Urinary Oxidative Damage Markers and Their Association with Obesity-Related Metabolic Risk Factors
Abstract
:1. Introduction
2. Methods
2.1. Anthropometric and Clinical Measurements
2.2. Biochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Oxidative Damage Marker Levels at Baseline and Follow-Up
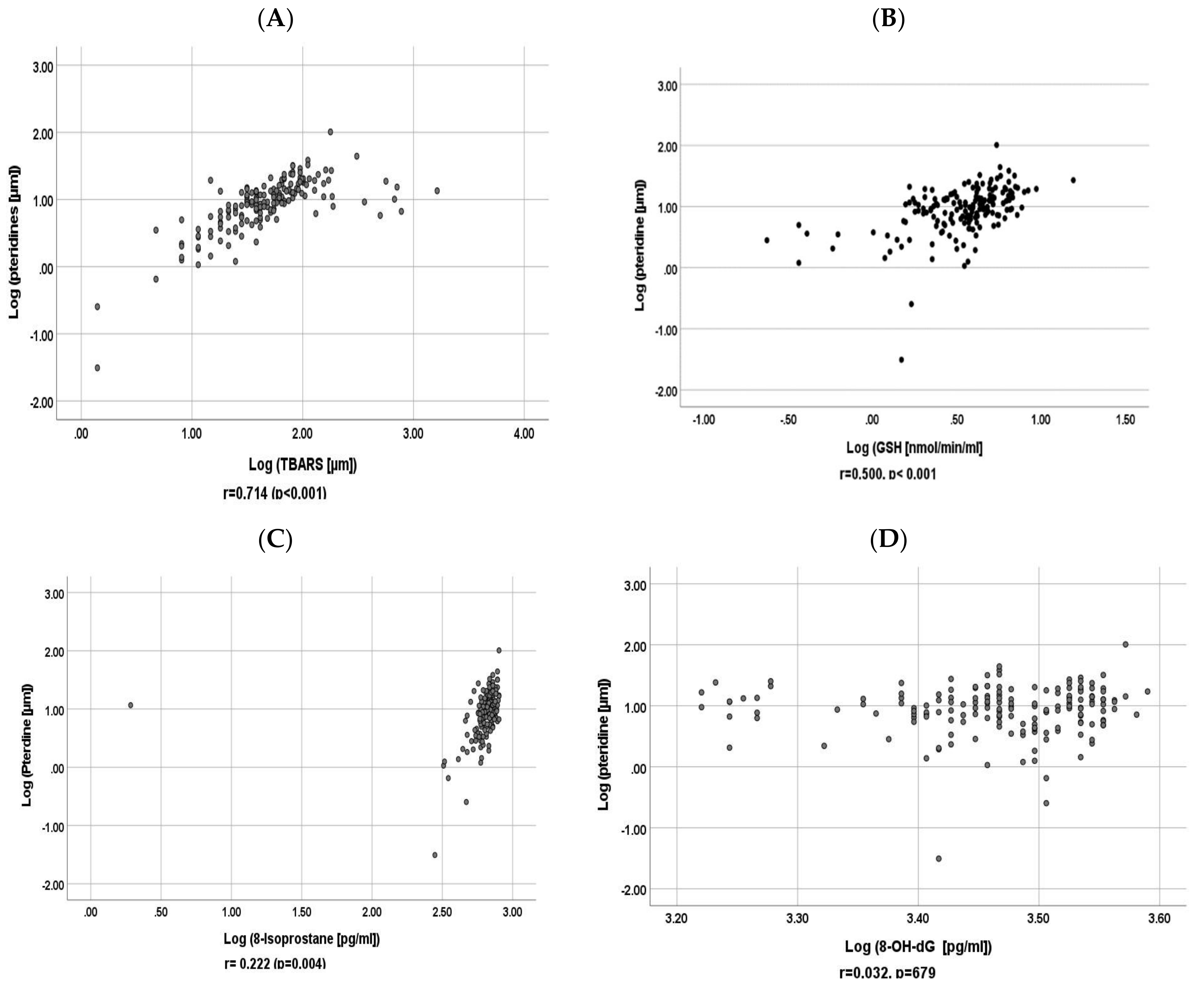
3.3. Correlation between Baseline Urinary Pteridine and Baseline Urinary GSH, 8-Isoprostane, 8-OH-dG and TBARS
3.4. Correlation between Cardiovascular Disease Risk Factors and Oxidative Damage Markers
3.5. Differences in Oxidant Damage Markers between Male and Female, Diabetic, and Hypertensive Subjects, and Healthy Subjects
3.6. Relationship between Smoking, BMI, and Physical Activity, and Oxidant Damage Markers
3.7. Multivariate Analysis of Urinary Markers Most Correlated with Clinical and Metabolic Risk Factors, and Other Prognostic Clinical Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKinsey Global Institute. Overcoming Obesity: An Initial Economic Analysis. Available online: https://www.mckinsey.com/~/media/mckinsey/business%20functions/economic%20studies%20temp/our%20insights/how%20the%20world%20could%20better%20fight%20obesity/mgi_overcoming_obesity_full_report.ashx (accessed on 5 April 2022).
