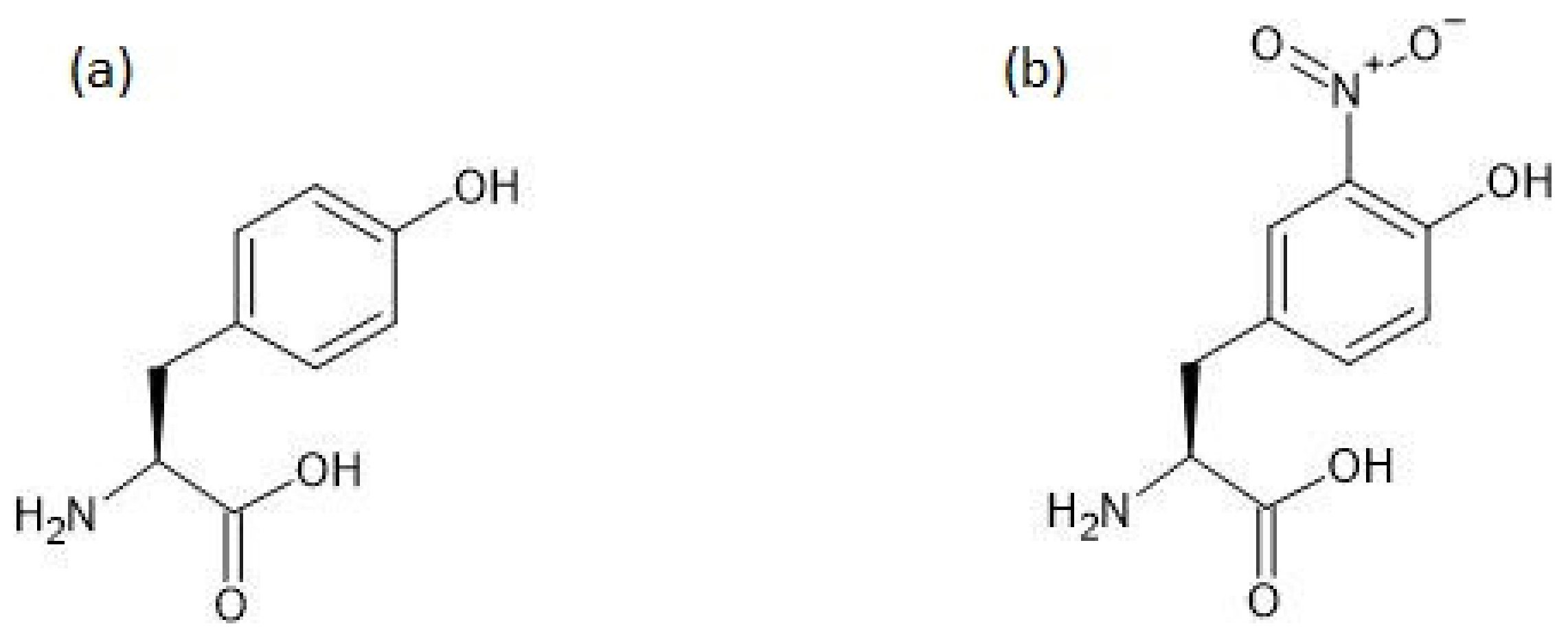Abstract
Diabetes mellitus (DM) is a strong risk factor for the development of cardiovascular diseases (CVDs), which are the most important cause of morbidity and mortality in the population of patients living with DM. DM is associated with lipid metabolism disorders characterized by a decrease in the high-density lipoprotein blood concentration, an increase in the triglyceride blood concentration, and the presence of modified lipoproteins not routinely measured in clinical practice. Nitrated lipoproteins are produced by the nitration of the tyrosyl residues of apolipoproteins by myeloperoxidase. There is some evidence from the research conducted showing that nitrated lipoproteins may play a role in the development of cardiovascular dysfunction, but this issue requires further investigation. It was found that the nitration of HDL particles was associated with a decrease in caspase-3 and paraoxonase-1 activity, as well as a decrease in the activity of cholesterol transport via ABCA1, which reduces the protective effect of HDL particles on the cardiovascular system. Less information has been collected about the role of nitrated LDL particles. Thus far, much more information has been obtained on the relationship of nitrotyrosine expression with the presence of cardiovascular risk factors and the development of cardiovascular dysfunction. The purpose of this paper is to provide an extensive review of the literature and to present the most important information on the current state of knowledge on the association between nitrotyrosine and nitrated lipoproteins with dysfunction of the cardiovascular system, especially in patients living with DM. Moreover, directions for future research in this area were discussed.
1. Introduction
1.1. Cardiovascular Disease in Patients Living with Diabetes Mellitus
Diabetes mellitus (DM) and its complications are one of the most important problems in public health worldwide. The number of patients living with DM is systematically increasing, which generates significant costs for healthcare systems [1]. DM is well documented to be a strong risk factor for the development of cardiovascular diseases (CVDs). DM predisposes the development of microvascular and macrovascular complications. CVDs are the leading cause of morbidity and mortality in the population of patients living with DM [2].
Coronary heart disease (CHD), ischemic cerebrovascular disease (ICVD), and peripheral arterial disease (PAD) are the most important clinical manifestations of atherosclerotic CVD [3]. DM accelerates and modifies the course of atherosclerotic CVD. PAD in patients with DM is characterized by a predisposition to multilevel stenosis and occlusion, as well as arteries below the knee more often being affected [4]. DM is also a risk factor for restenosis in patients after percutaneous balloon angioplasty with or without stent implantation, which worsens the prognosis and may lead to the need for reintervention [5].
1.2. Lipid Disorders in the Course of Diabetes Mellitus
The parameters of the lipid profile routinely determined in clinical practice, such as the concentrations of total cholesterol (TC), low-density lipoprotein (LDL) cholesterol (LDL-C), high-density lipoprotein (HDL) cholesterol (HDL-C), and triglycerides (TG), provide only some information about the state of a patient’s lipid metabolism, because lipoproteins can undergo different modifications in vivo that alter their pro- or anti-atherogenic profile. The mentioned modifications can be of enzymatic or non-enzymatic character. Lipoproteins can be modified through oxidation, glycation, desialylation, carbamylation, nitration, and chlorination, among other processes [6,7,8].
Lipid metabolism has been shown to be disordered in patients living with DM. The typical patterns of dyslipidemia in T2DM include an increased TG level, decreased HDL-C level, and higher susceptibility to forming more atherogenic small dense low-density lipoproteins (sdLDLs) [9]. In patients with normal sensitivity to insulin, insulin inhibits the secretion of very low-density lipoproteins (VLDLs) from the liver [10]. Insulin resistance (IR) is associated with a decrease in the activity of lipoprotein lipase, leading to the ineffective clearance of VLDL cholesterol and an increase in its blood concentration. Moreover, an increased plasma level of VLDL leads to a situation in which the plasma cholesteryl ester transfer protein (CETP) exchanges the triglycerides in VLDL for cholesterol in HDL. This is associated with the formation of more atherogenic VLDL particles and less protective HDL particles [10,11]. IR is associated with the inhibition of lipogenesis and stimulation of lipolysis, leading to an increase in the blood concentration of free fatty acids (FFAs) [12]. An increased delivery of FFAs to peripheral tissue activates the production of triglyceride-rich lipoproteins [10]. Moreover, an increase in the level of FFAs leads to the exacerbation of IR [13].
