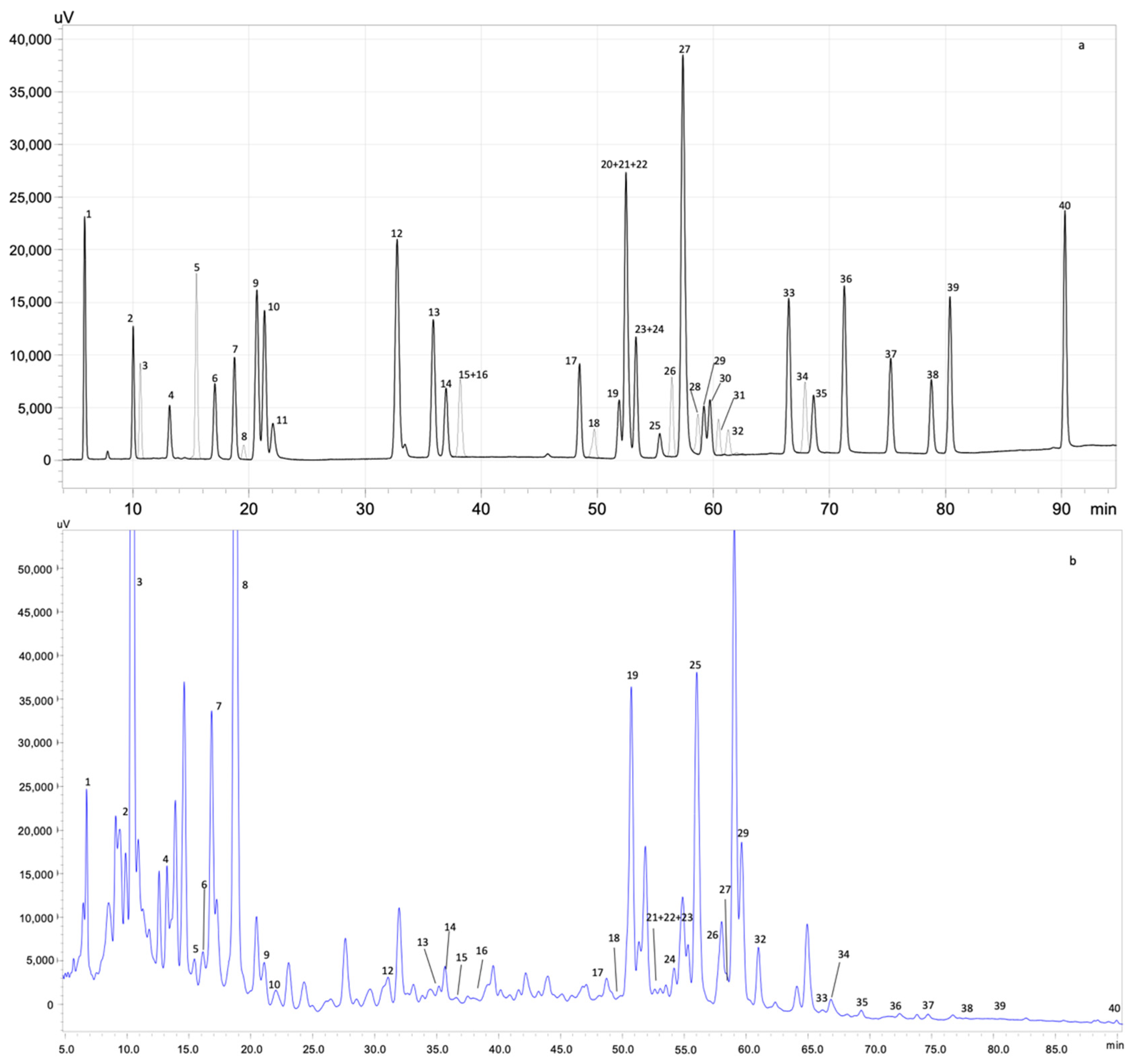

| Independent Variables | Dependent Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Point | Extraction Conditions | Y1, TPC (mg GAE/g dw) | Y2, ABTS (IC50, µg/mL) | Y3, DPPH (IC50, µg/mL) | Y4, FRAP (µmol FSE/g dw) | ||||||
| Run | X1 (Solid: Liquid Ratio, % w/v) | X2 (t, min) | X3 (Intensity, W/m2) | Predicted | Experimental | Predicted | Experimental | Predicted | Experimental | Predicted | Experimental |
| 1 | 10 | 40 | 30 | 113.39 | 117.22 ± 6.08 | 265.97 | 288.74 ± 3.18 | 365.83 | 304.02 ± 32.87 | 1339.78 | 1440.13 ± 63.43 |
| 2 | 2.5 | 40 | 70 | 97.91 | 94.07 ± 3.32 | 482.29 | 459.52 ± 8.35 | 566.06 | 627.87 ± 30.46 | 950.32 | 849.96 ± 48.09 |
| 3 | 10 | 40 | 70 | 104.22 | 108.56 ± 9.19 | 307.85 | 275.37 ± 7.78 | 443.04 | 469.99 ± 40.84 | 1228.93 | 1261.83 ± 34.60 |
| 4 | 6.25 | 40 | 50 | 69.33 | 69.83 ± 8.91 | 503.37 | 506.84 ± 40.24 | 802.61 | 669.04 ± 4.57 | 725.32 | 584.88 ± 47.81 |
| 5 | 10 | 60 | 50 | 72.55 | 67.45 ± 4.35 | 486.34 | 483.79 ± 55.63 | 663.07 | 785.75 ± 49.22 | 941.95 | 887.67 ± 90.80 |
| 6 | 6.25 | 20 | 70 | 75.29 | 74.03 ± 4.85 | 497.67 | 517.88 ± 32.68 | 704.90 | 765.77 ± 37.67 | 788.86 | 834.93 ± 61.66 |
| 7 | 10 | 20 | 50 | 106.73 | 103.66 ± 11.98 | 336.13 | 348.40 ± 7.97 | 438.66 | 350.84 ± 19.09 | 1192.43 | 1113.46 ± 96.87 |
| 8 | 6.25 | 40 | 50 | 69.33 | 78.41 ± 6.55 | 503.37 | 583.04 ± 14.37 | 802.61 | 759.46 ± 43.04 | 725.32 | 818.59 ± 64.82 |
| 9 | 6.25 | 40 | 50 | 69.33 | 68.86 ± 3.08 | 503.37 | 637.54 ± 72.35 | 802.61 | 691.25 ± 11.80 | 725.32 | 847.63 ± 81.65 |
| 10 | 6.25 | 40 | 50 | 69.33 | 64.04 ± 3.33 | 503.37 | 265.02 ± 5.35 | 802.61 | 978.72 ± 6.19 | 725.32 | 662.02 ± 90.97 |
| 11 | 6.25 | 40 | 50 | 69.33 | 65.49 ± 3.35 | 503.37 | 524.40 ± 36.11 | 802.61 | 914.57 ± 9.49 | 725.32 | 713.47 ± 84.80 |
| 12 | 2.5 | 60 | 50 | 74.95 | 78.02 ± 7.87 | 576.25 | 563.98 ± 33.54 | 593.76 | 681.58 ± 11.61 | 753.57 | 832.54 ± 75.51 |
| 13 | 2.5 | 20 | 50 | 65.11 | 70.20 ± 8.88 | 625.46 | 628.02 ± 71.38 | 889.47 | 766.80 ± 53.25 | 613.72 | 668.00 ± 92.03 |
