Natural Polyphenols May Normalize Hypochlorous Acid-Evoked Hemostatic Abnormalities in Human Blood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Blood Collection and Preparation
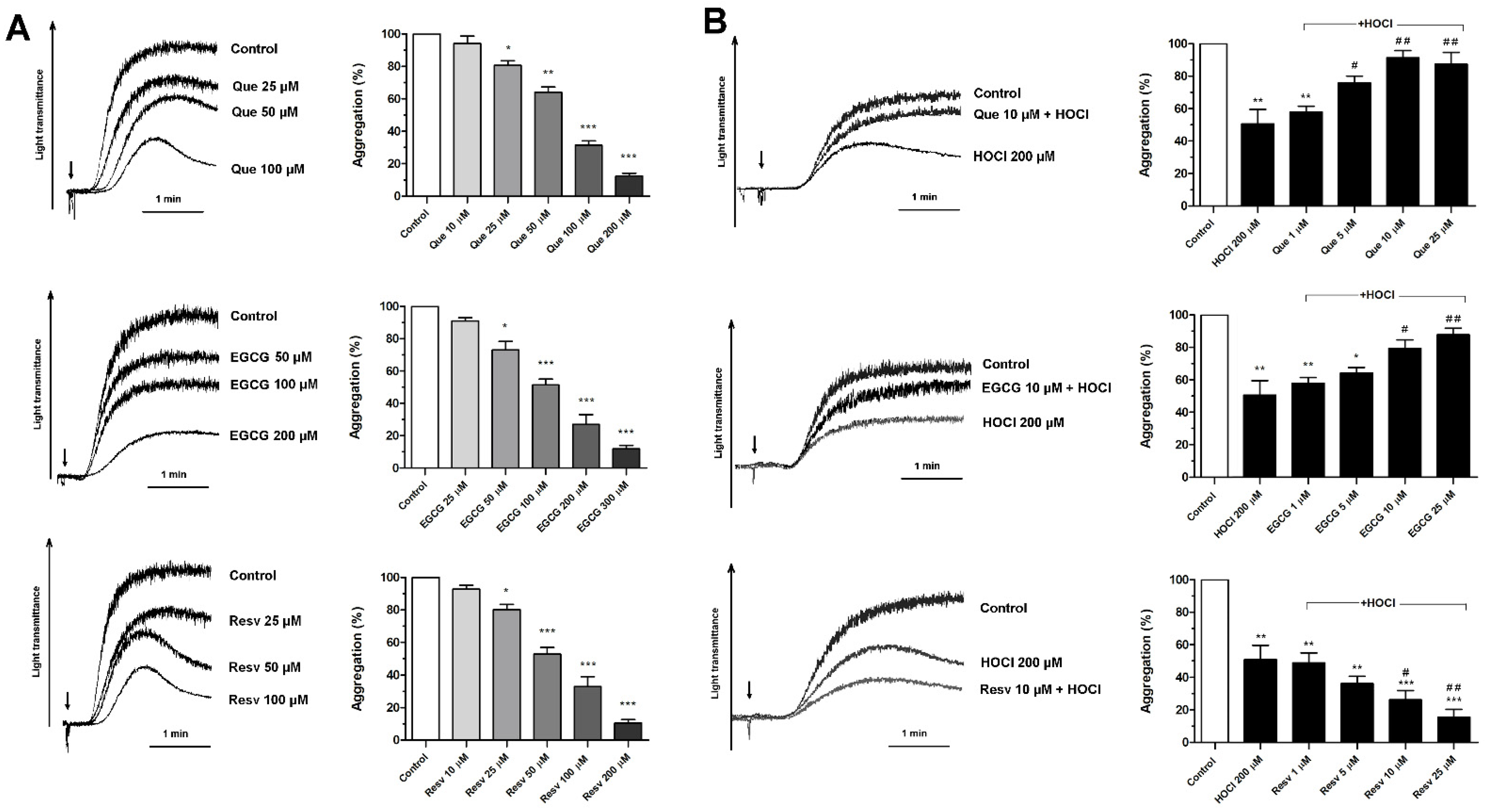
2.3. Platelet Aggregation Measurements
2.4. Thrombus Formation under Flow Study
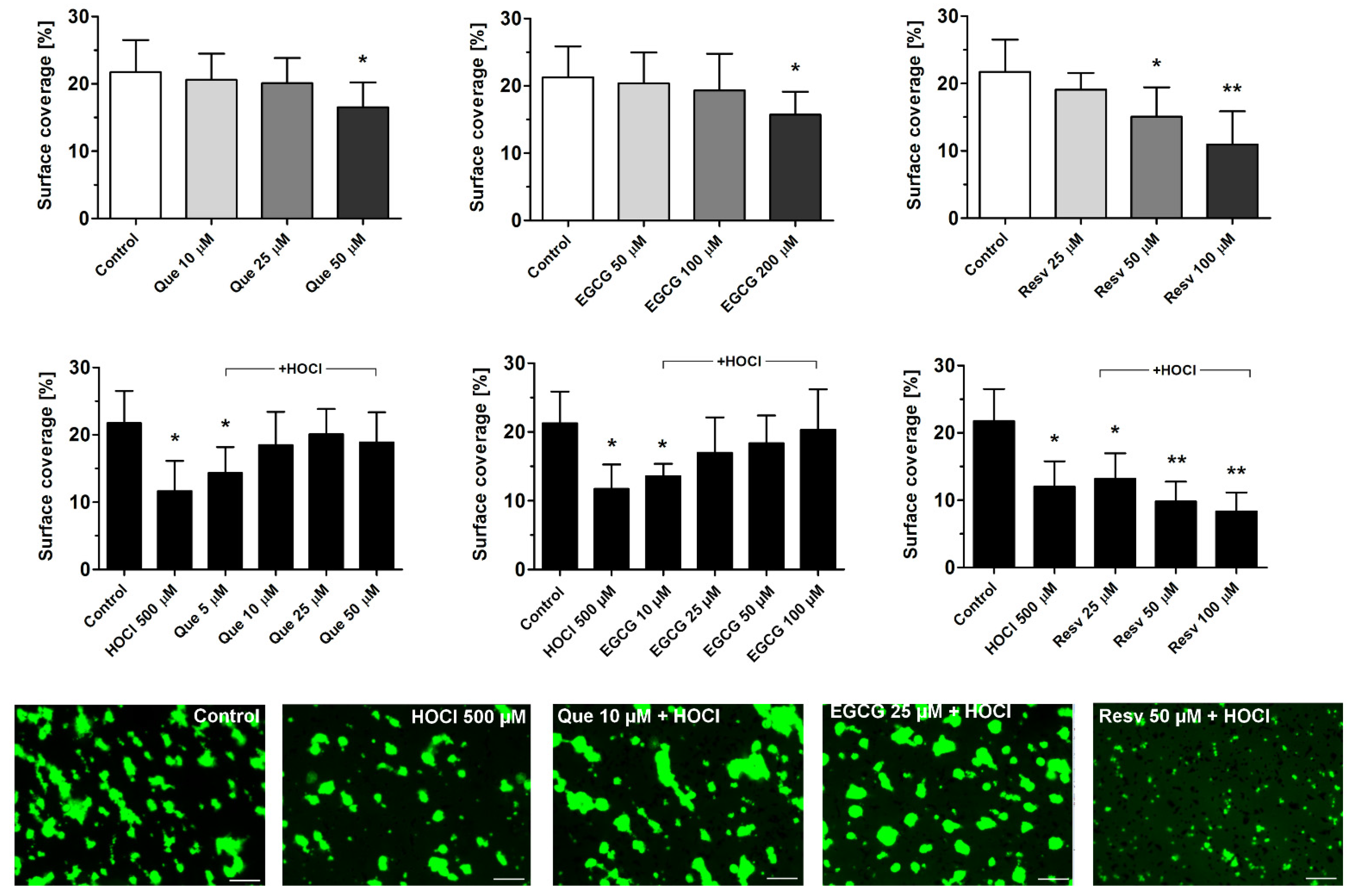
2.5. Confocal Analyses of Clot Structure
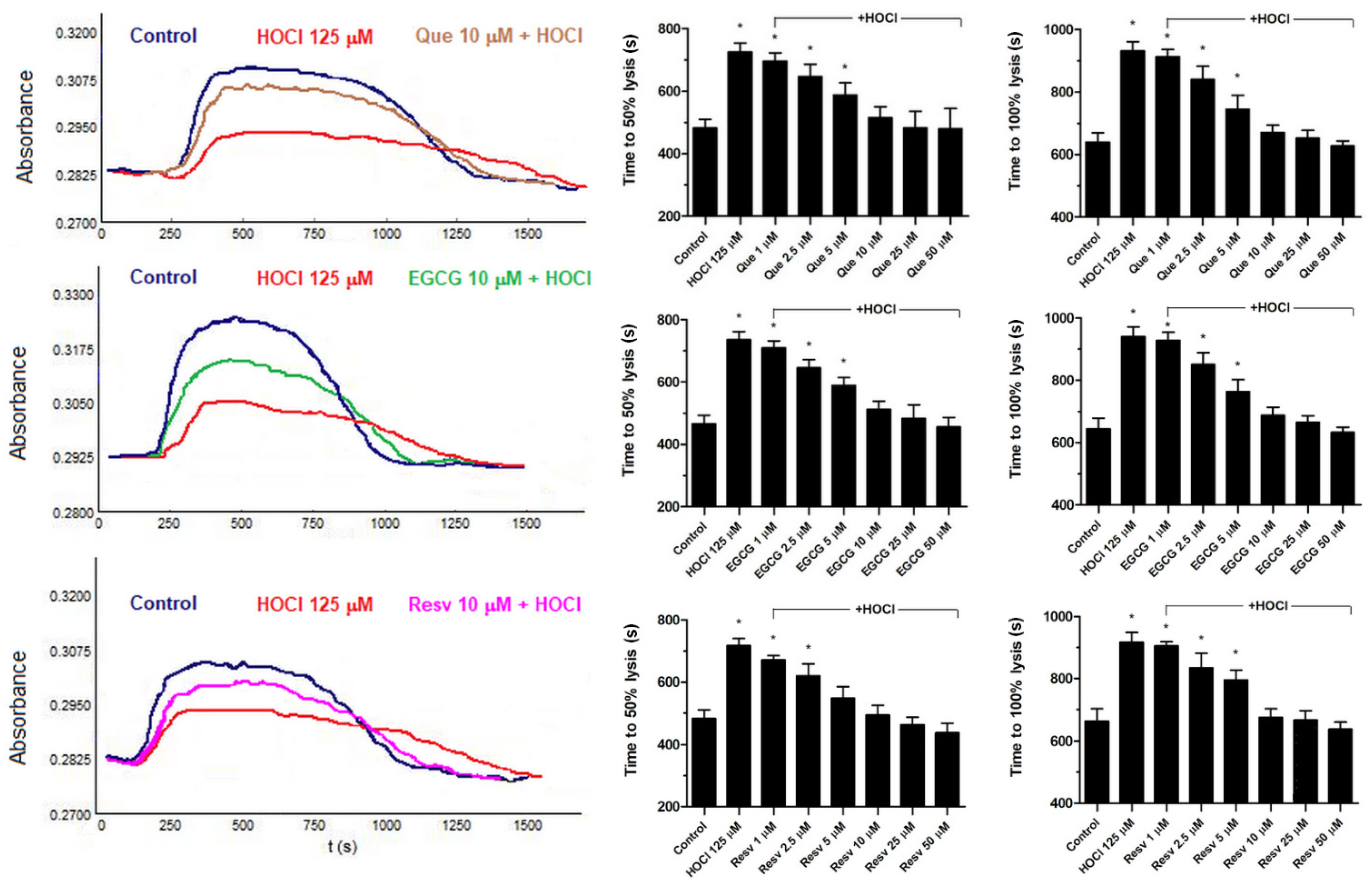
2.6. Fibrinolysis Assessment in Plasma
2.7. Determination of Thiol Groups in HOCl-Exposed Plasma
2.8. Data Analyses
3. Results
3.1. Concentration-Dependent Effects of Que, EGCG, and Resv on Platelet Aggregation
3.2. Effects of Que, EGCG, and Resv on Thrombus Formation under Flow
3.3. Normalization of HOCl-Evoked Densification of Fibrin Clots by Que, EGCG, and Resv
3.4. Normalization of HOCl-Associated Impairment of Fibrinolysis by Que, EGCG, and Resv
3.5. Counteraction against HOCl-Mediated Oxidations of Sulfhydryl Groups in Plasma by Que, EGCG, and Resv
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podrez, E.A.; Abu-Soud, H.M.; Hazen, S.L. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic. Biol. Med. 2000, 28, 1717–1725. [Google Scholar] [CrossRef]
- Pullar, J.M.; Vissers, M.C.; Winterbourn, C.C. Living with a killer: The effects of hypochlorous acid on mammalian cells. IUBMB Life 2000, 50, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase, Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Björnsdottir, H.; Welin, A.; Michaëlsson, E.; Osla, V.; Berg, S.; Christenson, K.; Sundqvist, M.; Dahlgren, C.; Karlsson, A.; Bylund, J. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic. Biol. Med. 2015, 89, 1024–1035. [Google Scholar] [CrossRef]
