Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review
Abstract
:1. Introduction
2. Nutritional Composition of Amla
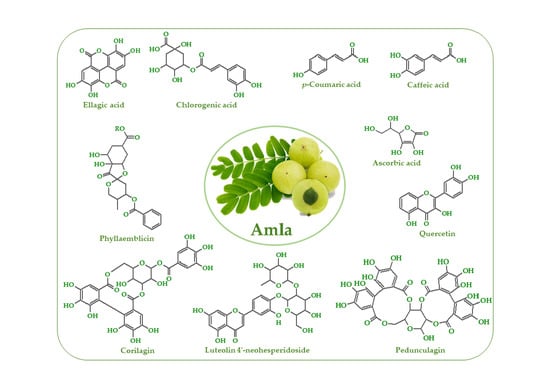
3. Phytochemistry of Amla
4. Potential Health Benefits
4.1. Antioxidant Activity
4.2. Cardioprotective Activity
4.3. Antidiabetic Activity
4.4. Anticancer Activity
4.5. Anti-Inflammatory Activity
4.6. Digestive Tract Protection
4.7. Neurological Protection
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walia, K.; Boolchandani, R.; Dhand, S.; Antony, B. Improving glycemic & lipidemic profile with amla powder (Emblica officinalis) supplementation in adults with type 2 diabetes mellitus. Int. J. Basic Appl. Med. Sci. 2015, 5, 251–258. [Google Scholar]
- Ramakrishna, N.; Singh, D.R. Ethno-Botanical Studies of Edible Plants Used by Tribal Women of Nirmal District. Int. J. Sci. Res. Sci. Eng. Technol. 2020, 3, 307–310. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Complement. Med. 2017, 7, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Pria, F.F.; Islam, M.S. Phyllanthus emblica Linn. (Amla)—A Natural Gift to Humans: An Overview. J. Dis. Med. Plants 2019, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Madka, V.; Pathuri, G.; Ganta, V.; Rao, C.V. Molecular Mechanisms of Cancer Prevention by Gooseberry (Phyllanthus emblica). Nutr. Cancer, 2021; in press. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Suzuki, K.; Derek, T.; Ozeki, M.; Okubo, T. Clinical evaluation of Emblica officinalis Gatertn (Amla) in healthy human subjects: Health benefits and safety results from a randomized, double-blind, crossover placebo-controlled study. Contemp. Clin. Trials Commun. 2020, 17, 100499. [Google Scholar] [CrossRef]
- Khan, K.H. Roles of Emblica officinalis in medicine—A review. Bot. Res. Int. 2009, 2, 218–228. [Google Scholar]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Anola (Emblica officinalis): Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas; Hussain, S.Z., Naseer, B., Qadri, T., Fatima, T., Bhat, T.A., Eds.; Springer: Cham, Switzerland, 2021; pp. 193–206. ISBN 30755027_15. [Google Scholar]
- KC, Y.; Rayamajhi, S.; Dangal, A.; Shiwakoti, L.D. Phytochemical, Nutritional, Antioxidant Activity and Sensorial Characteristics of Amala (Phyllanthus emblica L.) Chutney. Asian Food Sci. J. 2020, 18, 43–52. [Google Scholar] [CrossRef]
- Tewari, R.; Kumar, V.; Sharma, H.K. Physical and chemical characteristics of different cultivars of Indian gooseberry (Emblica officinalis). J. Food Sci. Technol. 2019, 56, 1641–1648. [Google Scholar] [CrossRef]
- Parveen, K.; Khatkar, B.S. Physico-chemical properties and nutritional composition of aonla (Emblica officinalis) varieties. Int. Food Res. J. 2015, 22, 2358–2363. [Google Scholar]
- Sonkar, N.; Rajoriya, D.; Chetana, R.; Venkatesh Murthy, K. Effect of cultivars, pretreatment and drying on physicochemical properties of Amla (Emblica officinalis) gratings. J. Food Sci. Technol. 2020, 57, 980–992. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarwadi, K.; Agte, V. Antioxidant and micronutrient potential of common fruits available in the Indian subcontinent. Int. J. Food Sci. Nutr. 2007, 58, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Khurdiya, D.S. Vitamin C enrichment of fruit juice based ready-to-serve beverages through blending of Indian gooseberry (Emblica officinalis Gaertn.) juice. Plant Foods Hum. Nutr. 2004, 59, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L. Coupling of chromatographic analyses with pretreatment for the determination of bioactive compounds in Emblica officinalis juice. Anal. Methods 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L. Rapid HPLC Method for determination of vitamin C, phenolic acids, hydroxycinnamic acid, and flavonoids in seasonal samples of Emblica officinalis juice. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 619–624. [Google Scholar] [CrossRef]
