Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases
Abstract
:1. Introduction
2. Proteins Involved in Carnosine Metabolism and Transport
3. Mechanism of Action of Carnosine
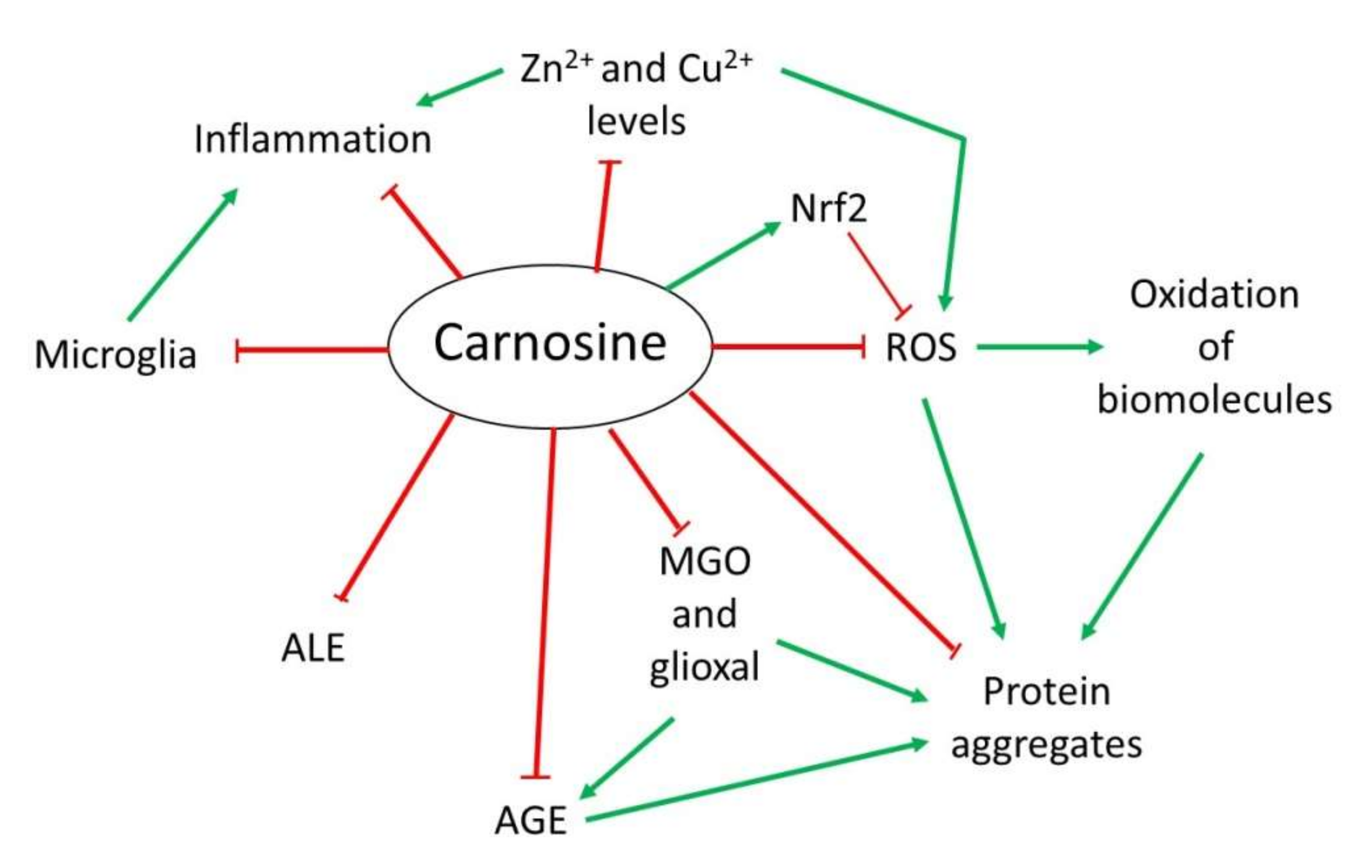
3.1. Antioxidant Activity
3.2. Anti-Glycating and Anti-Aggregant Activity
3.3. Anti-Inflammatory and Metal Ion Chelator Activity
4. Alterations of Carnosine Homeostasis in Neurodegenerative Diseases
4.1. Alzheimer’s Disease
4.2. Parkinson’s Disease
5. Therapeutic Potential of Carnosine in Neurodegenerative Diseases
5.1. Alzheimer’s Disease
5.2. Parkinson’s Disease
5.3. Aging-Related Neurodegeneration
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations:
| Aβ | Amyloid-beta |
| AD | Alzheimer’s disease |
| AGEs | Advanced glycation end-products |
| AKT | Protein kinase B |
| Ala | Alanine |
| ALEs | Advanced lipoxidation end-products |
| ARE | Antioxidant response element |
| ATP | Adenosine 5′-triphosphate |
| BBB | Blood-brain-barrier |
| BDNF | Brain-derived neurotrophic factor |
| CARNS1 | Carnosine synthase 1 |
| CARNMT | Carnosine N-methyltransferase |
| CN | Carnosinase |
| GPX | Glutathione peroxidase |
| CNDP | Cytosolic nonspecific dipeptidase |
| GSH | Glutathione |
| HCD | His-containing dipeptides |
| His | Histidine |
| HOCl | Hypochlorous acid |
| JNK | Jun N-terminal kinase |
| MD | Mixed dementia |
| MGO | Methylglyoxal |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| ND | Neurodegenerative disease |
| NMDA | N-methyl-D-aspartate |
| Nrf2 | Nuclear factor erytroid 2-related factor 2 |
| OS | Oxidative stress |
| pAD | Probable AD |
| PD | Parkinson’s disease |
| PEPT | Peptide transporter |
| PHT | Peptide/histidine transporter |
| PI3K | Phosphatidylinositol 3-kinase |
| POT | Proton-coupled oligopeptide transporter |
| ROS | Reactive oxygen species |
| SOD1 | Superoxide dismutase 1 |
| SLC | Solute carrier |
| SNpc | Substantia nigra pars compacta |
| 6-OHDA | 6-hydroxydopamine |
References
- Solana-Manrique, C.; Moltó, M.D.; Calap-Quintana, P.; Sanz, F.J.; Llorens, J.V.; Paricio, N. Drosophila as a model system for the identification of pharmacological therapies in neurodegenerative diseases. In Insights into Human Neurodegeneration: Lessons Learnt from Drosophila; Mutsuddi, M., Mukrherjee, A., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 433–467. [Google Scholar]
- Di Paolo, M.; Papi, L.; Gori, F.; Turillazzi, E. Natural Products in Neurodegenerative Diseases: A Great Promise but an ethical challenge. Int. J. Mol. Sci. 2019, 20, 5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsteins, G.; Hakosalo, V.; Jaronen, M.; Keuters, M.H.; Lehtonen, Š.; Koistinaho, J. CNS redox homeostasis and dysfunction in neurodegenerative diseases. Antioxidants 2022, 11, 405. [Google Scholar] [CrossRef]
- Durães, F.; Pinto, M.; Sousa, E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulewitsch, W.; Amiradžibi, S. Ueber das Carnosin, eine neue organische Base des Fleischextractes. Berichte Dtsch. Chem. Gesellschaft 1900, 33, 1902–1903. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Poddar, M.K. Carnosine research in relation to aging brain and neurodegeneration: A blessing for geriatrics and their neuronal disorders. Arch. Gerontol. Geriatr. 2020, 91, 104239. [Google Scholar] [CrossRef] [PubMed]
- Berezhnoy, D.S.; Stvolinsky, S.L.; Lopachev, A.V.; Devyatov, A.A.; Lopacheva, O.M.; Kulikova, O.I.; Abaimov, D.A.; Fedorova, T.N. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids 2019, 51, 139–150. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, their substrates and diseases. Molecules 2014, 19, 2299–2329. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; de Courten, B. The potential of carnosine in brain-related disorders: A comprehensive review of current evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, M.E.; Brosnan, J.T. Histidine metabolism and function. J. Nutr. 2020, 150, 2570S–2575S. [Google Scholar] [CrossRef]