- Foote, C.S.; Goyne, T.E.; Lehrer, R.I. Assessment of chlorination by human neutrophils. Nature 1983, 301, 715–716. [Google Scholar] [CrossRef]
- Thomas, E.L.; Grisham, M.B.; Jefferson, M.M. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J. Clin. Investig. 1983, 72, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef]
- Weigandt, K.M.; White, N.; Chung, D.; Ellingson, E.; Wang, Y.; Fu, X.; Pozzo, D.C. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys. J. 2012, 103, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Weisel, J.W.; Ischiropoulos, H. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free Radic. Biol. Med. 2013, 65, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, N.J.; Wang, Y.; Fu, X.; Cardenas, J.C.; Martin, E.J.; Brophy, D.F.; Wade, C.E.; Wang, X.; St John, A.E.; Lim, E.B.; et al. Posttranslational oxidative modification of fibrinogen is associated with coagulopathy after traumatic injury. Free Radic. Biol. Med. 2016, 96, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Cristofaro, R.; Landolfi, R. Oxidation of human alpha-thrombin by the myeloperoxidase-H2O2-chloride system: Structural and functional effects. Thromb. Haemost. 2000, 83, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Nalian, A.; Iakhiaev, A.V. Possible mechanisms contributing to oxidative inactivationof activated protein C: Molecular dynamics study. Thromb. Haemost. 