- Nambiar, S.S.; Paramesha, M.; Shetty, N.P. Comparative analysis of phytochemical profile, antioxidant activities and foam prevention abilities of whole fruit, pulp and seeds of Emblica officinalis. J. Food Sci. Technol. 2015, 52, 7254–7262. [Google Scholar] [CrossRef]
- Nambiar, S.S.; Shetty, N.P. Phytochemical Profiling and Assessment of Low-Density Lipoprotein Oxidation, Foam Cell-Preventing Ability and Antioxidant Activity of Commercial Products of Emblica officinalis Fruit. J. Food Biochem. 2015, 39, 218–229. [Google Scholar] [CrossRef]
- Poltanov, E.A.; Shikov, A.N.; Dorman, H.J.D.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R. Chemical and antioxidant evaluation of Indian gooseberry (Emblica officinalis Gaertn., syn. Phyllanthus emblica L.) supplements. Phyther. Res. 2009, 23, 1309–1315. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Abe, T.; Tanaka, T.; Yang, C.R.; Kouno, I. Two new acylated flavanone glycosides from the leaves and branches of Phyllanthus emblica. Chem. Pharm. Bull. 2002, 50, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Ur-Rehman, H.; Yasin, K.A.; Choudhary, M.A.; Khaliq, N.; Ur-Rahman, A.; Choudhary, M.I.; Malik, S. Studies on the chemical constituents of Phyllanthus emblica. Nat. Prod. Res. 2007, 21, 775–781. [Google Scholar] [CrossRef]
- Liu, X.; Cui, C.; Zhao, M.; Wang, J.; Luo, W.; Yang, B.; Jiang, Y. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem. 2008, 109, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Pingali, U.; Muralidhar, N. Study of pharmacodynamic interaction of Phyllanthus emblica extract with clopidogrel and ecosprin in patients with type II diabetes mellitus. Phytomedicine 2014, 21, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Abe, T.; Tanaka, T.; Yang, C.R.; Kouno, I. Phyllanemblinins A-F, new ellagitannins from Phyllanthus emblica. J. Nat. Prod. 2001, 64, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Al-Samman, A.M.M.A.; Siddique, N.A. Gas chromatography-mass spectrometry (GC-MS/MS) analysis, ultrasonic assisted extraction, antibacterial and antifungal activity of Emblica officinalis fruit extract. Pharmacogn. J. 2019, 11, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Sheoran, S.; Nidhi, P.; Kumar, V.; Singh, G.; Lal, U.R.; Sourirajan, A.; Dev, K. Altitudinal variation in gallic acid content in fruits of Phyllanthus emblica L. and its correlation with antioxidant and antimicrobial activity. Vegetos 2019, 32, 387–396. [Google Scholar] [CrossRef]
- Fitriansyah, S.N.; Aulifa, D.L.; Febriani, Y.; Sapitri, E. Correlation of total phenolic, flavonoid and carotenoid content of Phyllanthus emblica extract from bandung with DPPH scavenging activities. Pharmacogn. J. 2018, 10, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Bar, F.M.A.; Habib, M.M.A.; Badria, F.A. A new hexagalloyl compound from Emblica officinalis Gaertn.: Antioxidant, cytotoxicity, and silver ion reducing activities. Chem. Pap. 2021, 75, 6509–6518. [Google Scholar] [CrossRef]