- McLellan, F. Obesity rising to alarming levels around the world. Lancet 2002, 359, 1412. [Google Scholar] [CrossRef]
- Malik, A.; Babir, A.; Abi Saab, B.; Roglic, G.; King, H. Glucose intolerance and associated factors in the UAE. Diabetes Res. Clin. Pract. 2005, 69, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Middle East and North Africa Diabetes Report. IDF Diabetes Atlas, 10th ed.; 2021. Available online: https://www.diabetesatlas.org/data/en/region/4/mena.html (accessed on 5 April 2022).
- Evans, J.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress activated Signalling pathways: A unifying hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.F. Emerging role for antioxidant therapy in protection against diabetic cardiac complications: Experimental and clinical evidence for utilization of classic and new antioxidants. Curr. Cardiligy Rev. 2008, 4, 259–268. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef]
- Gariballa, S.; Kosanovic, M.; Yasin, J.; Essa, A.E. Oxidative damage and inflammation in obese diabetic emirati subjects. Nutrients 2014, 6, 4872–4880. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.; Hemmendinger, M.; Canu, I.G.; Hopf, N.B. Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Cejvanovic, V.; Asferg, C.; Kjær, L.K.; Andersen, U.B.; Linneberg, A.; Frystyk, J.; Henriksen, T.; Flyvbjerg, A.; Christiansen, M.; Weimann, A.; et al. Markers of oxidative stress in obese men with and without hypertension. Scand. J. Clin. Lab. Investig. 2016, 76, 620–625. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Nakanishi, M.; Ohki, M.; Suehiro, A.; Uchida, K. A simple and useful method for evaluation of oxidative stress in vivo by spectrofluorometric estimation of urinary pteridines. Sci. Rep. 2020, 10, 11223. [Google Scholar] [CrossRef]
- Murr, C.; Fuith, L.C.; Widner, B.; Wirleitner, B.; Baier-Bitterlich, G.; Fuchs, D. Increased neopterin concentrations in patients with cancer: Indicator of oxidative stress? Anticancer Res. 1999, 19, 1721–1728. [Google Scholar]
- Goutzourelas, N.; Orfanou, M.; Charizanis, I.; Leon, G.; Spandidos, D.A.; Kouretas, D. GSH levels affect weight loss in individuals with metabolic syndrome and obesity following dietary therapy. Exp. Ther. Med. 2018, 16, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Oxidative damage and associated inflammatory risk factors in obese Emirati women: Body mass index versus waist circumference. Saudi Med. J. 2017, 38, 960–964. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; John, A.; Raza, H.; Ali, B.H. Cardiovascular effects of nose-only water-pipe smoking exposure in mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H740–H746. [Google Scholar] [CrossRef]
- Gariballa, S.; Afandi, B.; Abuhaltem, M.; Yassin, J.; Habib, H.; Ibrahim, W. Oxidative damage and inflammation in obese diabetic Emirati subjects supplemented with antioxidants and B-vitamins. Nutr. Metab. 2013, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef] [Green Version]
- John, J.H.; Ziebland, S.; Yudkin, P.; Roe, L.S.; Neil, H.A.W. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet 2002, 359, 1969–1974. [Google Scholar] [CrossRef]
- Pereira, M.A.; Ludwig, D.S. Dietary fiber and body-weight regulation: Observations and mechanisms. Pediatr. Clin. N. Am. 2001, 48, 969–980. [Google Scholar] [CrossRef]
- Du, H.; van der A Daphne, L.; Boshuizen, H.C.; Forouhi, N.G.; Wareham, N.J.; Halkjær, J.; Tjønneland, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H.; et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am. J. Clin. Nutr. 2010, 91, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Global Burden of Diseases, Injuries, and Risk Factors Study. 2013. Available online: http://www.thelancet.com/themed/global-burden-of-disease (accessed on 5 April 2022).