2008, 100, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Misztal, T.; Rusak, T.; Tomasiak, M. Clinically relevant HOCl concentrations reduce clot retraction rate via the inhibition of energy production in platelet mitochondria. Free Radic. Res. 2014, 48, 1443–1453. [Google Scholar] [CrossRef]
- Misztal, T.; Golaszewska, A.; Tomasiak-Lozowska, M.M.; Iwanicka, M.; Marcinczyk, N.; Leszczynska, A.; Chabielska, E.; Rusak, T. The myeloperoxidase product, hypochlorous acid, reduces thrombus formation under flow and attenuates clot retraction and fibrinolysis in human blood. Free Radic. Biol. Med. 2019, 141, 426–437. [Google Scholar] [CrossRef]
- Mita, H.; Higashi, N.; Taniguchi, M.; Higashi, A.; Kawagishi, Y.; Akiyama, K. Urinary 3-bromotyrosine and 3-chlorotyrosine concentrations in asthmatic patients: Lack of increase in 3-bromotyrosine concentration in urine and plasma proteins in aspirin induced asthma after intravenous aspirin challenge. Clin. Exp. Allergy 2004, 34, 931–938. [Google Scholar] [CrossRef]
- Tomasiak-Lozowska, M.M.; Misztal, T.; Rusak, T.; Branska-Januszewska, J.; Bodzenta-Lukaszyk, A.; Tomasiak, M. Asthma is associated with reduced fibrinolytic activity, abnormal clot architecture, and decreased clot retraction rate. Allergy 2017, 72, 314–319. [Google Scholar] [CrossRef]
- McMillen, T.S.; Heinecke, J.W.; LeBoeuf, R.C. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation 2005, 111, 2798–2804. [Google Scholar] [CrossRef] [Green Version]