- Tewari, R.; Kumar, V.; Sharma, H.K. Pretreated Indian Gooseberry (Emblica officinalis) Segments: Kinetic, Quality and Microstructural Parameters. J. Inst. Eng. Ser. A 2021, 102, 523–534. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, L.; Guo, X.; Li, C.; Li, H.; Lou, H.; Ren, D. Chemical constituents from Phyllanthus emblica and the cytoprotective effects on H2O2-induced PC12 cell injuries. Arch. Pharm. Res. 2016, 39, 1202–1211. [Google Scholar] [CrossRef]
- Chahal, A.K.; Chandan, G.; Kumar, R.; Chhillar, A.K.; Saini, A.K.; Saini, R.V. Bioactive constituents of Emblica officinalis overcome oxidative stress in mammalian cells by inhibiting hyperoxidation of peroxiredoxins. J. Food Biochem. 2020, 44, e13115. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivananjappa, M.M.; Joshi, M.K. Influence of Emblica officinalis aqueous extract on growth and antioxidant defense system of human hepatoma cell line (HepG2). Pharm. Biol. 2012, 50, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Chen, R.; Li, Y.; Miao, J.; Liu, G.; Lan, Y.; Chen, Y.; Cao, Y. HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J. Ethnopharmacol. 2020, 254, 112740. [Google Scholar] [CrossRef]
- Yamamoto, H.; Morino, K.; Mengistu, L.; Ishibashi, T.; Kiriyama, K.; Ikami, T.; Maegawa, H. Amla Enhances Mitochondrial Spare Respiratory Capacity by Increasing Mitochondrial Biogenesis and Antioxidant Systems in a Murine Skeletal Muscle Cell Line. Oxid. Med. Cell. Longev. 2016, 2016, 1735841. [Google Scholar] [CrossRef] [Green Version]
- Nain, P.; Saini, V.; Sharma, S.; Nain, J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012, 142, 65–71. [Google Scholar] [CrossRef]
- Singh, M.K.; Yadav, S.S.; Gupta, V.; Khattri, S. Immunomodulatory role of Emblica officinalis in arsenic induced oxidative damage and apoptosis in thymocytes of mice. BMC Complement. Altern. Med. 2013, 13, 193. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Chattopadhyay, S.; Acharyya, N.; Deb, B.; Hati, A.K. Emblica officinalis (amla) ameliorates arsenic-induced liver damage via DNA protection by antioxidant systems. Mol. Cell. Toxicol. 2014, 10, 75–82. [Google Scholar] [CrossRef]
- Saha, S.; Verma, R.J. Antioxidant activity of polyphenolic extract of Phyllanthus emblica against lead acetate induced oxidative stress. Toxicol. Environ. Health Sci. 2015, 7, 82–90. [Google Scholar] [CrossRef]
- Biswas, T.K.; Chakrabarti, S.; Pandit, S.; Jana, U.; Dey, S.K. Pilot study evaluating the use of Emblica officinalis standardized fruit extract in cardio-respiratory improvement and antioxidant status of volunteers with smoking history. J. Herb. Med. 2014, 4, 188–194. [Google Scholar] [CrossRef]
- Usharani, P.; Merugu, P.L.; Nutalapati, C. Evaluation of the effects of a standardized aqueous extract of Phyllanthus emblica fruits on endothelial dysfunction, oxidative stress, systemic inflammation and lipid profile in subjects with metabolic syndrome: A randomised, double blind, placebo. BMC Complement. Altern. Med. 2019, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.D.; Padmavathi, P.; Paramahamsa, M.; Varadacharyulua, N.C. Amelioration of alcohol-induced oxidative stress by Emblica officinalis (Amla) in rats. Indian J. Biochem. Biophys. 2010, 47, 20–25. [Google Scholar] [PubMed]
- Variya, B.C.; Bakrania, A.K.; Chen, Y.; Han, J.; Patel, S.S. Suppression of abdominal fat and anti-hyperlipidemic potential of Emblica officinalis: Upregulation of PPARs and identification of active moiety. Biomed. Pharmacother. 2018, 108, 1274–1281. [Google Scholar] [CrossRef]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef]