- Solis, M.Y.; Cooper, S.; Hobson, R.M.; Artioli, G.G.; Otaduy, M.C.; Roschel, H.; Robertson, J.; Martin, D.; S Painelli, V.; Harris, R.C.; et al. Effects of beta-alanine supplementation on brain homocarnosine/carnosine signal and cognitive function: An exploratory study. PLoS ONE 2015, 10, e0123857. [Google Scholar] [CrossRef] [Green Version]
- Aldini, G.; de Courten, B.; Regazzoni, L.; Gilardoni, E.; Ferrario, G.; Baron, G.; Altomare, A.; D’Amato, A.; Vistoli, G.; Carini, M. Understanding the antioxidant and carbonyl sequestering activity of carnosine: Direct and indirect mechanisms. Free Radic. Res. 2020, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, K.; Dzierzbicka, K.; Inkielewicz-Stępniak, I.; Przybyłowska, M. Therapeutic potential of carnosine and its derivatives in the treatment of human diseases. Chem. Res. Toxicol. 2020, 33, 1561–1578. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of pro-oxidant and pro-inflammatory activities of M1 macrophages by the natural dipeptide carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaas, J.; Franssen, W.M.A.; Keytsman, C.; Blancquaert, L.; Vanmierlo, T.; Bogie, J.; Broux, B.; Hellings, N.; van Horssen, J.; Posa, D.K.; et al. Carnosine quenches the reactive carbonyl acrolein in the central nervous system and attenuates autoimmune neuroinflammation. J. Neuroinflammation 2021, 18, 255. [Google Scholar] [CrossRef]
- Caruso, G.; Benatti, C.; Musso, N.; Fresta, C.G.; Fidilio, A.; Spampinato, G.; Brunello, N.; Bucolo, C.; Drago, F.; Lunte, S.M.; et al. Carnosine protects macrophages against the toxicity of Aβ1-42 oligomers by decreasing oxidative stress. Biomedicines 2021, 9, 477. [Google Scholar] [CrossRef]
- Mahootchi, E.; Cannon Homaei, S.; Kleppe, R.; Winge, I.; Hegvik, T.-A.; Megias-Perez, R.; Totland, C.; Mogavero, F.; Baumann, A.; Glennon, J.C.; et al. GADL1 is a multifunctional decarboxylase with tissue-specific roles in β-alanine and carnosine production. Sci. Adv. 2020, 6, eabb3713. [Google Scholar] [CrossRef]
- Zhao, J.; Conklin, D.J.; Guo, Y.; Zhang, X.; Obal, D.; Guo, L.; Jagatheesan, G.; Katragadda, K.; He, L.; Yin, X.; et al. Cardiospecific overexpression of ATPGD1 (carnosine synthase) increases histidine dipeptide levels and prevents myocardial ischemia reperfusion injury. J. Am. Heart Assoc. 2020, 9, e015222. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Yee, S.W.; Buitrago, D.; Stecula, A.; Ngo, H.X.; Chien, H.-C.; Zou, L.; Koleske, M.L.; Giacomini, K.M. Deorphaning a solute carrier 22 family member, SLC22A15, through functional genomic studies. FASEB J. 2020, 34, 15734–15752. [Google Scholar] [CrossRef]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Drozak, J.; Piecuch, M.; Poleszak, O.; Kozlowski, P.; Chrobok, L.; Baelde, H.J.; de Heer, E. UPF0586 Protein C9orf41 Homolog Is Anserine-producing Methyltransferase. J. Biol. Chem. 2015, 290, 17190–17205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppermann, H.; Heinrich, M.; Birkemeyer, C.; Meixensberger, J.; Gaunitz, F. The proton-coupled oligopeptide transporters PEPT2, PHT1 and PHT2 mediate the uptake of carnosine in glioblastoma cells. Amino Acids 2019, 51, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Lopachev, A.V.; Abaimov, D.A.; Filimonov, I.S.; Kulichenkova, K.N.; Fedorova, T.N. An assessment of the transport mechanism and intraneuronal stability of L-carnosine. Amino Acids 2021. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Uhlén, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Licker, V.; Côte, M.; Lobrinus, J.A.; Rodrigo, N.; Kövari, E.; Hochstrasser, D.F.; Turck, N.; Sanchez, J.-C.; Burkhard, P.R. Proteomic profiling of the substantia nigra demonstrates CNDP2 overexpression in Parkinson’s disease. J. Proteom. 2012, 75, 4656–4667. [Google Scholar] [CrossRef]
