Online Extraction–DPPH–HPLC–DAD–QTOF-MS System for Efficient Screening and Identification of Antioxidants from Citrus aurantium L. var. amara (Rutaceae): Integrating Sample Preparation and Antioxidants Profiling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Reference Extract
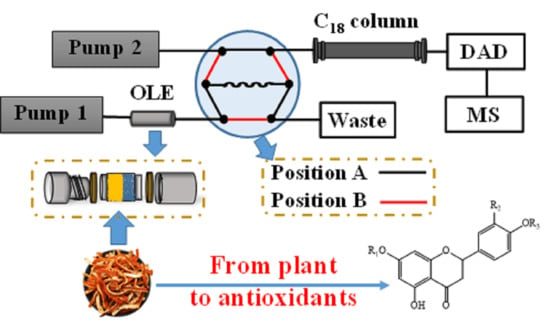
2.3. OLE–DPPH–HPLC−DAD−QTOF-MS Analysis
2.4. Antioxidant Activity Evaluation
2.5. DFT Calculations
3. Results and Discussion
3.1. OLE–DPPH–HPLC−DAD−QTOF-MS System Setup
3.1.1. Optimization of the OLE–HPLC System
3.1.2. OLE–DPPH–HPLC Assay
3.1.3. HPLC–QTOF-MS Analysis
3.2. Identification of Antioxidants in CAVA
3.3. Quantification of Antioxidants in CAVA
3.4. Antioxidant Activity Evaluation
3.5. DFT Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martín-García, B.; De Montijo-Prieto, S.; Jiménez-Valera, M.; Carrasco-Pancorbo, A.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Comparative extraction of phenolic compounds from olive leaves using a sonotrode and an ultrasonic bath and the evaluation of both antioxidant and antimicrobial activity. Antioxidants 2022, 11, 558. [Google Scholar]
- Jiang, S.; Liu, H.; Li, C. Dietary regulation of oxidative stress in chronic metabolic diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tian, T.; Wu, D.; Guo, D.; Lu, J. Prevention and treatment effects of edible berries for three deadly diseases: Cardiovascular disease, cancer and diabetes. Crit. Rev. Food Sci. Nutr. 2019, 59, 1903–1912. [Google Scholar] [CrossRef]
- Mirian, P.; Julián, A.G.; Mariana, J.P.; María, E.S.; José, M.L. Plant extracts obtained with green solvents as natural antioxidants in fresh meat products. Antioxidants 2021, 10, 181. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 1 1981 to 9 2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M.; Zappia, C.; Capocasale, M. Physicochemical stability of blood orange juice during frozen storage. Intern. J. Food Prop. 2017, 20, S1930–S1943. [Google Scholar]
- Giuffrè, A.M. Bergamot (Citrus bergamia, Risso): The effects of cultivar and harvest date on functional properties of juice and cloudy juice. Antioxidants 2019, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Guo, K.; Tong, R.; Liu, Y.; Tong, C.; Peng, M. Online extraction−HPLC−FRAP system for direct identification of antioxidants from solid Du-zhong brick tea. Food Chem. 2019, 288, 215–220. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, H.; Li, X.; He, T.; Wang, D.; Wang, W.; Jia, W.; Lin, Z.; Chen, S. One injection to profile the chemical composition and dual-antioxidation activities of Rosa chinensis Jacq. J. Chromatogr. A 2020, 1613, 460663. [Google Scholar] [CrossRef]
- Qian, Z.M.; Chen, L.; Wu, M.Q.; Li, D.Q. Rapid screening and characterization of natural antioxidants in Polygonum viviparum by an on-line system integrating the pressurized liquid microextraction, HPLC-DAD-QTOF-MS/MS analysis and antioxidant assay. J. Chromatogr. B 2020, 1137, 121926. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiao, L.; Tao, Y.; Shao, Y.; Wang, Q.; Yu, R.; Mei, L.; Dang, J. On-line HPLC-DPPH bioactivity-guided assay for isolated of antioxidative phenylpropanoids from Qinghai-Tibet Plateau medicinal plant Lancea tibetica. J. Chromatogr. B 2019, 1106–1107, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Shi, S.Y.; Xiong, X.; Chen, X.Q.; Peng, M.J. Comparative evaluation of three methods based on high-performance liquid chromatography analysis combined with a 2,2'-diphenyl-1-picrylhydrazyl assay for the rapid screening of antioxidants from Pueraria lobata flowers. Anal. Bioanal. Chem. 2012, 402, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.Y.; Xia, Y.; Guo, H.; He, X.Q.; Li, H.; Wu, D.T.; Geng, F.; Lin, F.J.; Li, H.B.; et al. Screening and process optimization of ultrasound-assisted extraction of main antioxidants from sweet tea (Lithocarpus litseifolius [Hance] Chun). Food Biosci. 2021, 43, 101277. [Google Scholar] [CrossRef]
- Tong, C.; Peng, M.; Tong, R.; Ma, R.; Guo, K.; Shi, S. Use of an online extraction liquid chromatography quadrupole time-of-flight tandem mass spectrometry method for the characterization of polyphenols in Citrus paradisi cv. Changshanhuyu peel. J. Chromatogr. A 2018, 1533, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China, 2020th ed.; Part I; China Medical Science and Technology Press: Beijing, China, 2020; p. 257.