- Yang, C.J.; Wang, C.S.; Hung, J.Y.; Huang, H.W.; Chia, Y.C.; Wang, P.H.; Weng, C.F.; Huang, M.S. Pyrogallol induces G2-M arrest in human lung cancer cells and inhibits tumor growth in an animal model. Lung Cancer 2009, 66, 162–168. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Bhatia, J.; Khan, S.I.; Vasisth, S.; Tomar, A.; Goyal, S.; Kumar, R.; Arya, D.S.; Ojha, S.K. Therapeutic potential and molecular mechanisms of Emblica officinalis gaertn in countering nephrotoxicity in rats induced by the chemotherapeutic agent cisplatin. Front. Pharmacol. 2016, 7, 350. [Google Scholar] [CrossRef] [Green Version]
- Golechha, M.; Sarangal, V.; Ojha, S.; Bhatia, J.; Arya, D.S. Anti-inflammatory effect of Emblica officinalis in rodent models of acute and chronic inflammation: Involvement of possible mechanisms. Int. J. Inflam. 2014, 2014, 178408. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, M.D.; Thenmozhi, A.J. Attenuation of Aluminum-Induced Neurotoxicity by Tannoid Principles of Emblica officinalis in Wistar Rats. Int. J. Nutr. Pharmacol. Neurol. Dis. 2018, 8, 35. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Selvaraju, V.; Tapias, L.; Sanchez, J.A.; Palesty, J.A.; Maulik, N. Protective effects of Phyllanthus emblica against myocardial ischemia-reperfusion injury: The role of PI3-kinase/glycogen synthase kinase 3β/β-catenin pathway. J. Physiol. Biochem. 2015, 71, 623–633. [Google Scholar] [CrossRef]
- Bhatia, J.; Tabassum, F.; Sharma, A.K.; Bharti, S.; Golechha, M.; Joshi, S.; Akhatar, M.S.; Srivastava, A.K.; Arya, D.S. Emblica officinalis exerts antihypertensive effect in a rat model of DOCA-salt-induced hypertension: Role of (p) eNOS, NO and Oxidative Stress. Cardiovasc. Toxicol. 2011, 11, 272–279. [Google Scholar] [CrossRef]
- Khanna, S.; Das, A.; Spieldenner, J.; Rink, C.; Roy, S. Supplementation of a standardized extract from Phyllanthus emblica improves cardiovascular risk factors and platelet aggregation in overweight/class-1 obese adults. J. Med. Food 2015, 18, 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenmozhi, A.J.; Dhivyabharathi, M.; Raja, T.R.W.; Manivasagam, T.; Essa, M.M. Tannoid principles of Emblica officinalis renovate cognitive deficits and attenuate amyloid pathologies against aluminum chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2016, 19, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Okubo, T.; Juneja, L.R.; Yokozawa, T. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. Br. J. Nutr. 2010, 103, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middha, S.K.; Goyal, A.K.; Lokesh, P.; Yardi, V.; Mojamdar, L.; Keni, D.S.; Babu, D.; Usha, T. Toxicological evaluation of Emblica officinalis fruit extract and its anti-inflammatory and free radical scavenging properties. Pharmacogn. Mag. 2015, 11, S427–S433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenmozhi, A.J.; Dhivyabharathi, M.; Manivasagam, T.; Essa, M.M. Tannoid principles of Emblica officinalis attenuated aluminum chloride induced apoptosis by suppressing oxidative stress and tau pathology via Akt/GSK-3βsignaling pathway. J. Ethnopharmacol. 2016, 194, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Akhtar, M.; Madaan, T.; Vohora, D.; Abdin, M.Z.; Islamuddin, M.; Najmi, A.K. Tannins enriched fraction of Emblica officinalis fruits alleviates high-salt and cholesterol diet-induced cognitive impairment in rats via Nrf2-ARE pathway. Front. Pharmacol. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, S.; Pandhi, P.; Malhotra, S.; Vaiphei, K.; Khanduja, K.L. Beneficial effects of Emblica officinalis in L-arginine-induced acute pancreatitis in rats. J. Med. Food 2011, 14, 147–155. [Google Scholar] [CrossRef]