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Capewell, S. United Nations’ dietary policies to prevent cardiovascular disease. BMJ 2011, 343, d5747. [Google Scholar] [CrossRef] [Green Version]
- Loft, S.; Vistisen, K.; Ewertz, M.; Tjønneland, A.; Overvad, K.; Poulsen, H.E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: Infuence of smoking, gender and body mass index. Carcinogenesis 1992, 13, 2241–2247. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Urinary biomarkers of oxidative/nitrosative stress in healthy smokers. Inhal. Toxicol. 2011, 23, 148–156. [Google Scholar] [CrossRef]
- Cross, C.E.; Traber, M.; Eiserich, J.; Van Der Vilet, A. Micronutrient antioxidants and smoking. Br. Med. Bull. 1999, 55, 691–704. [Google Scholar] [CrossRef] [Green Version]
- Reilly, M.; Delanty, N.; Lawson, J.A.; Fitzgerald, G.A. Modulation od oxidant stressing in vivo chronic cigarette smokers. Circulation 1996, 94, 19–25. [Google Scholar] [CrossRef]
- Hamling, J.S.; Coombs, K.J.; Lee, P.N. Misclassification of smoking habits: An updated review of the literature. World. J. Meta-Anal 2019, 7, 31–50. [Google Scholar] [CrossRef]
- Il’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative status. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef] [Green Version]


| Variables | Baseline (n = 168) | Follow Up (n = 168) | |
|---|---|---|---|
| Age (years) | 41 (12) | ||
| Sex, female n (%) | 119 (71) | ||
| Smoking n (%) | |||
| Current | 19 (11) | ||
| Ex-smoker | 8 (5.0) | ||
| Never smoked | 141 (84) | ||
| Body mass index (BMI) n (%) * | |||
| Normal weight (BMI 18.5–25) | 40 (24) | 38 (23) | |
| Overweight (BMI 25.1–29.9) | 54 (32) | 53 (32) | |
| Obese (BMI ≥ 30) | 74 (44) | 73 (45) | |
| Physical activity n (%) | |||
| Not active | 31 (18) | 20 (12) | |
| Moderately active | 104 (62) | 115 (69) | |
| Very active | 33 (20) | 32 (19) | |
| Diabetes n (%) | 33 (20) | ||
| Hypertension n (%) | 27 (16) | ||
| Systolic blood pressure (SBP; mm Hg) | 123 (13) | 121 (10) | |
| Diastolic blood pressure (DBP; mm Hg) | 76 (8.0) | 78 (8) | |
| Hs CRP (mg/L) | 3.5 (3.0) | 3.8 (4) | |
| HBA1c (%) | 5.8 (0.9) | 5.7 (1) | |
| Total cholesterol (mmol/L) | 4.9 (0.9) | ||
| Urea (mmol/L) | 4.1 (1.5) |
| 2A | |||||||
| Model | Unstandardized Coefficients | Standardized Coefficients | p-Value | 95.0% Confidence Interval for B | |||
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| 1 | (Constant) | −5.715 | 1.401 | <0.001 | −8.497 | −2.933 | |
| GSH | 0.223 | 0.095 | 0.201 | 0.021 | 0.035 | 0.412 | |