In Table 1, the results of selected studies contributing to the understanding of the cellular and molecular mechanisms responsible for the pathogenesis of dyslipidemia in the course of IR are shown.

Table 1.
Results of selected studies on the relationship between insulin and lipid metabolism.
1.3. Modified Lipoproteins in Atherogenesis
Modified lipoproteins promote the development of the inflammatory process within the arterial wall, macrophage activation, and the synthesis and secretion of proinflammatory cytokines, chemokines, and enzymes. The following modified lipoproteins were shown to play a role in the pathogenesis of CVDs: vortexed LDL, acetylated LDL, electronegative LDL, enzymatically modified LDL, lysosomal acid lipase-modified LDL, phospholipase A2-modified LDL, sphingomyelinase-modified LDL, oxidized LDL, oxidized VLDL, carbamylated LDL, carbamylated HDL, and advanced glycation end-product-modified LDL [23]. Glycated lipoproteins, which may be of particular interest for patients with DM, are also characterized by greater atherogenicity [24].
To date, the most data have been collected on the importance of oxLDL [25]. The formation of oxLDL and its subsequent unregulated phagocytosis by macrophages within the arterial wall via scavenger receptors is considered to be one of the most important elements in the pathogenesis of atherosclerosis [26].
1.4. Oxidative Stress
Reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as hydroxyl radicals, singlet oxygen, superoxide anions, hypochlorite, hydrogen peroxide, nitric oxide (NO), and peroxynitrite, are radical or non-radical compounds that arise in metabolic pathways and play an important role in physiological processes such as the control of gene expression, cell growth, transcription-factor activation, autophagy, the cell cycle, apoptosis, cell–cell interactions, intracellular signaling, and cellular defenses against pathogens [27,28]. However, under pathological conditions they can damage macromolecules essential for the function of cells, such as proteins, lipids, carbohydrates, and nucleic acids [29]. The human body has an antioxidant defense system that is responsible for protection against the excessive influence of pro-oxidative factors. Antioxidants can be divided into exogenous (such as vitamin C, vitamin E, carotenoids, and flavonoids) and endogenous types; among the endogenous antioxidants, there are enzymatic (such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase) and non-enzymatic (such as glutathione, ferritin, transferrin, ceruloplasmin, albumin, bilirubin, and uric acid) antioxidants [30]. The endogenous sources of ROS and RNS include the mitochondria, xanthine oxidase, lipoxygenase, myeloperoxidase (MPO), NADPH oxidase, cytochrome P450, and inducible nitric oxide synthase, among others [31,32].
The term oxidative stress (OS) refers to a greater activity of pro-oxidative factors over antioxidant factors [33]. OS has been shown to be related to the pathogenesis of various diseases such as cancer, CVDs, diabetes, obesity, neurodegenerative diseases, ankylosing spondylitis, chronic obstructive pulmonary disease, and obstructive sleep apnea [34,35,36]. Significant differences in the parameters of OS were found between obese, metabolically unhealthy patients, and healthy volunteers with normal body weight [37]. Interestingly, “obesity and insulin resistance” is the component of the metabolic syndrome most strongly associated with the presence of OS (according to principal component analysis) [38].
The possible use of nutraceuticals and supplements with antioxidant properties in the prevention and treatment of diseases remains an issue of great interest and is widely researched and discussed [39,40,41].
1.5. The Purpose of This Paper
The purpose of this paper is to provide an extensive review of the literature and to present the most important information on the current state of knowledge on the association of nitrotyrosine and nitrated lipoproteins with the development of the cardiovascular system dysfunction observed in patients living with DM. Moreover, directions for future research in this area are discussed.
2. Nitrotyrosine, Diabetes, and Cardiovascular Disease
Nitrotyrosine (NT-Tyr) is a product of tyrosine modification under the influence of peroxynitrite. Peroxynitrite is a potent prooxidative factor produced due to the interaction between superoxide anion with nitric oxide [42].
The chemical structures of tyrosine and 3-nitrotyrosine are shown in Figure 1.
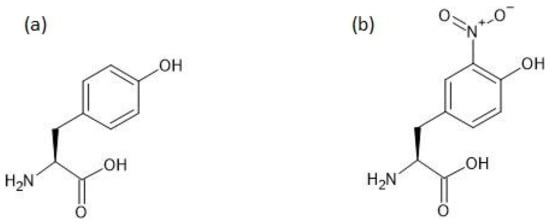
Figure 1.
The chemical structures of tyrosine (a) and 3-nitrotyrosine (b).
The NT-Tyr level was shown to be significantly higher in patients with T2DM than in non-diabetic controls, without a significant difference between patients with and without microalbuminuria among patients with T2DM [43]. On the other hand, according to Bo et al., the NT-Tyr level is significantly increased in patients with DM only in the population with a lower-than-recommended dietary intake of vitamins with antioxidant properties [44]. In a study performed by Segre et al., there was no significant difference between the level of NT-Tyr in patients with DM and angiographically confirmed CHD, and the level of NT-Tyr in patients with DM without CHD. It is worth noting that there was also no significant difference in the concentration of oxLDL, although its participation in the pathogenesis of CVD is unquestionable [45].
In vitro studies on aortic rings from non-diabetic rats have shown that canagliflozin alleviates the endothelial dysfunction induced by ischemia–reperfusion injury. The improvement in endothelial function was associated with a significant reduction in NT-Tyr content in the vessel wall. Endothelial function was assessed by the comparison of the relaxation of the smooth muscle of the aortic rings induced by an endothelium-dependent vasorelaxant (acetylcholine) and induced by an endothelium-independent vasorelaxant (sodium nitroprusside). Smooth muscles were previously contracted with phenylephrine [46].
In a study carried out on rats, it was shown that the 3-NT-Tyr level was significantly higher in an acute-blood-glucose-fluctuation group than in a constant-high-glucose group. Similarly, in the group with acute fluctuations in blood glucose, the acetylcholine-induced endothelium-dependent vascular relaxation was significantly lower than that in the group with constant high blood glucose, and the blood concentrations and expression of IL-6, TNF-α, and ICAM-1 mRNA were significantly higher. Moreover, the apoptosis index of endothelial cells was significantly higher in the group with the higher 3-NT-Tyr level [47]. It was found that supplementation with n-3 polyunsaturated fatty acids reversed endothelial dysfunction and normalized the reduction in endothelial nitric oxide synthase expression in aortas from rats with chronic kidney disease (CKD), which is associated with a substantial reduction in 3-NT-Tyr levels [48].
The Mediterranean diet was shown to significantly reduce the NT-Tyr level at three-month follow-up in patients with T2DM (0.64 ± 0.03 µmol/L at baseline vs. 0.35 ± 0.02 µmol/L at three-month follow-up; p < 0.05). It was associated with a significant improvement in endothelial function, as assessed by the flow-mediated dilation (FMD) method (5.6 ± 0.5% at baseline vs. 7.9 ± 0.4% at three-month follow-up; p < 0.05) [49]. In a study on OS and endothelial dysfunction in patients with human immunodeficiency virus (HIV) infection, it was found that NT-Tyr levels were significantly associated with carotid-radial pulse wave velocity (PWV) and that participants’ HIV status had a significant influence on this relationship [50].