| 14 | 2.5 | 40 | 30 | 80.50 | 76.17 ± 10.55 | 470.76 | 503.25 ± 64.32 | 624.32 | 597.37 ± 24.74 | 851.30 | 818.40 ± 62.44 |
| 15 | 6.25 | 60 | 30 | 59.00 | 60.27 ± 9.40 | 521.46 | 501.25 ± 3.94 | 659.79 | 598.92 ± 11.35 | 739.45 | 693.38 ± 64.38 |
| 16 | 6.25 | 20 | 30 | 72.05 | 71.29 ± 4.06 | 615.89 | 580.86 ± 21.28 | 642.03 | 791.67 ± 15.04 | 731.69 | 710.31 ± 64.18 |
| 17 | 6.25 | 60 | 70 | 64.00 | 64.76 ± 4.08 | 693.10 | 728.13 ± 38.94 | 615.85 | 466.22 ± 30.70 | 670.47 | 691.86 ± 66.16 |
| Source | Sum of Squares | Mean Squares | F Value | p-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y1 | Y2 | Y3 | Y4 | Y1 | Y2 | Y3 | Y4 | Y1 | Y2 | Y3 | Y4 | |
| Model | 4346.36 | 192,100 | 355,700 | 724,300 | 482.93 | 21,339.45 | 395,24.97 | 80,474.35 | 12.63 | 1.69 | 1.52 | 6.03 | 0.0015 * | 0.2506 | 0.2983 | 0.0136 ** |
| X1. % w/v | 768.69 | 71,906.90 | 72,773.42 | 294,200 | 768.69 | 71,906.90 | 72,773.42 | 294,200 | 20.11 | 5.70 | 2.79 | 22.06 | 0.0029 * | 0.0484 ** | 0.1387 | 0.0022 * |
| X2. min | 296.11 | 5100.15 | 2541.75 | 6119.17 | 296.11 | 5100.15 | 2541.75 | 6119.17 | 7.75 | 0.4041 | 0.0975 | 0.4588 | 0.0272 ** | 0.5452 | 0.7639 | 0.5199 |
| X3. W/m2 | 33.93 | 1426.03 | 179.32 | 69.86 | 33.93 | 1426.03 | 179.32 | 69.86 | 0.8876 | 0.1130 | 0.0069 | 0.0052 | 0.3775 | 0.7466 | 0.9362 | 0.9443 |
| X1.X2 | 484.48 | 9942.49 | 67,633.11 | 38,088.79 | 484.48 | 9942.49 | 67,633.11 | 38,088.79 | 12.67 | 0.7878 | 2.59 | 2.86 | 0.0092 * | 0.4042 | 0.1513 | 0.1349 |
| X1.X3 | 176.50 | 230.45 | 4588.16 | 11,010.93 | 176.50 | 230.45 | 4,588.16 | 11,010.93 | 4.62 | 0.0183 | 0.1760 | 0.8256 | 0.0687 | 0.8963 | 0.6874 | 0.3938 |
| X2.X3 | 0.7683 | 21,004.62 | 2851.78 | 3978.14 | 0.7683 | 21,004.62 | 2,851.78 | 3978.14 | 0.0201 | 1.66 | 0.1094 | 0.2983 | 0.8913 | 0.2380 | 0.7505 | 0.6019 |
| X12 | 1850.28 | 41,114.03 | 102,600 | 273,900 | 1850.28 | 41,114.03 | 102,600 | 273,900 | 48.40 | 3.26 | 3.94 | 20.53 | 0.0002 * | 0.1141 | 0.0877 | 0.0027 * |