- Kumar, N.P.; Annamalai, A.R.; Thakur, R.S. Antinociceptive property of Emblica officinalis Gaertn (Amla) in high fat diet fed/low dose streptozotocin induced diabetic neuropathy in rats. Indian J. Exp. Biol. 2009, 47, 737–742. [Google Scholar]
- Mehrotra, S.; Jamwal, R.; Shyam, R.; Meena, D.K.; Mishra, K.; Patra, R.; De, R.; Mukhopadhyay, A.; Kumar, A.; Nandi, S.P. Anti-Helicobacter pylori and antioxidant properties of Emblica officinalis pulp extract: A potential source for therapeutic use against gastric ulcer. J. Med. Plants Res. 2011, 5, 2577–2583. [Google Scholar]
- Al-Rehaily, A.J.; Al-Howiriny, T.S.; Al-Sohaibani, M.O.; Rafatullah, S. Gastroprotective effects of “Amla” Emblica officinalis on in vivo test models in rats. Phytomedicine 2002, 9, 515–522. [Google Scholar] [CrossRef]
- Huang, C.Z.; Tung, Y.T.; Hsia, S.M.; Wu, C.H.; Yen, G.C. The hepatoprotective effect of Phyllanthus emblica L. fruit on high fat diet-induced non-alcoholic fatty liver disease (NAFLD) in SD rats. Food Funct. 2017, 8, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Karkon Varnosfaderani, S.; Hashem-Dabaghian, F.; Amin, G.; Bozorgi, M.; Heydarirad, G.; Nazem, E.; Nasiri Toosi, M.; Mosavat, S.H. Efficacy and safety of amla (Phyllanthus emblica L.) in non-erosive reflux disease: A double-blind, randomized, placebo-controlled clinical trial. J. Integr. Med. 2018, 16, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Joshi, P.; Gupta, A.; Chhillar, R. Possible Involvement of Monoaminergic Neurotransmission in Antidepressant-like activity of Emblica officinalis Fruits in Mice. CNS Neurosci. Ther. 2012, 18, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kim, H.Y.; Kim, H.J.; Okubo, T.; Chu, D.C.; Juneja, L.R. Amla (Emblica officinalis Gaertn.) prevents dyslipidaemia and oxidative stress in the ageing process. Br. J. Nutr. 2007, 97, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.I.; Zhong, Z.G. Study of galic acid extracted from the leaves of Phyllanthus emblica on apoptotic mechanism of human hepatocellular carcinoma cells BEL-7404. J. Chin. Med. Mater. 2011, 34, 246–249. [Google Scholar]
- Goyal, M.R.; Suleria, H. Olive Oil Phenols: Chemistry, Synthesis, Metabolism, Fate, And Their Allied Health Claims. In Human Health Benefits of Plant Bioactive Compounds; Goyal, M.R., Suleria, H.A.R., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 95–127. ISBN 9780429457913. [Google Scholar]
- Madan, J.; Sindhu, S.; Gupta, M.; Poonia, J. Evaluation of Emblica officinalis and Mentha piperata supplementation on biochemical parameters in growing beetal kids. J. Cell Tissue Res. 2015, 15, 4811–4814. [Google Scholar]
- Kanthe, P.S.; Patil, B.S.; Bagali, S.C.; Reddy, C.R.; Aaithala, M.R.; Das, K.K. Protective effects of ethanolic extract of Emblica officinalis (amla) on cardiovascular pathophysiology of rats, fed with high fat diet. J. Clin. Diagn. Res. 2017, 11, CC05–CC09. [Google Scholar] [CrossRef]