Beckmann et al. demonstrated the presence of proteins whose tyrosyl residues had been nitrated in atherosclerotic lesions originating from human coronary arteries [51]. The study using immunohistochemical staining showed that the NT-Tyr content in sections taken from the renal artery and the external iliac artery was significantly higher in patients with end-stage renal disease (ESRD) eligible for transplantation than in the control group (donors of kidneys for transplantation). Moreover, among patients with ESRD, the NT-Tyr staining was significantly stronger in arteries with intima calcification, media calcification, and intima–media calcification than in arteries without the calcification of the appropriate layer of the artery wall [52]. In patients with ESRD, the expression of NT-Tyr was also shown to be significantly higher than in controls in the walls of small arteries [53]. According to Paier et al., the NT-Tyr expression in small arteries taken from subcutaneous tissue is significantly higher in patients with CHD but only in women [54]. Thus, the histopathological studies carried out to date on the sections of the artery wall suggest that, on the one hand, the amount of NT-Tyr is greater in patients with a worse cardiovascular condition, and on the other hand, in specific patients, it is greater in vascular beds with more advanced atherosclerotic processes.
The use of metformin in patients with prediabetes undergoing coronary artery bypass grafting (CABG) due to acute myocardial infarction was recently shown to be associated with a significant decrease in the concentration of NT-Tyr and proinflammatory cytokines in homogenates obtained from pericoronary fat [55]. The beneficial effect of metformin on cardiovascular risk has been documented in several meta-analyses, although it is not unambiguous [56,57,58,59].
In studies carried out in rats, it was found that drugs such as empagliflozin [60], sitagliptin [61], and spironolactone [62] showed cardioprotective potential and were able to reduce oxidative and nitrosative stress, which is associated with a reduction in NT-Tyr expression. Liraglutide has been shown to improve endothelial function in mice with polymicrobial sepsis, and to decrease the plasma concentration of 3-NT-Tyr [63].
NT-Tyr expression was shown to be significantly higher in small mesenteric arteries in mice with T2DM compared to control mice, as well as being reduced after bariatric surgery [64]. In rats with diabetic cardiomyopathy, valsartan has been shown to inhibit cardiomyocyte apoptosis by inhibiting the expression of 3-NT-Tyr [65].
2.1. Cellular and Molecular Mechanisms
The level of 3-NT-Tyr is a biomarker of OS and nitrosative stress that leads to cell death through mechanisms such as apoptosis, autophagy, ferroptosis, pyroptosis, NETosis, and parthanatos [66]. It was found that the nitration of protein and peptide tyrosyl residues was associated with a decrease in their ability to be degraded in proteasomes [67].
The overexpression of 3-NT-Tyr induced by a high glucose level was shown to be associated with the downregulation of peroxisome proliferator-activated receptor β (PPARβ) [68], whose activity has previously been documented to prevent endothelial dysfunction in diabetic rats [69]. This phenomenon shows a potential pathophysiological relationship between increased expression of 3-NT-Tyr and the development of CVD over the course of DM.
In a study using the rat thoracic aorta, free 3-NT-Tyr was documented to contribute to the development of endothelial dysfunction by promoting DNA damage and apoptosis [70]. NT-Tyr may also directly increase aortic smooth muscle cell migration in vitro and contribute to the overexpression of migration-related molecules through ROS production and the activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway [71].
Interestingly, in a human study, it was shown that the intravenous infusion of angiotensin II led to a significant increase in the concentration of NT-Tyr in the blood, and the prior inhibition of the activity of cyclooxygenase-2 (COX-2) was associated with a significantly smaller increase in the concentration of NT-Tyr [72]. It should be noted that the role of angiotensin II in the pathogenesis of CVD is well established [73] and that COX-2 plays an important role in the development of angiotensin II-induced inflammation within the vascular wall [74]. The presented relationship is, therefore, a factor supporting the role of NT-Tyr in the development of cardiovascular dysfunction, at least as a biomarker.
2.2. Association between Nitrotyrosine and Selected Risk Factors of CVD
The mean concentration of 3-NT-Tyr was shown to be significantly higher in smokers. Furthermore, eating foods with possibly high levels of acrylamide and drinking alcohol is associated with an increase in 3-NT-Tyr levels [75]. In an animal model study, it was shown that a diet rich in oxidized plant sterols increases the production of 3-NT-Tyr and the synthesis of cytokines (TNF-α, IL-1β, and IL-6) that can lead to a secondary disorder of lipid metabolism [76]. According to another publication, NT-Tyr expression was documented to be significantly higher in smokers than in control subjects, as well as similar in passive and active smokers [77]. On the other hand, Jin et al. documented significantly lower 3-NT-Tyr levels in the plasma proteins of smokers. It is worth noting that the methodology used in this study was used to determine the level of 3-NT-Tyr for twenty-four plasma proteins separately and not to determine the total level of protein-bound NT-Tyr. Furthermore, in the same study, it was elucidated that smokers diagnosed with chronic obstructive pulmonary disease (COPD) had significantly higher levels of NT-Tyr than smokers without COPD [78].
The NT-Tyr plasma concentration is significantly higher in sixty-year-old and older morbidly obese subjects than in morbidly obese individuals aged between twenty and thirty-nine years [79]. According to Fenster et al., weight loss (to the normal range) is associated with a significant decrease in the NT-Tyr blood level in overweight Caucasian women, but not in overweight African American women [80]. The 3-NT-Tyr levels in obese children aged three to six years are significantly higher than those in normal-weight children [81]. According to Choromańska et al., the plasma level of NT-Tyr was shown to be significantly higher in morbidly obese subjects than in lean individuals and to decrease significantly after bariatric surgery [82].
The blood levels of NT-Tyr are significantly higher in subjects with metabolic syndrome (MS) than in healthy controls (234.3 ± 158.2 µmol/mol tyrosine vs. 53.7 ± 46.8 µmol/mol tyrosine; p < 0.0001). Moreover, lifestyle modifications (supervised aerobic exercise and the Mediterranean diet) lead to significant decreases in NT-Tyr levels (234.3 ± 158.2 µmol/mol tyrosine vs. 58.9 ± 55.0 µmol/mol tyrosine; p < 0.0001) [83]. According to another study, the blood NT-Tyr levels are also significantly higher in MS subjects than in healthy controls, and a linear increase in the number of features of MS with the NT-Tyr level was observed (p < 0.02) [84]. In non-obese women with polycystic ovary syndrome, IR was shown to be associated with an increase in NT-Tyr levels [85]. Treatment with irbesartan (150 mg twice daily) was shown to be associated with a significant reduction in fasting NT-Tyr blood levels, as well as the levels during oral glucose tolerance tests in non-hypertensive individuals with T2DM [86].
2.3. Significance of Nitrotyrosine Measurements in Diagnosis of CVD and Prognosis
To date, only a small amount of research on the diagnostic and prognostic value of determining NT-Tyr concentrations in the context of CVDs has been conducted, with no consensus.