- Schindhelm, R.K.; van der Zwan, L.P.; Teerlink, T.; Scheffer, P.G. Myeloperoxidase, a useful biomarker for cardiovascular disease risk stratification? Clin. Chem. 2009, 55, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Pinto, B.; Costa, L.; Felgueira, E.; Fonseca, B.M.; Rebelo, I. Low doses of resveratrol protect human granulosa cells from induced-oxidative stress. Antioxidants 2021, 10, 561. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of resveratrol in clinical management of chronic diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef] [Green Version]
- Marumo, M.; Ekawa, K.; Wakabayashi, I. Resveratrol inhibits Ca2+ signals and aggregation of platelets. Environ. Health Prev. Med. 2020, 25, 70. [Google Scholar] [CrossRef]
- Shen, M.Y.; Hsiao, G.; Liu, C.L.; Fong, T.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 MAPK and NO/cyclic GMP. Br. J. Haematol. 2007, 139, 475–485. [Google Scholar] [CrossRef]
- Lill, G.; Voit, S.; Schrör, K.; Weber, A.-A. Complex effects of different green tea catechins on human platelets. FEBS Lett. 2003, 546, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Wright, B.; Moraes, L.A.; Kemp, C.F.; Mullen, W.; Crozier, A.; Lovegrove, J.A.; Gibbins, J.M. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. Br. J. Pharmacol. 2010, 159, 1312–1325. [Google Scholar] [CrossRef] [Green Version]