- Yu, L.; Chen, M.; Liu, J.; Huang, X.; He, W.; Qing, Z.; Zeng, J. Systematic detection and identification of bioactive ingredients from Citrus aurantium L. var. amara using HPLC-Q-TOF-MS combined with a screening method. Molecules 2020, 25, 357. [Google Scholar]
- Li, X.Y.; Hao, Y.F.; Hao, Z.X.; Jiang, J.G.; Liu, Q.; Shen, Q.; Liu, L.; Yi, Y.K.; Shen, C.Y. Inhibitory effect of chloroform extracts from Citrus aurantium L. var. amara Engl. on fat accumulation. Phytomedicine 2021, 90, 153634. [Google Scholar] [CrossRef]
- Shen, C.Y.; Wang, T.X.; Jiang, J.G.; Huang, C.L.; Zhu, W. Bergaptol from blossoms of Citrus aurantium L. var. amara Engl inhibits LPS-induced inflammatory responses and ox-LDL-induced lipid deposition. Food Funct. 2020, 11, 4915–4926. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yang, L.; Wei, J.; Huang, M.; Jiang, J.G. Bioactivity evaluations of ingredients extracted from the flowers of Citrus aurantium L. var. amara Engl. Food Chem. 2012, 135, 2175–2181. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.; Xie, L.; Tong, X.; Tang, C.; Shi, S. Red pitaya peels-based carbon dots for real-time fluorometric and colorimetric assay of Au3+, cellular imaging, and antioxidant activity. Anal. Bioanal. Chem. 2021, 413, 935–943. [Google Scholar] [CrossRef]
- Rouhani, M. Evaluation of structural properties and antioxidant capacity of Proxison: A DFT investigation. Comput. Theor. Chem. 2021, 1195, 113096. [Google Scholar] [CrossRef]
- Tong, C.; Guo, K.; Xu, J.; Tong, X.; Shi, S. Online extraction and cleanup–quadrupole time-of-flight tandem mass spectrometry for rapid analysis of bioactive components in natural products. Anal. Bioanal. Chem. 2019, 411, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Park, S.; Kim, E.; Kwon, H.; Park, H.J.; Nam, J.W.; Roh, S.S.; Choi, H. Antioxidant, pancreatic lipase inhibitory, and tyrosinase inhibitory activities extracts of the invasive plant Spartina anglica (Cord-Grass). Antioxidants 2021, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Darkal, A.K.; Zuraik, M.M.; Ney, Y.; Nasim, M.J.; Jacob, C. Unleashing the biological potential of Fomes fomentarius via dry and wet milling. Antioxidants 2021, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ladaniya, M.S.; Gurjar, M.; Kumar, S.; Mendke, S. Quantification of flavonoids, phenols and antioxidant potential from dropped Citrus reticulata Blanco fruits influenced by drying techniques. Molecules 2021, 26, 4159. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Zhang, R.; Zhao, Z.; Xu, X.; Chen, B.; Yang, D.; Zheng, G. Characterization of antioxidant compounds extracted from Citrus reticulata cv. Chachiensis using UPLC-Q-TOF-MS/MS, FT-IR and scanning electron microscope. J. Pharmaceut. Biomed. Anal. 2021, 192, 113683. [Google Scholar] [CrossRef]
- Zhou, X.; Qin, D.; Xiang, B.; Xi, J. Cyclodextrin-based liquid-phase pulsed discharge extraction of flavonoids from tangerine (Citrus reticulata) pericarp: Optimization, antioxidant activity and storage stability. Sep. Purif. Technol. 2022, 278, 119603. [Google Scholar] [CrossRef]