There was no significant difference in NT-Tyr blood levels between middle-aged men with arterial hypertension, middle-aged men with arterial hypertension and other cardiovascular disorders, and a control group [87]. According to Shishehbor et al., the NT-Tyr levels were significantly higher in patients with CHD than in control subjects (9.1 µmol/mol vs. 5.2 µmol/mol, respectively; p < 0.001). It should be noted that the correlation remained significant after adjustment for the Framingham Global Risk Score, age, sex, DM, arterial hypertension, current smoking, HDL-C, LDL-C, TG, and CRP. Moreover, statin therapy was shown to significantly reduce NT-Tyr levels (25%; p < 0.02) [88]. Four-week rosuvastatin therapy (10 mg daily) was shown to be associated with a significant reduction in NT-Tyr blood concentrations [89]. According to Ferlazzo et al., the plasma 3-NT-Tyr level was significantly higher in patients with periodontitis and CHD in comparison to healthy subjects, although there was no significant difference between patients with periodontitis and healthy subjects, or between patients with CHD and healthy subjects [90].
The NT-Tyr concentration was shown to have no prognostic value in terms of survival after acute coronary syndrome at a four-year follow-up [91]. In a study conducted by Heslop et al., it was shown that there were no significant differences in the concentrations of NT-Tyr in the blood between patients with confirmed or excluded features of CHD using angiography (72.1 vs. 71.9 nmol/L, respectively; p = 0.965). In the same study, the risk of cardiovascular mortality increased across tertiles of blood concentrations of NT-Tyr. The difference between the highest and lowest tertiles became statistically significant after four years of follow-up. However, after multivariate adjustment for factors such as age, sex, TC/HDL-C ratio, body mass index, smoking, DM, and hypertension, attenuation of the correlation was observed (p = 0.08), which indicates that the NT-Tyr level is not an independent predictor of cardiovascular mortality [92].
The NT-Tyr blood level was suggested to be a marker of transient cardiac ischemia following coronary vasospasm. In patients with vasospastic angina pectoris (VSAP), the blood levels of NT-Tyr increased significantly three and twelve hours after an acetylcholine provocation test, with no significant differences fifteen minutes after the test, while in the control group, the serum level of NT-Tyr decreased significantly [93]. In another study, no significant differences in the levels of NT-Tyr thirty minutes after an exercise test in patients with positive exercise tests, or in patients with negative exercise tests, were observed [94].
In 2021, a paper was published in which a real-time 3-NT-Tyr detection system using a smartphone-based electrochemiluminescence system was presented [95]. Undoubtedly, it is an interesting invention, but its usefulness in clinical practice requires further research.
3. Nitrated Lipoproteins and Diabetes Mellitus
Postprandial hyperglycemia elevates the risk of ROS overproduction, as well as increasing the synthesis of non-enzymatic early glycated and nitrated proteins, and it makes the lipoprotein profile more atherogenic [96]. Lipoproteins can undergo MPO-catalyzed enzymatic nitration, and the reaction concerns the apolipoprotein apoA-I in HDL particles and apoB in LDL particles [97].
Alterations in MPO activity have been investigated over the course of DM. According to Peng et al., the concentrations of serum extracellular-vesicle-derived MPO as well as serum MPO were shown to be significantly higher in patients with T2DM than in controls without DM [98]. A similar conclusion related to an increase in MPO levels in DM patients was previously documented in other publications [99,100,101]. On the other hand, papers reporting no significant differences in MPO levels between patients with and without DM have been published [102,103]. Thus, the results of studies on the relationship between DM and MPO levels are not conclusive and further research is needed.
The blood levels of nitrated apolipoprotein A-I (NT-apoA-I) were significantly higher in patients living with T2DM than in age-matched control subjects [104]. In patients with T2DM, an increased blood level of NT-apoA-I is associated with a decrease in serum paraoxonase-1 (PON-1) activity [105]. PON-1 is responsible for the antioxidant properties of HDL particles [106]. DM was shown to be an independent predictor of a higher NT-apoA-I/apoA-I ratio, and this value is negatively correlated with cholesterol efflux from macrophages independently of total apoA-I levels [107].
According to Lu et al., MPO bound to apoA-I in serum taken from patients with T2DM facilitated the selective oxidative modification of HDL particles. Furthermore, using liquid chromatography with tandem mass spectrometry, Tyr192 was shown to be the main nitration and chlorination site in the apoA-I present in serum taken from patients living with T2DM. It was also documented that the apoA-I nitration and chlorination levels increase in patients with T2DM. The oxidative modification of apoA-I mediated by MPO is associated with a decrease in the cholesterol efflux properties and antiapoptotic activity (through diminished possibilities for the inhibition of caspase-3 activity) of HDL particles [108].
4. Nitrated Lipoproteins and Cardiovascular Disease
According to Shao et al., Tyr192 is the major nitration site in the apoA-I of circulating HDL particles, while the highest nitration level in the apoA-I in HDL particles within atherosclerotic lesions is suspected to be associated with Tyr18, although without a significant difference from that for Tyr100 [109]. In atheromatous plaques taken from human aortas, apoA-I particles were shown to have undergone MPO-dependent nitration at residues Tyr192 and Tyr166 [110]. Apolipoprotein A-I nitrated at Tyr166 accounts for 8% of the total apoA-I in atherosclerotic coronary arteries, but over 100-fold less in normal coronary arteries [111].
The MPO-dependent oxidative modification of HDL particles leads to a change in cholesterol transport through the ATP-binding cassette transporter A1 (ABCA1) pathway [112]. LDL nitration is associated with the formation of cholesterol-rich cells, a key feature of atherosclerosis [113].