| 8-Isoprostane | 1.789 | 0.495 | 0.316 | <0.001 | 0.807 | 2.772 | |
| 8-OH-dG | 0.259 | 0.294 | 0.061 | 0.381 | −0.325 | 0.842 | |
| TBARS | 0.350 | 0.076 | 0.396 | <0.001 | 0.199 | 0.502 | |
| Urine creatinine | 0.001 | 0.006 | 0.012 | 0.881 | −0.012 | 0.014 | |
| 2B | |||||||
| Model | Unstandardized Coefficients | Standardized Coefficients | p-Value | 95.0% Confidence Interval for B | |||
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| (Constant) | −5.861 | 1.199 | <0.001 | −8.241 | −3.481 | ||
| GSH | 0.239 | 0.105 | 0.167 | 0.025 | 0.031 | 0.447 | |
| 8-Isoprostane | 2.471 | 0.416 | 0.482 | <0.001 | 1.645 | 3.296 | |
| 8-OH-dG | −0.262 | 0.258 | −0.067 | 0.312 | −0.774 | 0.250 | |
| TBARS | 0.357 | 0.074 | 0.381 | <0.001 | 0.211 | 0.503 | |
| Urine creatinine | 0.001 | 0.007 | 0.006 | 0.939 | −0.014 | 0.015 | |
| GSH (nmol/mL) | TBARS (µmL) | 8-Isoprostane (pg/mL) | Pteridine (µmL) | 8-OH-dG (pg/mL) | |
|---|---|---|---|---|---|
| SBP (mm Hg) | r = −0.296 (p = 0.015) | r = 0.337 (p < 0.001) | r = −0.550 (p = 0.513) | r = −0.047 (p = 0.596) | r = −0.104 (p = 0.238) |
| HbA1c (%) | r = −0.144 (p = 0.062) | r = 0.550 (p < 0.001) | r = 0.100 (p = 0.198) | r = −0.030 (p = 0.697) | r = −0.176 (p = 0.022) |
| Glucose (mmol/L) | r = −0.062 (p = 0.427) | r = 0.603 (p < 0.001) | r = 0.059 (p = 0.447) | r = 0.073 (p = 0.347) | r = −0.254 (p = 0.001) |
| C-reactive proteins (mg/L) | r = 0.139 (p = 0.072) | r = 0.094 (p = 0.224) | r = 0.038 (p = 0.662) | r = 0.178 (p = 0.021) | r = 0.147 (p = 0.056) |
| Total Cholesterol (mmol/L) | r = 0.004 (p = 0.963) | r = −0.083 (p = 0.286) | r = −0.032 (p = 0.676) | r = 0.029 (p = 0.705) | r = −0.110 (p = 0.153) |
| High densitylipoprotein (mmol/L) | r = −0.047 (p = 0.545) | r = −0.152 (p = 0.049) | r = 0.007 (p = 0.924) | r- = −0.101 (p = 0.192) | r = 0.077 (p = 0.317) |
| GSH (nmol/mL) | TBARS (µmL) | 8-Isdoprostane (pg/mL) | Pteridine (µmL) | 8-OH-dG (pg/mL) | |
|---|---|---|---|---|---|
| Body weight (kg) | r = −0.015 (p = 0.844) | r = 0.172 (p = 0.028) | r = 0.016 (p = 0.834) | r = 0.019 (p = 0.209) | r = −0.030 (p = 0.699) |
| SBP (mm Hg) | r = −0.103 (p = 0.398) | r = 0.209 (p = 0.089) | r = −0.005 (p = 0.966) | r = −0.003 (p = 0.979) | r = −0.054 (p = 0.652) |
| HBA1c (%) | r = −0.019 (p = 0.811) | r = 0.036 (p = 0.655) | r = 0.166 (p = 0.139) | r = 0.166 (p = 0.139) | r = −0.043 (p = 0.586) |
| GSH (nmol/mL) | TBARS (µmL) | 8-Isoprostane (pg/mL) | Pteridine (µmL) | 8-OH-dG (pg/mL) | ||