- Turner, M.D.; Sale, C.; Garner, A.C.; Hipkiss, A.R. Anti-cancer actions of carnosine and the restoration of normal cellular homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119117. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihara, H.; Kakihana, Y.; Yamakage, A.; Kai, K.; Shibata, T.; Nishida, M.; Yamada, K.-I.; Uchida, K. 2-Oxo-histidine-containing dipeptides are functional oxidation products. J. Biol. Chem. 2019, 294, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Carroll, L.; Karton, A.; Radom, L.; Davies, M.J.; Pattison, D.I. Carnosine and carcinine derivatives rapidly react with hypochlorous acid to form chloramines and dichloramines. Chem. Res. Toxicol. 2019, 32, 513–525. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: Implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry 2006, 45, 8152–8162. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Wang, Z.; Han, N.; Wang, Y. Carnosine protects mouse podocytes from high glucose induced apoptosis through PI3K/AKT and Nrf2 pathways. BioMed Res. Int. 2019, 2019, 4348973. [Google Scholar] [CrossRef]
- Alsheblak, M.M.; Elsherbiny, N.M.; El-Karef, A.; El-Shishtawy, M.M. Protective effects of L-carnosine on CCl4 -induced hepatic injury in rats. Eur. Cytokine Netw. 2016, 27, 6–15. [Google Scholar] [CrossRef]
- Ahshin-Majd, S.; Zamani, S.; Kiamari, T.; Kiasalari, Z.; Baluchnejadmojarad, T.; Roghani, M. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: Possible involved mechanisms. Peptides 2016, 86, 102–111. [Google Scholar] [CrossRef]
- Mou, Y.; Wen, S.; Li, Y.-X.; Gao, X.-X.; Zhang, X.; Jiang, Z.-Y. Recent progress in Keap1-Nrf2 protein-protein interaction inhibitors. Eur. J. Med. Chem. 2020, 202, 112532. [Google Scholar] [CrossRef]
- Hecker, M.; Wagner, A.H. Role of protein carbonylation in diabetes. J. Inherit. Metab. Dis. 2018, 41, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, R.; Kheirouri, S. Carnosine and advanced glycation end products: A systematic review. Amino Acids 2018, 50, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. Glycotoxins: Dietary and metabolic origins; possible amelioration of neurotoxicity by carnosine, with special reference to Parkinson’s disease. Neurotox. Res. 2018, 34, 164–172. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Kalaz, E.B.; Aydın, A.F.; Soluk-Tekkeşin, M.; Doğru-Abbasoğlu, S.; Uysal, M.; Koçak-Toker, N. The effect of carnosine on methylglyoxal-induced oxidative stress in rats. Arch. Physiol. Biochem. 2017, 123, 192–198. [Google Scholar] [CrossRef]
- Hobart, L.J.; Seibel, I.; Yeargans, G.S.; Seidler, N.W. Anti-crosslinking properties of carnosine: Significance of histidine. Life Sci. 2004, 75, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Sanz, F.J.; Solana-Manrique, C.; Torres, J.; Masiá, E.; Vicent, M.J.; Paricio, N. A high-throughput chemical screen in DJ-1β mutant flies identifies Zaprinast as a potential Parkinson’s disease treatment. Neurotherapeutics 2021, 18, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Son, D.O.; Satsu, H.; Kiso, Y.; Totsuka, M.; Shimizu, M. Inhibitory effect of carnosine on interleukin-8 production in intestinal epithelial cells through translational regulation. Cytokine 2008, 42, 265–276. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. A review of zinc-L-carnosine and its Positive effects on oral mucositis, taste disorders, and gastrointestinal disorders. Nutrients 2020, 12, 665. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, M.; Tanaka, K.-I.; Kato-Negishi, M. Zinc, carnosine, and neurodegenerative diseases. Nutrients 2018, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Odashima, M.; Otaka, M.; Jin, M.; Wada, I.; Horikawa, Y.; Matsuhashi, T.; Ohba, R.; Hatakeyama, N.; Oyake, J.; Watanabe, S. Zinc L-carnosine protects colonic mucosal injury through induction of heat shock protein 72 and suppression of NF-κB activation. Life Sci. 2006, 79, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Almeida Rezende, M.S.; Dantas, S.H.; de Lima Silva, S.; de Oliveira, J.C.P.L.; de Lourdes Assunção Araújo de Azevedo, F.; Alves, R.M.F.R.; de Menezes, G.M.S.; Dos Santos, P.F.; Gonçalves, T.A.F.; et al. Unveiling the role of inflammation and oxidative stress on age-related cardiovascular diseases. Oxid. Med. Cell. Longev. 2020, 2020, 1954398. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Calabrese, V.; Guarino, F.; Cavallaro, M.; Cornelius, C.; De Pinto, V.; Rizzarelli, E. Carnosinase levels in aging brain: Redox state induction and cellular stress response. Antioxid. Redox Signal. 2009, 11, 2759–2775. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s disease: Current therapeutic significance and future prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Shankar, G.M.; Walsh, D.M. Alzheimer’s disease: Synaptic dysfunction and Abeta. Mol. Neurodegener. 2009, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Silva-Spínola, A.; Baldeiras, I.; Arrais, J.P.; Santana, I. The road to personalized medicine in Alzheimer’s disease: The use of artificial intelligence. Biomedicines 2022, 10, 315. [Google Scholar] [CrossRef]
- d’Errico, P.; Meyer-Luehmann, M. Mechanisms of pathogenic Tau and Aβ protein spreading in Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 265. [Google Scholar] [CrossRef]
- Fernàndez-Busquets, X.; Ponce, J.; Bravo, R.; Arimon, M.; Martiáñez, T.; Gella, A.; Cladera, J.; Durany, N. Modulation of amyloid β peptide1-42 cytotoxicity and aggregation in vitro by glucose and chondroitin sulfate. Curr. Alzheimer Res. 2010, 7, 428–438. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef]
- Perrin, R.J.; Craig-Schapiro, R.; Malone, J.P.; Shah, A.R.; Gilmore, P.; Davis, A.E.; Roe, C.M.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer’s disease. PLoS ONE 2011, 6, e16032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balion, C.M.; Benson, C.; Raina, P.S.; Papaioannou, A.; Patterson, C.; Ismaila, A.S. Brain type carnosinase in dementia: A pilot study. BMC Neurol. 2007, 7, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, J.; Ohara, T.; Katakura, Y.; Shimizu, K.; Yamashita, S.; Yoshida, D.; Honda, T.; Hirakawa, Y.; Shibata, M.; Sakata, S.; et al. Association between serum β-Alanine and risk of dementia. Am. J. Epidemiol. 2019, 188, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Green, B.D. Temporal effects of neuron-specific beta-secretase 1 (BACE1) knock-in on the mouse brain metabolome: Implications for Alzheimer’s disease. Neuroscience 2019, 397, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouda, N.A.; Elkamhawy, A.; Cho, J. Emerging therapeutic strategies for Parkinson’s disease and future prospects: A 2021 update. Biomedicines 2022, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Feng, Y.; Li, X.; Zhu, X.-Y.; Truong, D.; Ondo, W.G.; Wu, Y.-C. Prodromal markers of Parkinson’s disease in patients with essential tremor. Front. Neurol. 2020, 11, 874. [Google Scholar] [CrossRef]