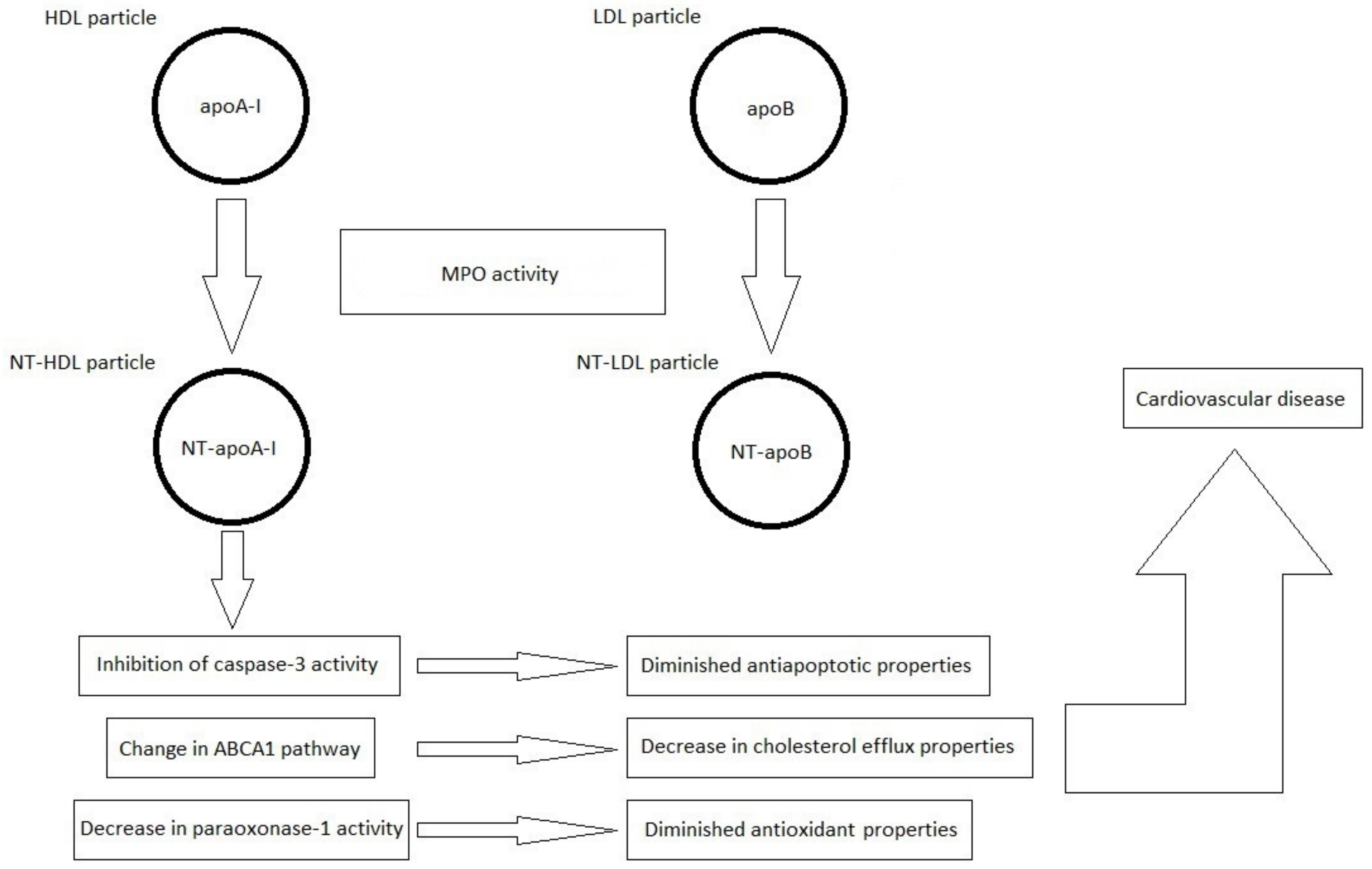
The most important information in the field of current knowledge on the formation of nitrated lipoproteins and their importance in the pathogenesis of CVDs is schematically summarized in Figure 2.
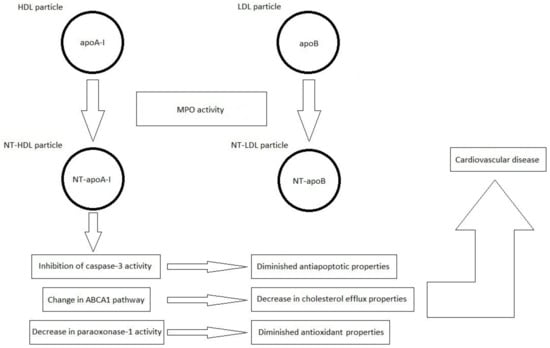
Figure 2.
Formation of nitrated lipoproteins (NT-HDL and NT-LDL) under the influence of myeloperoxidase (MPO) activity. Nitrated lipoproteins are characterized by the presence of 3-NT-Tyr in the polypeptide chains of apolipoprotein A-I (apoA-I) and apolipoprotein B (apoB), respectively, resulting in the formation of nitrated apoA-I (NT-apoA-I) and nitrated apoB (NT-apoB), respectively.
The blood concentration of nitrated high-density lipoproteins (NT-HDL) was shown to be a useful early marker of microangiopathic dysfunction, as assessed using the vascular reactivity index (VRI), in African American patients with T2DM, in the total population, and in subjects with HbA1c ≤ 7.0%. In the same study, the blood NT-LDL concentration was shown to be significantly associated with the carotid intima–media thickness (CIMT) outcome in the total population and in patients with HbA1c > 7% but only in a univariable analysis. In a multivariate analysis, the significant association was lost. Sex and the duration of DM were confounding variables that affected the association between NT-LDL and CIMT [114].
5. Factors Influencing Blood Concentration of Nitrated Lipoproteins
According to Mathew et al., a twelve-week lifestyle modification (exercise and the Mediterranean diet) led to a significant decrease in the 3-NT-Tyr levels in HDL particles in subjects diagnosed with metabolic syndrome. Similarly, a significant decrease in the level of 3-chlorotyrosine was observed in HDLs. This is suspected to be the direct effect of the decrease in MPO activity [115].
To our knowledge, Mathew et al.’s study is currently the only one available in the literature that has shown the effect of lifestyle modifications on the concentration of nitrated lipoproteins. During the preparation of this paper, we did not find any study in which the influence of pharmacological treatment on the concentration of nitrated lipoproteins was examined. However, there are data that indicate a beneficial effect of lifestyle modifications and drugs used in CVD treatment on the activity of MPO, which is considered an important source of NT-Tyr within lipoprotein molecules.
Twelve-week treatment with atorvastatin (20–40 mg daily) significantly reduced the MPO activity in the blood in patients with moderate to very high risks of atherosclerotic CVD [116]. In patients with acute coronary syndrome, who received rosuvastatin on the first day, a significantly greater decrease in MPO activity was observed compared to that in the placebo group [117]. In patients admitted to hospital for coronary angiography, the use of drugs such as beta-blockers, statins, and angiotensin-converting-enzyme inhibitors was observed to be associated with significantly lower levels of MPO blood activity in patients with acute coronary syndrome, but not in patients with stable coronary syndrome. In multivariate analysis, the effect remained statistically significant for beta-blockers [118].
NT-apoA-I was documented to be significantly reduced at twelve months after kidney transplantation, as well as associated with a significant reduction in MPO activity. On the other hand, nitrated apolipoprotein B (NT-apoB) did not change significantly after kidney transplantation [97]. In a recently published epidemiological study, it was shown that, in a fifteen-year observation, the risk of death over the course of CVD was lower in the population of patients with CKD after transplantation than in patients who did not undergo transplantation (2.3% vs. 15.2%) [119]. This suggests that NT-apoA-I may become a new marker for the evaluation of cardiovascular risk in patients with CKD in the future, but its usefulness in clinical practice needs to be further evaluated.
6. Conclusions and Directions for Future Research
The results of this review of the literature show that the level of NT-Tyr is a widely used marker in research, and the results provide premises indicating a significant relationship between NT-Tyr and CVD. The most important findings of our review of the literature related to NT-Tyr are presented in Table 2.

Table 2.
Associations between 3-nitrotyrosine and cardiovascular disease—the most important findings.
Based on the studies conducted to date, the relationship between increased levels of NT-Tyr and dysfunction of the cardiovascular system seems to be unquestionable, although the data on the usefulness of this parameter in the assessment of prognosis in patients with CVD are more ambiguous.