| X22 | 460.39 | 43,374.07 | 0.3040 | 46,360.86 | 460.39 | 43,374.07 | 0.3040 | 46,360.86 | 12.04 | 3.44 | 0.0000 | 3.48 | 0.0104 ** | 0.1062 | 0.9974 | 0.1045 |
| X32 | 319.80 | 2194.89 | 90,608.08 | 53,035.74 | 319.80 | 2194.89 | 90,608.08 | 53,035.74 | 8.37 | 0.1739 | 3.48 | 3.98 | 0.0232 ** | 0.6892 | 0.1045 | 0.0864 |
| Residual | 267.61 | 88,347.51 | 182,500 | 93,361.93 | 38.23 | 12,621.07 | 26,065.91 | 13,337.42 | ||||||||
| Lack of fit | 141.97 | 6733.69 | 106,800 | 45,833.46 | 47.32 | 2244.56 | 35,602.49 | 15,277.82 | 1.51 | 0.1100 | 1.88 | 1.29 | 0.3415 | 0.9498 | 0.2736 | 0.3934 |
| Pure error | 125.64 | 81,613.82 | 75,653.91 | 475,28.47 | 31.41 | 20,403.45 | 18,913.48 | 11,882.12 | ||||||||
| Total | 4613.97 | 280,400 | 538,200 | 817,600 | ||||||||||||
| R2 pred (Y1)—0.4651 | R2 adjust (Y1)—0.8674 | Ratio—11.47 | ||||||||||||||
| R2 pred (Y2)—0.1610 | R2 adjust (Y2)—0.2798 | Ratio—4.96 | ||||||||||||||
| R2 pred (Y3)—-2.3950 | R2 adjust (Y3)—0.2251 | Ratio—4.23 | ||||||||||||||
| R2 pred (Y4)—0.0123 | R2 adjust (Y4)—0.7390 | Ratio—8.20 | ||||||||||||||
| TPC (mg GAE/g dw) | ABTS (IC50; µg/mL) | DPPH (IC50; µg/mL) | FRAP (µmol FSE/g dw) | |
|---|---|---|---|---|
| Experimental value | 97.50 ± 2.74 | 249.46 ± 20.89 | 547.34 ± 21.44 | 1154.10 ± 85.85 |
| Predicted value | 119.12 | 284.85 | 304.05 | 1360.69 |
| p | 0.053 | 0.689 | 0.129 | 0.123 |
| Compounds | (mg/ 100 g dw) |
|---|---|
| Phenolic acids | |
| Gallic acid | 91.9 ± 4.6 |
| Protocatechuic acid | 174 ± 9 |
| Neochlorogenic acid | 761 ± 38 |
| Caftaric acid | 22.6 ± 1.1 |
| Chlorogenic acid | 196 ± 10 |
| 4-O-caffeoylquinic acid | 338 ± 17 |
| Vanillic acid | <LOD |
| Caffeic acid | <LOQ |
| Syringic acid | ND |
| p-coumaric acid | <LOD |
| Ferulic acid | 4.13 ± 0.21 |
| Sinapic acid | <LOQ |
| 3,5-di-caffeoylquinic acid | 7.86 ± 0.39 |
| Ellagic acid | 15.6 ± 0.8 |
| 3,4-di-O-caffeoylquinic acid | 491 ± 25 |
| Cinnamic acid | 0.84 ± 0.04 |
| ∑Phenolic acids | 2103 ± 106 |
| Flavanols | |
| Catechin | 80.9 ± 4.0 |