- Prell, T.; Witte, O.W.; Grosskreutz, J. Biomarkers for dementia, fatigue, and depression in Parkinson’s disease. Front. Neurol. 2019, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Scott, L.; Dawson, V.L.; Dawson, T.M. Trumping neurodegeneration: Targeting common pathways regulated by autosomal recessive Parkinson’s disease genes. Exp. Neurol. 2017, 298, 191–201. [Google Scholar] [CrossRef]
- Anandhan, A.; Jacome, M.S.; Lei, S.; Hernandez-Franco, P.; Pappa, A.; Panayiotidis, M.I.; Powers, R.; Franco, R. Metabolic dysfunction in Parkinson’s disease: Bioenergetics, redox homeostasis and central carbon metabolism. Brain Res. Bull. 2017, 133, 12–30. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonninen, T.-M.; Hämäläinen, R.H.; Koskuvi, M.; Oksanen, M.; Shakirzyanova, A.; Wojciechowski, S.; Puttonen, K.; Naumenko, N.; Goldsteins, G.; Laham-Karam, N.; et al. Metabolic alterations in Parkinson’s disease astrocytes. Sci. Rep. 2020, 10, 14474. [Google Scholar] [CrossRef] [PubMed]
- Wassif, W.S.; Sherwood, R.A.; Amir, A.; Idowu, B.; Summers, B.; Leigh, N.; Peters, T.J. Serum carnosinase activities in central nervous system disorders. Clin. Chim. Acta 1994, 225, 57–64. [Google Scholar] [CrossRef]
- Solana-Manrique, C.; Sanz, F.J.; Torregrosa, I.; Palomino-Schätzlein, M.; Hernández-Oliver, C.; Pineda-Lucena, A.; Paricio, N. Metabolic alterations in a Drosophila model of Parkinson’s disease based on DJ-1 deficiency. Cells 2022, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, N. Expression of histidine decarboxylase and its roles in inflammation. Int. J. Mol. Sci. 2019, 20, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, S.M.; Pires, J.; Esteves, M.; Graça, B.; Bernardino, L. Histamine: A new immunomodulatory player in the neuron-glia crosstalk. Front. Cell. Neurosci. 2014, 8, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, R.-H.; Sun, H.-B.; Hu, Z.-L.; Lu, M.; Ding, J.-H.; Hu, G. Kir6.1/K-ATP channel modulates microglia phenotypes: Implication in Parkinson’s disease. Cell Death Dis. 2018, 9, 404. [Google Scholar] [CrossRef]
- Rinne, J.O.; Anichtchik, O.V.; Eriksson, K.S.; Kaslin, J.; Tuomisto, L.; Kalimo, H.; Röyttä, M.; Panula, P. Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J. Neurochem. 2002, 81, 954–960. [Google Scholar] [CrossRef]
- Aloisi, A.; Barca, A.; Romano, A.; Guerrieri, S.; Storelli, C.; Rinaldi, R.; Verri, T. Anti-aggregating effect of the naturally occurring dipeptide carnosine on aβ1-42 fibril formation. PLoS ONE 2013, 8, e68159. [Google Scholar] [CrossRef]
- Attanasio, F.; Convertino, M.; Magno, A.; Caflisch, A.; Corazza, A.; Haridas, H.; Esposito, G.; Cataldo, S.; Pignataro, B.; Milardi, D.; et al. Carnosine inhibits Aβ42 aggregation by perturbing the H-bond network in and around the central hydrophobic cluster. Chembiochem 2013, 14, 583–592. [Google Scholar] [CrossRef]
- Greco, V.; Naletova, I.; Ahmed, I.M.M.; Vaccaro, S.; Messina, L.; La Mendola, D.; Bellia, F.; Sciuto, S.; Satriano, C.; Rizzarelli, E. Hyaluronan-carnosine conjugates inhibit Aβ aggregation and toxicity. Sci. Rep. 2020, 10, 15998. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Sato, M.; Matsumoto, T.; Kadooka, K.; Hasegawa, T.; Fujimura, T.; Katakura, Y. Mechanisms of carnosine-induced activation of neuronal cells. Biosci. Biotechnol. Biochem. 2018, 82, 683–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou-Yang, L.; Liu, Y.; Wang, B.-Y.; Cao, P.; Zhang, J.-J.; Huang, Y.-Y.; Shen, Y.; Lyu, J.