The importance of peroxynitrite, under the influence of which NT-Tyr is formed, in the context of cardiovascular dysfunction in DM was the subject of a review published by Pacher and Szabó in 2006. The authors of this study drew similar conclusions indicating that NT-Tyr is, on the one hand, a marker of dysfunction of the cardiovascular system during DM. On the other hand, it also probably plays a direct pathogenetic role. We had more data in the preparation of this publication, as we, of course, also used papers published after 2006, which makes this review more up to date. In this paper, we are also concerned with the issue of nitrated lipoproteins, which was not discussed in the publication mentioned above [42].
The term nitrated lipoproteins refers to those lipoprotein molecules in which the tyrosyl residues are nitrated in the polypeptide chain of the apolipoprotein. Our review of the literature shows that, contrary to NT-Tyr research, little research on nitrated lipoproteins and their role in the pathogenesis of CVD has been conducted to date, the information on which remains limited. The limitation of this literature review is the relatively small number of publications on nitrated lipoproteins. The most important findings of our review of the literature related to nitrated lipoproteins and their role in the pathogenesis of CVDs are summarized in Table 3.

Table 3.
Nitrated lipoproteins, diabetes mellitus, and cardiovascular disease—the most important findings.
Undoubtedly, OS plays an important role in the pathogenesis of atherosclerotic CVD. An increased level of NT-Tyr is one of the markers of OS. It is difficult to say whether the increased level of nitrated lipoproteins is only a marker of OS that provides no additional information with the level of NT-Tyr, or whether it provides additional information and plays an independent role in the pathogenesis of atherosclerotic CVD. More detailed research is needed to dispel these doubts. The study of the importance of nitrated lipoproteins in the development of cardiovascular dysfunctions in patients with DM is an interesting direction for further research. In our opinion, the issue of the role of nitrated lipoproteins in the development of cardiovascular dysfunction could be the subject of a large clinical research project involving patients living with DM and controls. Table 4 presents the research questions that should be addressed. We hope that this may also inspire other research teams that wish to conduct research on such topics. More work is also required at the level of basic research to better understand how nitrated lipoproteins are formed, how nitration alters lipoprotein function, and how it can contribute to the development of CVD, including in patients with DM.

Table 4.
The role of nitrated lipoproteins in the pathogenesis of cardiovascular disease—perspectives for future research.
Author Contributions
Conceptualization, G.K.J.; writing—original draft preparation, G.K.J.; writing—review and editing, G.C. and A.S.; visualization, G.K.J., G.C. and A.S.; supervision, G.C. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| apoA-I | apolipoprotein A-I |
| apoB | apolipoprotein B |
| CABG | coronary artery bypass grafting |
| CETP | cholesteryl ester transfer protein |
| CHD | coronary heart disease |
| CIMT | carotid intima–media thickness |
| CKD | chronic kidney disease |
| COPD | chronic obstructive pulmonary disease |
| COX-2 | cyclooxygenase-2 |
| CVD | cardiovascular disease |
| DM | diabetes mellitus |
| ESRD | end-stage renal disease |
| FFA | free fatty acid |
| FMD | flow-mediated dilation |
| FoxO1 | transcription factor forkhead box O1 |
| Foxa2 | transcription factor forkhead box A2 |
| HDL | high-density lipoprotein |
| HDL-C | high-density lipoprotein cholesterol |
| HIV | human immunodeficiency virus |
| ICVD | ischemic cerebrovascular disease |
| IR | insulin resistance |
| LDL | low-density lipoprotein |
| LDL-C | low-density lipoprotein cholesterol |
| MPO | myeloperoxidase |
| MS | metabolic syndrome |
| MTP | microsomal triglyceride transfer protein |
| NO | nitric oxide |
| NT-apoA–I | nitrated apolipoprotein A–I |
| NT-apoB | nitrated apolipoprotein B |
| NT-HDL | nitrated high-density lipoprotein |
| NT-LDL | nitrated low-density lipoprotein |
| NT-Tyr | nitrotyrosine |
| OS | oxidative stress |
| oxLDL | oxidized LDL particle |
| PAD | peripheral arterial disease |
| PON-1 | paraoxonase-1 |
| PPARβ | peroxisome proliferator-activated receptor β |
| PTP-1B | protein-tyrosine phosphatase-1B |
| PWV | pulse-wave velocity |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| sdLDL | small dense low-density lipoprotein |
| SREBP1c | sterol regulatory element-binding protein 1c |
| TG | triglycerides |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| VLDL | very low-density lipoprotein |
| VRI | vascular reactivity index |
| VSAP | vasospastic angina pectoris |
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The growing epidemic of diabetes mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef]
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 8–16. [Google Scholar] [CrossRef]
- Mahtta, D.; Khalid, U.; Misra, A.; Samad, Z.; Nasir, K.; Virani, S.S. Premature atherosclerotic cardiovascular disease: What have we learned recently? Curr. Atheroscler. Rep. 2020, 22, 44. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic lower extremity ischemia and its association with the frailty syndrome in patients with diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and clinical significance of in-stent restenosis in patients with diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Norata, G.D.; Pirillo, A.; Catapano, A.L. Modified HDL: Biological and physiopathological consequences. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 371–386. [Google Scholar] [CrossRef]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.F.; Sobenin, I.A.; Orekhov, A.N. The atherogenic role of circulating modified lipids in atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [PubMed]
- Obama, T.; Itabe, H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 8312. [Google Scholar] [CrossRef]
- Warraich, H.J.; Rana, J.S. Dyslipidemia in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 27–32. [Google Scholar] [CrossRef]
- Bahiru, E.; Hsiao, R.; Phillipson, D.; Watson, K.E. Mechanisms and treatment of dyslipidemia in diabetes. Curr. Cardiol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Borén, J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Zozulińska, D. Atherogenic dyslipidaemia in type 2 diabetes. Kardiol. Pol. 2006, 64, S567–S571. [Google Scholar] [PubMed]
- Sivan, E.; Boden, G. Free fatty acids, insulin resistance, and pregnancy. Curr. Diab. Rep. 2003, 3, 319–322. [Google Scholar] [CrossRef]
- Chirieac, D.V.; Chirieac, L.R.; Corsetti, J.P.; Cianci, J.; Sparks, C.E.; Sparks, J.D. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1003–E1011. [Google Scholar] [CrossRef]