- Oh, W.J.; Endale, M.; Park, S.-C.; Cho, J.Y.; Rhee, M.H. Dual roles of quercetin in platelets: Phosphoinositide-3-kinase and MAP kinases inhibition, and cAMP-dependent vasodilator-stimulated phosphoprotein stimulation. Evid.-Based Complement. Alternat. Med. 2012, 2012, 485262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, O.K.; Floegel, A.; Chung, S.-J.; Chung, C.E.; Song, W.O.; Koo, S.I. Estimation of antioxidant intakes from diet and supplements in U.S. adults. J. Nutr. 2010, 140, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendt, I.; Eichner, G.; Fasshauer, M. Association of antioxidants use with all-cause and cause-specific mortality: A prospective study of the UK Biobank. Antioxidants 2020, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V.R.; Cross, M.J. The aggregation of blood platelets. J. Physiol. 1963, 168, 178–195. [Google Scholar] [CrossRef] [PubMed]
- de Witt, S.M.; Swieringa, F.; Cavill, R.; Lamers, M.M.E.; van Kruchten, R.; Mastenbroek, T.; Baaten, C.; Coort, S.; Pugh, N.; Schulz, A.; et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat. Commun. 2014, 5, 4257. [Google Scholar] [CrossRef] [Green Version]
- Golaszewska, A.; Misztal, T.; Marcinczyk, N.; Chabielska, E.; Rusak, T. Adrenaline may contribute to prothrombotic condition via augmentation of platelet procoagulant response, enhancement of fibrin formation, and attenuation of fibrinolysis. Front. Physiol. 2021, 12, 657881. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U. S. National Institutes of Health: Bethesda, MD, USA, 2016.