|---|---|---|---|---|---|---|
| Gender | Male | 3.4 (1.6) | 66 (3.3) | 670 (1.2) | 8.5 (2.7) | 2754 (1.2) |
| Female | 3.2 (2.0) | 40 (2.6) * | 603 (1.8) | 8.5 (2.5) | 3019 (1.4) * | |
| Smoking | Current | 3.7 (1.5) | 70 (3.4) | 657 (1.2) | 10.6 (2.1) | 2842 (1.2) |
| Ex-smoker | 3.24 (1.8) | 38 (3.2) | 650 (1.1) | 6.9 (2.0) | 2977 (1.2) | |
| Never smoked | 3.23 (1.9) | 44 (2.8) | 615 (1.7) | 8.3 (2.7) | 2975 (1.4) | |
| Body mass index | Normal weight | 3.4 (1.8) | 42 (3.0) | 566 (3.0) | 7.7 (3.0) | 3013 (2.0) |
| Overweight | 3.8 (1.8) | 54 (3.0) | 630 (2.0) | 9.3 (2.0) | 2791 (2.0) | |
| Obese | 3.2 (1.9) | 45 (2.8) | 649 (2.0) | 8.5 (2.6) | 3061 (1.5) | |
| Diabetes + | No | 3.4 (1.9) | 41.5 (1.1) | 614 (1.7) | 8.6 (2.7) | 3019 (1.4) |
| Yes | 2.9 (2.0) | 74 (3.7) * | 655 (1.1) | 8.1 (2.0) | 2708 (1.2) * | |
| Hypertension + | No | 3.4 (1.9) | 46 (3.0) | 615 (2.0) | 8.7 (2.7) | 3083 (2) |
| Yes | 2.8 (1.6) | 47 (2.3) | 650 (1) | 7.6 (2) | 2616 (1.3) * |
| Mod | Unstandardized Coefficients | Standardized Coefficients | p Value | 95.0% Confidence Interval for B | |||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| 1 | Age (years) | −0.588 | 1.327 | −0.041 | 0.659 | −3.209 | 2.034 |
| Gender (male/female) | −66.231 | 31.474 | −0.180 | 0.037 | −128.418 | −4.044 | |
| Physical activity (very active/moderately active/inactive) | −5.577 | 8.297 | −0.049 | 0.502 | −21.970 | 10.816 | |
| Smoking (current/ex/never) | −33.208 | 20.294 | −0.131 | 0.104 | −73.304 | 6.888 | |
| Diabetes (yes/no) | 165.406 | 34.343 | 0.392 | <0.001 | 97.550 | 233.261 | |
| Hypertension (yes/no) | −85.086 | 38.339 | −0.189 | 0.028 | −160.836 | −9.336 | |
| Body mass index | 0.937 | 2.532 | 0.029 | 0.712 | −4.066 | 5.940 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gariballa, S.; Nemmar, A.; Elzaki, O.; Zaaba, N.E.; Yasin, J. Urinary Oxidative Damage Markers and Their Association with Obesity-Related Metabolic Risk Factors. Antioxidants 2022, 11, 844. https://doi.org/10.3390/antiox11050844
Gariballa S, Nemmar A, Elzaki O, Zaaba NE, Yasin J. Urinary Oxidative Damage Markers and Their Association with Obesity-Related Metabolic Risk Factors. Antioxidants. 2022; 11(5):844. https://doi.org/10.3390/antiox11050844
Chicago/Turabian StyleGariballa, Salah, Abderrahim Nemmar, Ozaz Elzaki, Nur Elena Zaaba, and Javed Yasin. 2022. "Urinary Oxidative Damage Markers and Their Association with Obesity-Related Metabolic Risk Factors" Antioxidants 11, no. 5: 844. https://doi.org/10.3390/antiox11050844