- Gopa, B.; Bhatt, J.; Hemavathi, K.G. A comparative clinical study of hypolipidemic efficacy of Amla (Emblica officinalis) with 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitor simvastatin. Indian J. Pharmacol. 2012, 44, 238–242. [Google Scholar] [CrossRef]
- Koshy, S.M.; Bobby, Z.; Hariharan, A.P.; Gopalakrishna, S.M. Amla (Emblica officinalis) extract is effective in preventing high fructose diet-induced insulin resistance and atherogenic dyslipidemic profile in ovariectomized female albino rats. Menopause 2012, 19, 1146–1155. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef]
- Ansari, A.; Shahriar, M.S.Z.; Hassan, M.M.; Das, S.R.; Rokeya, B.; Haque, M.A.; Haque, M.E.; Biswas, N.; Sarkar, T. Emblica officinalis improves glycemic status and oxidative stress in STZ induced type 2 diabetic model rats. Asian Pac. J. Trop. Med. 2014, 7, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.S.; Goyal, R.K. Prevention of diabetes-induced myocardial dysfunction in rats using the juice of the Emblica officinalis fruit. Exp. Clin. Cardiol. 2011, 16, 87–91. [Google Scholar] [PubMed]
- Akhtar, M.S.; Ramzan, A.; Ali, A.; Ahmad, M. Effect of amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int. J. Food Sci. Nutr. 2011, 62, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.; Putnik, P.; Kovačević, D.B.; Muchenje, V.; Barba, F.J. Sources, Chemistry, and Biological Potential of Ellagitannins and Ellagic Acid Derivatives. Stud. Nat. Prod. Chem. 2018, 60, 189–221. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Zhang, W.; Dominguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Health benefits, extraction and development of functional foods with curcuminoids. J. Funct. Foods 2021, 79, 104392. [Google Scholar] [CrossRef]
- Rodríguez, M.L.; Estrela, J.M.; Ortega, Á.L. Natural Polyphenols and Apoptosis Induction in Cancer Therapy. J. Carcinog. Mutagen. 2013, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Soyal, D.; Goyal, P. Radioprotective potential of Emblica officinalis fruit extract against hematological alterations induced by gamma radiation. In Proceedings of the International Conference on Emerging Frontiers and Challenges in Radiation Biology, Bikaner, India, 24–25 January 2012. [Google Scholar]
- Zhu, X.; Wang, J.; Ou, Y.; Han, W.; Li, H. Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells. Eur. J. Med. Res. 2013, 18, 46. [Google Scholar] [CrossRef] [Green Version]
- Purena, R.; Seth, R.; Bhatt, R. Protective role of Emblica officinalis hydro-ethanolic leaf extract in cisplatin induced nephrotoxicity in Rats. Toxicol. Rep. 2018, 5, 270–277. [Google Scholar] [CrossRef]
- Singh, M.K.; Yadav, S.S.; Yadav, R.S.; Chauhan, A.; Katiyar, D.; Khattri, S. Protective effect of Emblica officinalis in arsenic induced biochemical alteration and inflammation in mice. SpringerPlus 2015, 4, 438. [Google Scholar] [CrossRef] [Green Version]
- Dang, G.K.; Parekar, R.R.; Kamat, S.K.; Scindia, A.M.; Rege, N.N. Antiinflammatory activity of Phyllanthus emblica, Plumbago zeylanica and Cyperus rotundus in acute models of inflammation. Phyther. Res. 2011, 25, 904–908. [Google Scholar] [CrossRef]
- Goel, B.; Pathak, N.; Nim, D.K.; Singh, S.K.; Dixit, R.K.; Chaurasia, R. Evaluation of analgesic activity of Emblica officinalis in albino rats. Int. J. Basic Clin. Pharmacol. 2014, 3, 365–368. [Google Scholar] [CrossRef]
- Deshmukh, C.D.; Bantal, V.; Pawar, A. Protective effect of Emblica officinalis fruit extract on acetic acid induced colitis in rats. J. Herb. Med. Toxicol. 2010, 4, 25–29. [Google Scholar]