- Chirieac, D.V.; Cianci, J.; Collins, H.L.; Sparks, J.D.; Sparks, C.E. Insulin suppression of VLDL apo B secretion is not mediated by the LDL receptor. Biochem. Biophys. Res. Commun. 2002, 297, 134–137. [Google Scholar] [CrossRef]
- Chirieac, D.V.; Collins, H.L.; Cianci, J.; Sparks, J.D.; Sparks, C.E. Altered triglyceride-rich lipoprotein production in Zucker diabetic fatty rats. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E42–E49. [Google Scholar] [CrossRef]
- Taghibiglou, C.; Rashid-Kolvear, F.; Van Iderstine, S.C.; Le-Tien, H.; Fantus, I.G.; Lewis, G.F.; Adeli, K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J. Biol. Chem. 2002, 277, 793–803. [Google Scholar] [CrossRef]
- Kuriyama, H.; Yamashita, S.; Shimomura, I.; Funahashi, T.; Ishigami, M.; Aragane, K.; Miyaoka, K.; Nakamura, T.; Takemura, K.; Man, Z.; et al. Enhanced expression of hepatic acyl-coenzyme A synthetase and microsomal triglyceride transfer protein messenger RNAs in the obese and hypertriglyceridemic rat with visceral fat accumulation. Hepatology 1998, 27, 557–562. [Google Scholar] [CrossRef]
- Lin, M.C.; Gordon, D.; Wetterau, J.R. Microsomal triglyceride transfer protein (MTP) regulation in HepG2 cells: Insulin negatively regulates MTP gene expression. J. Lipid Res. 1995, 36, 1073–1081. [Google Scholar] [CrossRef]
- Au, W.S.; Kung, H.F.; Lin, M.C. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: Roles of MAPKerk and MAPKp38. Diabetes 2003, 52, 1073–1080. [Google Scholar] [CrossRef][Green Version]
- Kamagate, A.; Qu, S.; Perdomo, G.; Su, D.; Kim, D.H.; Slusher, S.; Meseck, M.; Dong, H.H. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Investig. 2008, 118, 2347–2364. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Montminy, M. Fatty acids and insulin resistance: A perfect storm. Mol. Cell. 2006, 21, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Lorey, M.B.; Öörni, K.; Kovanen, P.T. Modified lipoproteins induce arterial wall inflammation during atherogenesis. Front. Cardiovasc. Med. 2022, 9, 841545. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.; Soran, H.; Sharma, R.; Charlton-Menys, K.; Durrington, P. Lipoprotein glycation in atherogenesis. Clin. Lipidol. 2009, 4, 781–790. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of oxLDL and its impact on cardiovascular health: Focus on atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- El Hadri, K.; Smith, R.; Duplus, E.; El Amri, C. Inflammation, oxidative stress, senescence in atherosclerosis: Thioredoxine-1 as an emerging therapeutic target. Int. J. Mol. Sci. 2021, 23, 77. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Kulbacka, J.; Saczko, J.; Chwiłkowska, A. Stres oksydacyjny w procesach uszkodzenia komórek. Pol. Merk. Lek. 2009, 27, 44–47. [Google Scholar]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant therapies for neuroprotection–a review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2013, 3, 424. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and oxidative stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Stanek, A.; Cholewka, A.; Wielkoszyński, T.; Romuk, E.; Sieroń, A. Decreased oxidative stress in male patients with active phase ankylosing spondylitis who underwent whole-body cryotherapy in closed cryochamber. Oxid. Med. Cell. Longev. 2018, 2018, 7365490. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. Oxidative stress markers among obstructive sleep apnea patients. Oxid. Med. Cell. Longev. 2021, 2021, 9681595. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Kasperczyk, S.; Osadnik, T.; Pawlas, N. Oxidative stress in association with metabolic health and obesity in young adults. Oxid. Med. Cell. Longev. 2021, 2021, 9987352. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and insulin resistance” is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants 2022, 11, 79. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and cancer: Potential for natural polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Schiffers, C.; Reynaert, N.L.; Wouters, E.F.M.; van der Vliet, A. Redox dysregulation in aging and COPD: Role of NOX enzymes and implications for antioxidant strategies. Antioxidants 2021, 10, 1799. [Google Scholar] [CrossRef]
- Rasaei, N.; Asbaghi, O.; Samadi, M.; Setayesh, L.; Bagheri, R.; Gholami, F.; Soveid, N.; Casazza, K.; Wong, A.; Suzuki, K.; et al. Effect of green tea supplementation on antioxidant status in adults: A systematic review and meta-analysis of randomized clinical trials. Antioxidants 2021, 10, 1731. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Szabó, C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr. Opin. Pharmacol. 2006, 6, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Stankova, T.R.; Delcheva, G.T.; Maneva, A.I.; Vladeva, S.V. Serum levels of carbamylated LDL, nitrotyrosine and soluble lectin-like oxidized low-density lipoprotein receptor-1 in poorly controlled type 2 diabetes mellitus. Folia Med. 2019, 61, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Gambino, R.; Guidi, S.; Silli, B.; Gentile, L.; Cassader, M.; Pagano, G.F. Plasma nitrotyrosine levels, antioxidant vitamins and hyperglycaemia. Diabet. Med. 2005, 22, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Segre, C.A.; Hueb, W.; Garcia, R.M.; Rezende, P.C.; Favarato, D.; Strunz, C.M.; Sprandel Mda, C.; Roggério, A.; Carvalho, A.L.; Maranhão, R.C.; et al. Troponin in diabetic patients with and without chronic coronary artery disease. BMC Cardiovasc. Disord. 2015, 15, 72. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Kocer, C.; Sayour, A.A.; Kraft, P.; Benker, M.I.; Abulizi, S.; Georgevici, A.I.; Brlecic, P.; Radovits, T.; Loganathan, S.; et al. The sodium-glucose cotransporter-2 inhibitor canagliflozin alleviates endothelial dysfunction following in vitro vascular ischemia/reperfusion injury in rats. Int. J. Mol. Sci. 2021, 22, 7774. [Google Scholar] [CrossRef]
- Wu, N.; Shen, H.; Liu, H.; Wang, Y.; Bai, Y.; Han, P. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc. Diabetol. 2016, 15, 109. [Google Scholar] [CrossRef]
- Zanetti, M.; Gortan Cappellari, G.; Barbetta, D.; Semolic, A.; Barazzoni, R. Omega 3 polyunsaturated fatty acids improve endothelial dysfunction in chronic renal failure: Role of eNOS activation and of oxidative stress. Nutrients 2017, 9, 895. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; La Sala, L.; Pujadas, G.; De Nigris, V.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. The protective effect of the Mediterranean diet on endothelial resistance to GLP-1 in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2014, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Chikopela, T.; Goma, F.; Kaluba, L.; Mutale, W.; Guure, C.; Heimburger, D.C.; Koethe, J.R. Arterial stiffness is associated with oxidative stress and endothelial activation among persons with treated HIV in Zambia. South. Afr. J. HIV Med. 2021, 22, 1298. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Ye, Y.Z.; Anderson, P.G.; Chen, J.; Accavitti, M.A.; Tarpey, M.M.; White, C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler 1994, 375, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Guilgen, G.; Werneck, M.L.; de Noronha, L.; Martins, A.P.; Varela, A.M.; Nakao, L.S.; Pecoits-Filho, R. Increased calcification and protein nitration in arteries of chronic kidney disease patients. Blood Purif. 