- Misztal, T.; Rusak, T.; Branska-Januszewska, J.; Ostrowska, H.; Tomasiak, M. Peroxynitrite may affect fibrinolysis via the reduction of platelet-related fibrinolysis resistance and alteration of clot structure. Free Radic. Biol. Med. 2015, 89, 533–547. [Google Scholar] [CrossRef]
- Eyer, P.; Worek, F.; Kiderlen, D.; Sinko, G.; Stuglin, A.; Simeon-Rudolf, V.; Reiner, E. Molar absorption coefficients for the reduced Ellman reagent: Reassessment. Anal. Biochem. 2003, 312, 224–227. [Google Scholar] [CrossRef]
- Carter, A.M.; Cymbalista, C.M.; Spector, T.D.; Grant, P.J. Heritability of clot formation, morphology, and lysis: The EuroCLOT study, Arterioscler Throm. Vasc. Biol. 2007, 27, 2783–2789. [Google Scholar] [CrossRef] [Green Version]
- Marcinczyk, N.; Golaszewska, A.; Misztal, T.; Gromotowicz-Poplawska, A.; Rusak, T.; Chabielska, E. New approaches for the assessment of platelet activation status in thrombus under flow condition using confocal microscopy. Naunyn-Schmiedebergs Arch. Pharmacol. 2020, 393, 727–738. [Google Scholar] [CrossRef]
- Marcinczyk, N.; Misztal, T.; Gromotowicz-Poplawska, A.; Zebrowska, A.; Rusak, T.; Radziwon, P.; Chabielska, E. Utility of platelet endothelial cell adhesion molecule 1 in the platelet activity assessment in mouse and human blood. Int. J. Mol. Sci. 2021, 22, 9611. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Louie, S.; Cross, C.E.; Motchnik, P.; Halliwel, B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993, 121, 257–262. [Google Scholar] [PubMed]
- Jackson, S.P.; Nesbitt, W.S.; Kulkarni, S. Signaling events underlying thrombus formation. J. Thromb. Haemost. 2003, 1, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Secrets of platelet exocytosis—What do we really know about platelet secretion mechanisms? Br. J. Haematol. 2014, 165, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Brüne, B.; Ullrich, V. Different calcium pools in human platelets and their role in thromboxane A2 formation. J. Biol. Chem. 1991, 266, 19232–19237. [Google Scholar] [CrossRef]
- Misztal, T.; Golaszewska, A.; Branska-Januszewska, J.; Marcinczyk, N.; Chabielska, E.; Tomasiak, M.; Rusak, T. HAuCl4, putative general aquaporins blocker, reduces platelet spreading, filopodia formation, procoagulant response, and thrombus formation under flow. Front. Physiol. 2020, 11, 1025. [Google Scholar] [CrossRef]
- Park, J.-Y.; Hong, S.J.; Kim, K.-A.; Lee, S.H.; Cho, J.-Y.; Park, J.H.; Yu, C.W.; Lim, D.-S. Anti-platelet effects of epigallocatechin-3-gallate in addition to the concomitant aspirin, clopidogrel or ticagrelor treatment. Korean J. Intern. Med. 2018, 33, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Stainer, A.R.; Sasikumar, P.; Bye, A.P.; Unsworth, A.J.; Holbrook, L.M.; Tindall, M.; Lovegrove, J.A.; Gibbins, J.M. The metabolites of the dietary flavonoid quercetin possess potent antithrombotic activity, and interact with aspirin to enhance antiplatelet effects. TH Open 2019, 3, e244–e258. [Google Scholar] [CrossRef]
- Dumore, N.; Dumore, M.; Gupta, R.A. Effect of resveratrol on adverse functions of platelets. Int. Res. J. Pharm. 2017, 8, 125–131. [Google Scholar] [CrossRef]
- Stef, G.; Csiszar, A.; Lerea, K.; Ungvari, Z.; Veress, G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J. Cardiovasc. Pharmacol. 2006, 48, 1–5. [Google Scholar] [CrossRef]
- Crescente, M.; Jessen, G.; Momi, S.; Höltje, H.-D.; Gresele, P.; Cerletti, C.; de Gaetano, G. Interactions of gallic acid, resveratrol, quercetin and aspirin at the platelet cyclooxygenase-1 level. Functional and modelling studies. Thromb. Haemost. 2009, 102, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Pereillo, J.-M.; Maftouh, M.; Andrieu, A.; Uzabiaga, M.-F.; Fedeli, O.; Savi, P.; Pascal, M.; Herbert, J.-M.; Maffrand, J.-P.; Picard, C. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab. Dispos. 2002, 30, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.; Schrör, K.; Wojta, J.; Huber, K. Thienopyridines in cardiovascular disease: Focus on clopidogrel resistance. Thromb. Haemost. 2007, 97, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.; Ung, A.; Abdelkawy, K. Studying the inhibitory effect of quercetin and thymoquinone on human cytochrome P450 enzyme activities. Pharmacogn Mag. 2017, 13, S895–S899. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory effects of quercetin and its main methyl, sulfate, and glucuronic acid conjugates on cytochrome P450 enzymes, and on OATP, BCRP and MRP2 transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef]