| Epicatechin | 20.2 ± 1.0 |
| ∑Flavanols | 101 ± 5 |
| Flavanones | |
| Naringin | 64.3 ± 3.2 |
| Naringenin | 7.92 ± 0.40 |
| ∑Flavanones | 72.2 ± 3.6 |
| Flavonols | |
| Quercetin-3-O-galactoside | 22.4 ± 1.1 |
| Quercetin-3-O-glucopyranoside | 7.08 ± 0.35 |
| Rutin | 9.18 ± 0.46 |
| Myricetin | 25.6 ± 1.28 |
| Kaempferol-3-O-glucoside | 27.6 ± 1.4 |
| Isorhamnetin-3-O-glucoside | ND |
| Kaempferol-3-O-rutinoside | ND |
| Isorhamnetin-3-O-rutinoside | 103 ± 5 |
| Quercetin | 4.96 ± 0.25 |
| Tiliroside | 0.85 ± 0.04 |
| Kaempferol | 2.79 ± 0.14 |
| ∑Flavonols | 203 ± 10 |
| Flavones | |
| Apigenin | <LOD |
| Chrysin | <LOQ |
| ∑ Flavones | – |
| Others | |
| Caffeine | 55.9 ± 2.8 |
| trans-polydatin | 2.11 ± 0.11 |
| Resveratrol | <LOQ |
| Phloridzin | 7.69 ± 0.38 |
| trans-ε-viniferin | 14.9 ± 0.7 |
| Phloretin | <LOQ |
| ∑Others | 80.6 ± 4.0 |
| Samples | ROS | |
|---|---|---|
| O2●− | HOCl | |
| IC50 (µg/mL) | ||
| Optimal extract | 220.13 ± 3.41 b | 10.26 ± 0.35 b |
| Positive controls | ||
| Catechin | 590.18 ± 14.31 c | 0.10 ± 0.01 a |
| Gallic acid | 52.49 ± 1.58 a | 0.60 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.M.; Pinto, D.; Moreira, M.M.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach. Antioxidants 2022, 11, 763. https://doi.org/10.3390/antiox11040763
Silva AM, Pinto D, Moreira MM, Costa PC, Delerue-Matos C, Rodrigues F. Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach. Antioxidants. 2022; 11(4):763. https://doi.org/10.3390/antiox11040763
Chicago/Turabian StyleSilva, Ana Margarida, Diana Pinto, Manuela M. Moreira, Paulo C. Costa, Cristina Delerue-Matos, and Francisca Rodrigues. 2022. "Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach" Antioxidants 11, no. 4: 763. https://doi.org/10.3390/antiox11040763
APA StyleSilva, A. M., Pinto, D., Moreira, M. M., Costa, P. C., Delerue-Matos, C., & Rodrigues, F. (2022). Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach. Antioxidants, 11(4), 763. https://doi.org/10.3390/antiox11040763