- Romanet, R.; Sarhane, Z.; Bahut, F.; Uhl, J.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Exploring the chemical space of white wine antioxidant capacity: A combined DPPH, EPR and FT-ICR-MS study. Food Chem. 2021, 355, 129566. [Google Scholar] [CrossRef]
- Mohanraj, S.K.P.; Tulasi, R.; Subramanian, V.C.; Dandu, B.S.R.; Guvvala, V.; Kota, S.R. A study on structural characterization of potential impurities of Sugammadex sodium using LC/ESI/QTOF/MS/MS and NMR. J. Pharmaceut. Biomed. Anal. 2021, 207, 114419. [Google Scholar] [CrossRef]
- Poletto, P.; Álvarez-Rivera, G.; López, G.; Borges, O.M.A.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Recovery of ascorbic acid, phenolic compounds and carotenoids from acerola by-products: An opportunity for their valorization. LWT 2021, 146, 111654. [Google Scholar] [CrossRef]
- Daniel, V.M.; Pedro, L.R.; Masaharu, I.; Anna, P.S.S.; Adna, P.M.; Severino, M.A. Active antioxidant phenolics from Brazilian red propolis: An optimization study for their recovery and identification by LC-ESI-QTOF-MS/MS. Antioxidants 2021, 10, 297. [Google Scholar]
- Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A comparative investigation on phenolic composition, characterization and antioxidant potentials of five different Australian grown pear varieties. Antioxidants 2021, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; He, M.; Yin, C.; Jiang, Y.; Luo, D.; Yang, B. Phenolics in Citrus aurantium fruit identified by UHPLC-MS/MS and their bioactivities. LWT 2021, 147, 111671. [Google Scholar] [CrossRef]
- Mazzotti, F.; Bartella, L.; Talarico, I.R.; Napoli, A.; Donna, L.D. High-throughput determination of flavanone-O-glycosides in citrus beverages by paper spray tandem mass spectrometry. Food Chem. 2021, 360, 130060. [Google Scholar] [CrossRef]
- Deng, M.; Jia, X.; Dong, L.; Liu, L.; Huang, F.; Chi, J.; Ma, Q.; Zhao, D.; Zhang, M.; Zhang, R. Structural elucidation of flavonoids from Shatianyu (Citrus grandis L. Osbeck) pulp and screening of key antioxidant components. Food Chem. 2022, 366, 130605. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, Y.; Shen, S.; Zhi, Z.; Cheng, H.; Chen, S.; Ye, X. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chem. 2020, 326, 126785. [Google Scholar] [CrossRef] [PubMed]
- Villar-Lopez, M.; Soto-Becerra, P.; Choque, R.C.; Al-Kassab-Cordova, A.; Bernuy-Barrera, F.; Palomino, H.; Rojas, P.A.; Vera, C.; Lugo-Martinez, G.; Mezones-Holguin, E. Safety and tolerability of a natural supplement containing glucosinolates, phytosterols and citrus flavonoids in adult women: A randomized phase I, placebo-controlled, multi-arm, double-blinded clinical trial. Gynecol. Endocrinol. 2021, 37, 906–913. [Google Scholar] [CrossRef]




| No. | tR (min) | λmax (nm) | [M + H]+ (∆ ppm) | Formula | Fragment Ions (m/z) | Identification |
|---|---|---|---|---|---|---|
| 1 | 16.9 | 287 | 743.2375 (−3.0) | C33H42O19 | 581.1853 [M + H − Glu]+ 435.1280 [M + H − Rut]+ 273.0747 [M + H – Glu − Rut]+ | Narirutin-4′-O-glucoside |
| 2 | 19.1 | 284 | 581.1882 (2.1) | C27H32O14 | 435.1257 [M + H − Rha]+ 273.0794 [M + H − Rut]+ | Narirutin |
| 3 | 19.9 | 284 | 581.1852 (−3.1) | C27H32O14 | 435.1284 [M + H − Rha]+ 273.0749 [M + H − Neo]+ | Naringin |