- Misaka, S.; Kawabe, K.; Onoue, S.; Werba, J.P.; Giroli, M.; Tamaki, S.; Kan, T.; Kimura, J.; Watanabe, H.; Yamada, S. Effects of green tea catechins on cytochrome P450 2B6, 2C8, 2C19, 2D6 and 3A activities in human liver and intestinal microsomes. Drug Metab. Pharmacokinet. 2013, 28, 244–249. [Google Scholar] [CrossRef]
- Yu, C.; Shin, Y.G.; Kosmeder, J.W.; Pezzuto, J.M.; van Breemen, R.B. Liquid chromatography/tandem mass spectrometric determination of inhibition of human cytochrome P450 isozymes by resveratrol and resveratrol-3-sulfate. Rapid Commun. Mass Spectrom. 2003, 17, 307–313. [Google Scholar] [CrossRef]
- Orsini, F.; Verotta, L.; Klimo, K.; Gerhäuser, C. Synthesis of resveratrol derivatives and in vitro screening for potential cancer chemopreventive activities. Arch. Pharm. (Weinheim) 2016, 349, 414–427. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [Green Version]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Burak, C.; Brüll, V.; Langguth, P.; Zimmermann, B.F.; Stoffel-Wagner, B.; Sausen, U.; Stehle, P.; Wolffram, S.; Egert, S. Higher plasma quercetin levels following oral administration of an onion skin extract compared with pure quercetin dihydrate in humans. Eur. J. Nutr. 2017, 56, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of tea catechins and its improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002, 11 Pt 1, 1025–1032. [Google Scholar] [PubMed]
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-encapsulation of EGCG and quercetin in liposomes for optimum antioxidant activity. J. Food Sci. 2019, 84, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef] [Green Version]
- Briskey, D.; Rao, A. Trans-resveratrol oral bioavailability in humans using LipiSperse™ dispersion technology. Pharmaceutics 2020, 12, 1190. [Google Scholar] [CrossRef]
- Murina, M.A.; Savel’eva, E.L.; Roshchupkin, D.I. Mechanism of action of biogenic chloramines and hypochlorite on initial aggregation of blood platelets. Biofizika 2006, 51, 299–305. [Google Scholar]
- Reifenberger, M.S.; Arnett, K.L.; Gatto, C.; Milanick, M.A. The reactive nitrogen species peroxynitrite is a potent inhibitor of renal Na-K-ATPase activity. Am. J. Physiol. Renal. Physiol. 2008, 295, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Misztal, T.; Rusak, T.; Tomasiak, M. Peroxynitrite may affect clot retraction in human blood through the inhibition of platelet mitochondrial energy production. Thromb. Res. 2014, 133, 402–411. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misztal, T.; Golaszewska, A.; Marcińczyk, N.; Tomasiak-Łozowska, M.; Szymanowska, M.; Chabielska, E.; Rusak, T. Natural Polyphenols May Normalize Hypochlorous Acid-Evoked Hemostatic Abnormalities in Human Blood. Antioxidants 2022, 11, 779. https://doi.org/10.3390/antiox11040779
Misztal T, Golaszewska A, Marcińczyk N, Tomasiak-Łozowska M, Szymanowska M, Chabielska E, Rusak T. Natural Polyphenols May Normalize Hypochlorous Acid-Evoked Hemostatic Abnormalities in Human Blood. Antioxidants. 2022; 11(4):779. https://doi.org/10.3390/antiox11040779
Chicago/Turabian StyleMisztal, Tomasz, Agata Golaszewska, Natalia Marcińczyk, Maria Tomasiak-Łozowska, Małgorzata Szymanowska, Ewa Chabielska, and Tomasz Rusak. 2022. "Natural Polyphenols May Normalize Hypochlorous Acid-Evoked Hemostatic Abnormalities in Human Blood" Antioxidants 11, no. 4: 779. https://doi.org/10.3390/antiox11040779
APA StyleMisztal, T., Golaszewska, A., Marcińczyk, N., Tomasiak-Łozowska, M., Szymanowska, M., Chabielska, E., & Rusak, T. (2022). Natural Polyphenols May Normalize Hypochlorous Acid-Evoked Hemostatic Abnormalities in Human Blood. Antioxidants, 11(4), 779. https://doi.org/10.3390/antiox11040779