- Uddin, M.S.; Mamun, A.A.; Hossain, M.S.; Akter, F.; Iqbal, M.A.; Asaduzzaman, M. Exploring the effect of Phyllanthus emblica L. on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: Promising natural gift for the mitigation of Alzheimer’s disease. Ann. Neurosci. 2016, 23, 218–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variety | Moisture | Carbohydrate | Fiber | Minerals | Protein | Fat | Vitamin C | Ref. |
|---|---|---|---|---|---|---|---|---|
| Local variety (no name) | 81 g/100 g | 14 g/100 g | 3.2 g/100 g | 0.3 g/100 g | 1 g/100 g | 0.5 g/100 g | 720 mg/100 g | [8] |
| Local variety (no name) | 82.8 g/100 g | 7.6 g/100 g | 5.1 g/100 g | 2.3 g/100 g | 2.0 g/100 g | 0.3 g/100 g | 573 mg/100 g | [9] |
| NA-7 NA-9 NA-10 Balwant Chakaiya Hathijhool | 84.9–87.5 g/100 g | 77.2–81.9 g/100 g DW | 11.7–16.0 g/100 g DW | 2.1–3.0 g/100 g DW | 3.0–4.5 g/100 g DW | 0.2–0.5 g/100 g DW | 489.9–585.0 mg/100 g | [10] |
| NA-7, Banarasi, Kanchan, Chakaiya and Desi | 81.3–84.6 g/100 g | 73.8–87.1 g/100 g DW | 7.2–22.4 g/100 g DW | 2.2 to 3.1 g/100 g DW | 2.0 to 3.2 g/100 g DW | 0.4–0.5 g/100 g DW | 193–315 mg/100 g | [11] |
| Krishna, Kanchan, NA-7, Chakaiya | 85.6–87.7 g/100 g | 70.7–73.8 g/100 g DW | 13.9–16.5 g/100 g DW | 2.3–2.8 g/100 g DW | 2.9–3.6 g/100 g DW | 0.5–0.6 g/100 g DW | 421–506 mg/100 g | [12] |
| Source | Type of Study | Study Characteristics | Main Outcomes | Ref. |
|---|---|---|---|---|
| Fruit | In vitro (cell) | PC12 cells; dosage (10–50 µM); and incubation (2 h) | No toxicity; ethyl gallate was the most efficient antioxidant (10–50 µM) | [32] |
| Fruit | In vitro (cell) | HepG2 cells; dosage (5, 10, 20, 50, and 100 μg/mL); and incubation (4, 8, 12, 16, 20, and 24 h) | No Cytotoxicity (up to 100 μg/mL); reduced lipid hydroperoxides reactive oxygen species levels (50 and 100 μg/mL after 8 h); and increased GSH, total antioxidant capacity, SOD, CAT, GPx, GSH reductase, and GSH S-transferase (50 and 100 μg/mL after 12–24 h) | [35] |
| Fruit | In vitro (cell) | RAW 264.7 cells; dosage (25, 50, or 100 μg/mL); and incubation (24 h) | No Cytotoxicity (100 μg/mL); increased GSH and SOD activity when challenges with H2O2 (50 and 100 μg/mL); and reduced MDA level (100 μg/mL) | [36] |
| Fruit | In vitro (cell) | C2C12 myoblasts; dosage (100 and 200 µg/mL); and incubation (48 h) | Increased cell survivability (200 µg/mL) and reduced ROS levels with increased oxygen consumption (200 µg/mL) | [37] |
| Leaves | Animal (mice) | Diabetic wistar mice; 100–400 mg/kg BW; oral administration; and 45 days | Induced GSH, GPx, SOD, and CAT activity (200–400 mg/kg BW) and reduced lipid peroxidation (200–400 mg/kg BW) | [38] |
| Fruit | Animal (mice thymus) | Balb/c male mice; 500 mg/kg BW; oral administration; and 28 days | Improved cell viability, GSH, CAT, and SOD levels and reduced lipid peroxidation, ROS level | [39] |
| Fruit | Animal (mice liver) | Wistar mice; 5000 mg/kg BW; oral administration; and 24 days | Reduce lipid peroxidation; preserved CD, CAT, and NPSH; and ameliorated SOD reduction | [40] |
| Fruits | Animal (mice kidney) | Healthy wistar mice; dosage (50, 100, 150, 200, and 250 µg/mL); single application | Increased SOD and CAT (50–250 µg/mL); reduced lipid peroxidation (50–250 µg/mL); and no effect in GSH | [41] |
| Commercial supplement | Clinical trial | Male smoker subjects (20–60 y); randomized, double-blind placebo-controlled design; 250 mg (twice a day); and 60 days | Increased antioxidant status (FRAP assay) and reduced lipid peroxidation level | [42] |