| 4 | 20.8 | 286 | 611.1984 (1.3) | C28H34O15 | 465.1412 [M + H − Rha]+ 303.0886 [M + H − Rut]+ | Hesperidin |
| 5 | 21.4 | 284 | 611.1948 (−4.6) | C28H34O15 | 465.1373 [M + H − Rha]+ 303.0850 [M + H − Neo]+ | Neohesperidin |
| 6 | 24.7 | 276 340 | 653.1686 (−4.9) | C29H32O17 | 509.1389 [M + H − 144]+ 347.0749 [M + H – 144 − Glu]+ | Limocitrin-3-O-(3-hydroxy-3- methylglutarate)-glucoside |
| 7 | 26.0 | 284 | 595.1993 (−5.7) | C28H34O14 | 449.1433 [M + H − Rha]+ 287.0911 [M + H − Rut]+ | Didymin |
| 8 | 29.9 | 286 | 303.0854 (−4.9) | C16H14O6 | 153.0174 [0,2B]+ | Hesperitin |
| Compd | Regression Equation a | R2 | Linear Range (μg/mL) | LOD (μg/mL) | Matrix Effect (%) | Precision (n = 5) (RSD, %) | Recovery b (%) | Contents (mg/g) c | |
|---|---|---|---|---|---|---|---|---|---|
| Intraday | Interday | ||||||||
| 2 | y = 389.92x + 563.80 | 0.997 | 1.0–100 | 0.17 | 98.2 | 3.1 | 8.6 | 95.1 | 0.62 ± 0.07 |
| 3 | y = 349.11x + 170.89 | 0.995 | 3.0–400 | 0.68 | 100.3 | 4.5 | 5.9 | 99.2 | 50.37 ± 0.43 |
| 4 | y = 510.94x + 86.52 | 0.999 | 1.0–100 | 0.25 | 104.7 | 3.5 | 4.5 | 101.9 | 1.49 ± 0.04 |
| 5 | y = 635.27x + 91.49 | 0.994 | 3.0–400 | 0.50 | 96.3 | 4.6 | 6.3 | 105.2 | 38.20 ± 0.27 |
| 7 | y = 434.95x + 131.84 | 0.995 | 3.0–300 | 0.59 | 94.0 | 3.2 | 5.7 | 96.4 | 3.91 ± 0.03 |
| 8 | y = 873.60x − 49.25 | 0.999 | 0.5–20 | 0.09 | 105.4 | 2.7 | 4.8 | 94.7 | 0.73 ± 0.06 |
| Compounds | IC50 | EHOMO | ELUMO | Eg |
|---|---|---|---|---|
| 1 | - a | −6.545 | −1.766 | 4.779 |
| 2 | 257.06 ± 9.32 | −6.470 | −1.761 | 4.709 |
| 3 | 111.9 ± 10.06 | −6.128 | −1.687 | 4.441 |
| 4 | 361.50 ± 13.29 | −6.482 | −1.767 | 4.715 |
| 5 | 178.55 ± 11.28 | −6.139 | −1.565 | 4.574 |
| 6 | - a | −5.924 | −2.029 | 3.895 |
| 7 | 219.73 ± 16.45 | −6.438 | −1.765 | 4.673 |
| 8 | 39.07 ± 2.51 | −6.123 | −1.831 | 4.292 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Fu, F.; Wei, Y.; Shi, S.; Shan, Y. Online Extraction–DPPH–HPLC–DAD–QTOF-MS System for Efficient Screening and Identification of Antioxidants from Citrus aurantium L. var. amara (Rutaceae): Integrating Sample Preparation and Antioxidants Profiling. Antioxidants 2022, 11, 1014. https://doi.org/10.3390/antiox11051014
Xiao Y, Fu F, Wei Y, Shi S, Shan Y. Online Extraction–DPPH–HPLC–DAD–QTOF-MS System for Efficient Screening and Identification of Antioxidants from Citrus aurantium L. var. amara (Rutaceae): Integrating Sample Preparation and Antioxidants Profiling. Antioxidants. 2022; 11(5):1014. https://doi.org/10.3390/antiox11051014
Chicago/Turabian StyleXiao, Yecheng, Fuhua Fu, Youhe Wei, Shuyun Shi, and Yang Shan. 2022. "Online Extraction–DPPH–HPLC–DAD–QTOF-MS System for Efficient Screening and Identification of Antioxidants from Citrus aurantium L. var. amara (Rutaceae): Integrating Sample Preparation and Antioxidants Profiling" Antioxidants 11, no. 5: 1014. https://doi.org/10.3390/antiox11051014
APA StyleXiao, Y., Fu, F., Wei, Y., Shi, S., & Shan, Y. (2022). Online Extraction–DPPH–HPLC–DAD–QTOF-MS System for Efficient Screening and Identification of Antioxidants from Citrus aurantium L. var. amara (Rutaceae): Integrating Sample Preparation and Antioxidants Profiling. Antioxidants, 11(5), 1014. https://doi.org/10.3390/antiox11051014