-X. Carnosine suppresses oxygen-glucose deprivation/recovery-induced proliferation and migration of reactive astrocytes of rats in vitro. Acta Pharmacol. Sin. 2018, 39, 24–34. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine prevents Aβ-induced oxidative stress and inflammation in microglial cells: A key role of TGF-β1. Cells 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, J.E.; Hipkiss, A.R.; Himsworth, D.T.; Romero, I.A.; Abbott, J.N. Toxic effects of beta-amyloid(25–35) on immortalised rat brain endothelial cell: Protection by carnosine, homocarnosine and beta-alanine. Neurosci. Lett. 1998, 242, 105–108. [Google Scholar] [CrossRef]
- Joshi, P.; Perni, M.; Limbocker, R.; Mannini, B.; Casford, S.; Chia, S.; Habchi, J.; Labbadia, J.; Dobson, C.M.; Vendruscolo, M. Two human metabolites rescue a C. elegans model of Alzheimer’s disease via a cytosolic unfolded protein response. Commun. Biol. 2021, 4, 843. [Google Scholar] [CrossRef] [PubMed]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; La Sorda, R.; Piantelli, M.; Canzoniero, L.M.T.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS ONE 2011, 6, e17971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, W.H.; Nunes, A.K.; França, M.E.R.; Santos, L.A.; Lós, D.B.; Rocha, S.W.; Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016, 1644, 149–160. [Google Scholar] [CrossRef]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. β-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.A.; Abdelmonsif, D.A.; Zeitoun, T.M.; El-Sayed, N.S.; Samy, D.M. Swimming exercise versus L-carnosine supplementation for Alzheimer’s dementia in rats: Implication of circulating and hippocampal FNDC5/irisin. J. Physiol. Biochem. 2022, 78, 109–124. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, L.; Zhang, L.-R. Neuroprotective effect of carnosine against salsolinol-induced Parkinson’s disease. Exp. Ther. Med. 2017, 14, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, M.; Kobayashi, N.; Sugizaki, T.; Shimoda, M.; Kawahara, M.; Tanaka, K.-I. Carnosine suppresses neuronal cell death and inflammation induced by 6-hydroxydopamine in an in vitro model of Parkinson’s disease. PLoS ONE 2020, 15, e0240448. [Google Scholar] [CrossRef] [PubMed]
- Afshin-Majd, S.; Khalili, M.; Roghani, M.; Mehranmehr, N.; Baluchnejadmojarad, T. Carnosine exerts neuroprotective effect against 6-hydroxydopamine toxicity in hemiparkinsonian rat. Mol. Neurobiol. 2015, 51, 1064–1070. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Kuo, W.-W.; Liu, W.-H.; Yin, M.-C. Antioxidative and anti-inflammatory protection from carnosine in the striatum of MPTP-treated mice. J. Agric. Food Chem. 2010, 58, 11510–11516. [Google Scholar] [CrossRef]
- Bermúdez, M.-L.; Skelton, M.R.; Genter, M.B. Intranasal carnosine attenuates transcriptomic alterations and improves mitochondrial function in the Thy1-aSyn mouse model of Parkinson’s disease. Mol. Genet. Metab. 2018, 125, 305–313. [Google Scholar] [CrossRef]
- Bermúdez, M.-L.; Seroogy, K.B.; Genter, M.B. Evaluation of carnosine intervention in the Thy1-aSyn mouse model of Parkinson’s disease. Neuroscience 2019, 411, 270–278. [Google Scholar] [CrossRef]
- Brown, J.M.; Baker, L.S.; Seroogy, K.B.; Genter, M.B. Intranasal carnosine mitigates α-synuclein pathology and motor dysfunction in the Thy1-aSyn mouse model of Parkinson’s disease. ACS Chem. Neurosci. 2021, 12, 2347–2359. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y. L-histidine and L-carnosine exert anti-brain aging effects in D-galactose-induced aged neuronal cells. Nutr. Res. Pract. 2020, 14, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Aydin, F.; Kalaz, E.B.; Kucukgergin, C.; Coban, J.; Dogru-Abbasoglu, S.; Uysal, M. Carnosine treatment diminished oxidative stress and glycation products in serum and tissues of D-galactose-treated rats. Curr. Aging Sci. 2018, 11, 10–15. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, B.; Poddar, M.K.; Dunbar, G.L. Carnosine improves aging-induced cognitive impairment and brain regional neurodegeneration in relation to the neuropathological alterations in the secondary structure of amyloid beta (Aβ). J. Neurochem. 2021, 158, 710–723. [Google Scholar] [CrossRef]