| Commercial supplement | Clinical trial | Female and male subjects with metabolic syndrome (30–68 y); randomized, double-blind, and placebo-controlled; 250 and 500 mg per capsule (twice a day); and 12 weeks | Increased GSH level and reduced lipid peroxidation level | [43] |
| Commercial supplement | Clinical trial | Female and male healthy subjects (36–67 y); randomized, double-blind, placebo-controlled, and crossover; 125 mg per capsule (4 capsules/day) | A non-significant reduction in lipid peroxidation level | [6] |
| Source | Main Active Compounds | Biological Effect | Ref. |
|---|---|---|---|
| Fruit | Gallic acid | Cardioprotective activity | [45] |
| Fruit | Ellagic acid | Antidiabetic activity | [46] |
| Fruit | Pyrogallol | Anticancer activity | [47] |
| Fruit | Emblicanin A and B | Anticancer activity | [48] |
| Fruit | Emblicanin A and B | Anti-inflammatory activity | [49] |
| Fruit | Emblicanin A and B | Neuroprotective activity | [50] |
| Fruit | Myricetin, gallic acid, and kaempferol | Cardioprotective activity | [19] |
| Fruit | Gallic acid, corilagin, and ellagic acid | Anti-inflammatory activity | [36] |
| Fruit | Emblicanin A and B, punigluconin, and pedunculagin | Cardioprotective activity | [51,52,53] |
| Fruit | Emblicanin A and B, punigluconin, and pedunculagin | Anti-inflammatory activity | [24] |
| Fruit | Emblicanin A and B, punigluconin and pedunculagin | Neuroprotective activity | [54] |
| Fruit | Gallic acid, chebulagic acid, geraniin, ellagic acid, and corilagin | Cardioprotective activity | [55] |
| Fruit | Quercetin, rutin, gallic acid, mucic acid, and beta-glucogallin | Anti-inflammatory activity | [56] |
| Fruit | Emblicanin A and B, punigluconin, pedunculagin, rutin, and gallic acid | Neuroprotective activity | [57,58] |
| Fruit | Tannins and gallic acid | Gastrointestinal protective activity | [59] |
| Fruit | Flavonoids | Antidiabetic activity | [60] |
| Fruit | Polyphenols | Gastrointestinal protective activity | [61,62,63,64] |
| Fruit | Polyphenols | Neuroprotective activity | [65] |
| Fruit | Polyphenols | Cardioprotective activity | [66] |
| Leaves | Gallic acid | Anticancer activity | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, M.; Liu, Z.-W.; Iahtisham-Ul-Haq; Rabail, R.; Faheem, F.; Walayat, N.; Nawaz, A.; Shabbir, M.A.; Munekata, P.E.S.; Lorenzo, J.M.; et al. Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review. Antioxidants 2022, 11, 816. https://doi.org/10.3390/antiox11050816
Gul M, Liu Z-W, Iahtisham-Ul-Haq, Rabail R, Faheem F, Walayat N, Nawaz A, Shabbir MA, Munekata PES, Lorenzo JM, et al. Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review. Antioxidants. 2022; 11(5):816. https://doi.org/10.3390/antiox11050816
Chicago/Turabian StyleGul, Maryam, Zhi-Wei Liu, Iahtisham-Ul-Haq, Roshina Rabail, Fatima Faheem, Noman Walayat, Asad Nawaz, Muhammad Asim Shabbir, Paulo E. S. Munekata, José M. Lorenzo, and et al. 2022. "Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review" Antioxidants 11, no. 5: 816. https://doi.org/10.3390/antiox11050816
APA StyleGul, M., Liu, Z.-W., Iahtisham-Ul-Haq, Rabail, R., Faheem, F., Walayat, N., Nawaz, A., Shabbir, M. A., Munekata, P. E. S., Lorenzo, J. M., & Aadil, R. M. (2022). Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review. Antioxidants, 11(5), 816. https://doi.org/10.3390/antiox11050816