- Song, J.-H.; Yu, J.-T.; Tan, L. Brain-derived neurotrophic factor in Alzheimer’s disease: Risk, mechanisms, and therapy. Mol. Neurobiol. 2015, 52, 1477–1493. [Google Scholar] [CrossRef] [PubMed]
- Iulita, M.F.; Caraci, F.; Cuello, A.C. A link between nerve growth factor metabolic deregulation and amyloid-β-driven inflammation in Down syndrome. CNS Neurol. Disord. Drug Targets 2016, 15, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, O.I.; Berezhnoy, D.S.; Stvolinsky, S.L.; Lopachev, A.V.; Orlova, V.S.; Fedorova, T.N. Neuroprotective effect of the carnosine—α-lipoic acid nanomicellar complex in a model of early-stage Parkinson’s disease. Regul. Toxicol. Pharmacol. 2018, 95, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.; Fedorova, T.; Stepanova, M.; Dobrotvorskaya, I.; Kozlova, E.; Boldanova, N.; Bagyeva, G.; Ivanova-Smolenskaya, I.; Illarioshkin, S. Carnosine increases efficiency of DOPA therapy of Parkinson’s disease: A pilot study. Rejuvenation Res. 2008, 11, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.L.; Ousman, S.S. Astrocytes and Aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Ghosh, T.K.; Poddar, M.K. Carnosine reverses the aging-induced down regulation of brain regional serotonergic system. Mech. Ageing Dev. 2015, 152, 5–14. [Google Scholar] [CrossRef]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of anserine/carnosine supplementation on verbal episodic memory in elderly people. J. Alzheimer’s Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Menini, S.; Iacobini, C.; Fantauzzi, C.B.; Pugliese, G. L-carnosine and its derivatives as new therapeutic agents for the prevention and treatment of vascular complications of diabetes. Curr. Med. Chem. 2020, 27, 1744–1763. [Google Scholar] [CrossRef]
- Bae, O.-N.; Serfozo, K.; Baek, S.-H.; Lee, K.Y.; Dorrance, A.; Rumbeiha, W.; Fitzgerald, S.D.; Farooq, M.U.; Naravelta, B.; Bhatt, A.; et al. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke 2013, 44, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Heron, D.; Davenport, I.; Huckaba, T.; Graves, R.; Mandal, T.; Muniruzzaman, S.; Wang, S.; Bhattacharjee, P.S. Protective effects of bestatin in the retina of streptozotocin-induced diabetic mice. Exp. Eye Res. 2016, 149, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanini, D.; Jezdimirovic, T.; Stajer, V.; Ostojic, J.; Maksimovic, N.; Ostojic, S.M. Dietary supplementation with L-carnosine improves patient-reported outcomes, autonomic nervous system performance, and brain metabolism in 3 adult patients with multiple sclerosis. Nutr. Res. 2020, 84, 63–69. [Google Scholar] [CrossRef] [PubMed]

| Name | Symbols | Function | Predicted Location | Brain Expression a |
|---|---|---|---|---|
| Carnosine synthase 1 | CARNS1, ATPGD1, KIAA1394 | Synthesis | Intracellular | RNA: oligodendrocytes Protein: subsets of glial cells, possibly oligodendrocytes |
| Carnosine dipeptidase 1 (or serum carnosinase) | CN1, CNDP1, CPGL2, HsT2308, MGC10825 | Degradation | Intracellular, secreted | RNA: oligodendrocytes Protein: subsets of glial and neuronal cells |
| Carnosine dipeptidase 2 (or tissue carnosinase) | CN2, CNDP2, CPGL, FLJ10830,H2T2298, PEPA | Degradation | Intracellular | RNA: very low levels Protein: no data available |
| Carnosine N-methyltransferase 1 | CARNMT1, C9orf41, FLJ25795 | Methylation | Intracellular | RNA: very low levels Protein: no data available |
| Solute carrier family 15 member 1 | SLC15A1, PEPT1, HPEPT1, HEPCT1 | Transport | Membrane | RNA: no expression Protein: no data available |
| Solute carrier family 15 member 2 | SLC15A2, PEPT2 | Transport | Membrane | RNA: astrocytes, microglia Protein: no data available |
| Solute carrier family 15 member 3 | SLC15A3, hPTR3, PHT2 | Transport | Membrane | RNA: very low levels Protein: no data available |
| Solute carrier family 15 member 4 | SLC15A4, PTR4, PHT1 | Transport | Membrane | RNA: oligodendrocytes, microglia and several neurons Protein: no data available |
| Solute carrier family 22 member 15 | SLCA22A15, FLIPT1 | Transport | Membrane | RNA: oligodendrocytes and low in astrocytes Protein: no data available |
| Alzheimer’s Disease | |||
|---|---|---|---|
| Type of model | Description | Phenotypes modified | References |
| Cellular models | Cells supplemented with Aβ peptide or endogenously overexpressing Aβ | Reduction in Aβ aggregation, inflammation and OS, and neurotrophins induction | [80,81,82,83,84,85,86] |
| C. elegans | Aβ overexpression in large muscle cells | Induction of cytosolic unfolded proteins response | [87] |
| Rodents | Transgenic AD model mice Streptozotocin-induced AD rats | Reduction in cognitive impairment, OS, pro-inflammatory signaling, microglia activation and Aβ accumulation | [88,89,90,91] |
| Parkinson Disease | |||
| Type of model | Description | Phenotypes modified | References |
| Cellular model | Salsolinol-treated rat brain endothelial cells GT1-7 hypothalamic immortalized neurons treated with 6-OHDA | Increase in survival and upregulation of antioxidant enzymes Reduction in apoptosis, ROS levels, lipid peroxidation and pro-inflammatory cytokines | [92,93] |
| Rodents | Mice and rats treated with MPTP or 6-OHDA Mice model overexpressing α-synuclein (Thy1-aSyn) | Increase in antioxidant enzymes, improvement of mitochondrial function Reduction in α-synuclein aggregation, motor deficits and apoptosis | [94,95,96,97,98] |
| Aging | |||
| Type of model | Description | Phenotypes modified | References |
| Cellular models | Neurons with accelerated aging induced by galactose | Upregulation of antioxidant enzymes. Reduction in β amyloid protein and pro-inflammatory cytokines | [99] |
| Rodents | Elderly rats or rats supplemented with galactose | Decrease in oxidative stress and amyloid plaque formation. Improvement of cognitive function | [100,101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solana-Manrique, C.; Sanz, F.J.; Martínez-Carrión, G.; Paricio, N. Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases. Antioxidants 2022, 11, 848. https://doi.org/10.3390/antiox11050848
Solana-Manrique C, Sanz FJ, Martínez-Carrión G, Paricio N. Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases. Antioxidants. 2022; 11(5):848. https://doi.org/10.3390/antiox11050848
Chicago/Turabian StyleSolana-Manrique, Cristina, Francisco José Sanz, Guillermo Martínez-Carrión, and Nuria Paricio. 2022. "Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases" Antioxidants 11, no. 5: 848. https://doi.org/10.3390/antiox11050848
APA StyleSolana-Manrique, C., Sanz, F. J., Martínez-Carrión, G., & Paricio, N. (2022). Antioxidant and Neuroprotective Effects of Carnosine: Therapeutic Implications in Neurodegenerative Diseases. Antioxidants, 11(5), 848. https://doi.org/10.3390/antiox11050848