2011, 32, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Luksha, N.; Luksha, L.; Carrero, J.J.; Hammarqvist, F.; Stenvinkel, P.; Kublickiene, K. Impaired resistance artery function in patients with end-stage renal disease. Clin. Sci. 2011, 120, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Paier, A.; Agewall, S.; Kublickiene, K. Expression of heat shock proteins and nitrotyrosine in small arteries from patients with coronary heart disease. Heart Vessel. 2009, 24, 260–266. [Google Scholar] [CrossRef]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Mureddu, S.; Loreni, F.; Ferraraccio, F.; Panarese, I.; Trotta, M.C.; Gatta, G.; et al. Metformin therapy effects on the expression of sodium-glucose cotransporter 2, leptin, and SIRT6 levels in pericoronary fat excised from pre-diabetic patients with acute myocardial infarction. Biomedicines 2021, 9, 904. [Google Scholar] [CrossRef]
- Monami, M.; Candido, R.; Pintaudi, B.; Targher, G.; Mannucci, E.; SID-AMD joint panel for Italian guidelines on treatment of type 2 diabetes. Effect of metformin on all-cause mortality and major adverse cardiovascular events: An updated meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 699–704. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.; Dai, H.; Deng, Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta-analysis. Diabetes Res. Clin. Pract. 2020, 160, 108001. [Google Scholar] [CrossRef]
- Li, T.; Providencia, R.; Mu, N.; Yin, Y.; Chen, M.; Wang, Y.; Liu, M.; Yu, L.; Gu, C.; Ma, H. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 30. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Ye, Z.; Găman, M.A.; Tan, S.C.; Zhu, F. The effect of metformin on carotid intima-media thickness (CIMT): A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 2020, 886, 173458. [Google Scholar] [CrossRef]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.Á.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Man, F.; Guo, Z.; Xu, J.; Yan, W.; Li, J.; Pan, Q.; Wang, W. Sitagliptin protects cardiac function by reducing nitroxidative stress and promoting autophagy in Zucker diabetic fatty (ZDF) rats. Cardiovasc. Drugs Ther. 2018, 32, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Alzoubi, K.H.; Bonyan, R. The role of spironolactone on myocardial oxidative stress in rat model of streptozotocin-induced diabetes. Cardiovasc. Ther. 2017, 35, e12242. [Google Scholar] [CrossRef] [PubMed]
- Helmstädter, J.; Keppeler, K.; Aust, F.; Küster, L.; Frenis, K.; Filippou, K.; Vujacic-Mirski, K.; Tsohataridis, S.; Kalinovic, S.; Kröller-Schön, S.; et al. GLP-1 analog liraglutide improves vascular function in polymicrobial sepsis by reduction of oxidative stress and inflammation. Antioxidants 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Zhang, J.; Potter, B.J.; Sowers, J.R.; Zhang, C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wei, J.R. 3-nitrotyrosine, a biomarker for cardiomyocyte apoptosis induced by diabetic cardiomyopathy in a rat model. Mol. Med. Rep. 2013, 8, 989–994. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Q.; Chen, F.; Pang, J.; Pan, C.; Xu, F.; Chen, Y. Fundamental mechanisms of the cell death caused by nitrosative stress. Front. Cell Dev. Biol. 2021, 9, 742483. [Google Scholar] [CrossRef]
- Ott, C.; Tomasina, F.; Campolo, N.; Bartesaghi, S.; Mastrogiovanni, M.; Leyva, A.; Batthyány, C.; Meinl, W.; Grune, T.; Radi, R. Decreased proteasomal cleavage at nitrotyrosine sites in proteins and peptides. Redox Biol. 2021, 46, 102106. [Google Scholar] [CrossRef]
- Yang, C.; Xue, L.; Wu, Y.; Li, S.; Zhou, S.; Yang, J.; Jiang, C.; Ran, J.; Jiang, Q. PPARβ down-regulation is involved in high glucose-induced endothelial injury via acceleration of nitrative stress. Microvasc. Res. 2022, 139, 104272. [Google Scholar] [CrossRef]
- Quintela, A.M.; Jiménez, R.; Gómez-Guzmán, M.; Zarzuelo, M.J.; Galindo, P.; Sánchez, M.; Vargas, F.; Cogolludo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; et al. Activation of peroxisome proliferator-activated receptor-β/-δ (PPARβ/δ) prevents endothelial dysfunction in type 1 diabetic rats. Free Radic. Biol. Med. 2012, 53, 730–741. [Google Scholar] [CrossRef]
- Mihm, M.J.; Jing, L.; Bauer, J.A. Nitrotyrosine causes selective vascular endothelial dysfunction and DNA damage. J. Cardiovasc. Pharmacol. 2000, 36, 182–187. [Google Scholar] [CrossRef]
- Mu, H.; Wang, X.; Lin, P.; Yao, Q.; Chen, C. Nitrotyrosine promotes human aortic smooth muscle cell migration through oxidative stress and ERK1/2 activation. Biochim. Biophys. Acta 2008, 1783, 1576–1584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pialoux, V.; Poulin, M.J.; Hemmelgarn, B.R.; Muruve, D.A.; Chirico, E.N.; Faes, C.; Sola, D.Y.; Ahmed, S.B. Cyclooxygenase-2 inhibition limits angiotensin II-induced DNA oxidation and protein nitration in humans. Front. Physiol. 2017, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Cassis, L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc. Med. 2004, 14, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Vapaatalo, H.; Mervaala, E. Angiotensin II and vascular inflammation. Med. Sci Monit. 2005, 11, RA194–RA205. [Google Scholar] [PubMed]
- Szumska, M.; Wielkoszyński, T.; Tyrpień, K. 3-nitrotyrosine determination as nitrosative stress marker and health attitudes of medical students considering exposure to environmental tobacco smoke. Przegl. Lek. 2012, 69, 798–802. [Google Scholar] [PubMed]
- Wielkoszyński, T.; Zalejska-Fiolka, J.; Strzelczyk, J.K.; Owczarek, A.J.; Cholewka, A.; Kokoszczyk, K.; Stanek, A. 5α,6α-epoxyphytosterols and 5α,6α-epoxycholesterol increase nitrosative stress and inflammatory cytokine production in rats on low-cholesterol diet. Oxid. Med. Cell. Longev. 2020, 2020, 4751803. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.; Wan, E.; Wei, Y.; Wahab, R.; Castagna, F.; Wang, G.; Emin, M.; Russo, C.; Homma, S.; Le Jemtel, T.H.; et al. Secondhand smoking is associated with vascular inflammation. Chest 2015, 148, 112–119. [Google Scholar] [CrossRef]
- Jin, H.; Webb-Robertson, B.J.; Peterson, E.S.; Tan, R.; Bigelow, D.J.; Scholand, M.B.; Hoidal, J.R.; Pounds, J.G.; Zangar, R.C. Smoking, COPD, and 3-nitrotyrosine levels of plasma proteins. Environ. Health Perspect. 2011, 119, 1314–1320. [Google Scholar] [CrossRef][Green Version]
- Choromańska, B.; Myśliwiec, P.; Dadan, J.; Maleckas, A.; Zalewska, A.; Maciejczyk, M. Effects of age and gender on the redox homeostasis of morbidly obese people. Free Radic. Biol. Med. 2021, 175, 108–120. [Google Scholar] [CrossRef]
- Fenster, C.P.; Darley-Usmar, V.M.; Landar, A.L.; Gower, B.A.; Weinsier, R.L.; Hunter, G.R.; Patel, R.P. Weight loss and race modulate nitric oxide metabolism in overweight women. Free Radic. Biol. Med. 2004, 37, 695–702. [Google Scholar] [CrossRef]
- Fuentes-Venado, C.E.; Terán-Pérez, G.; Espinosa-Hernández, V.M.; Martínez-Herrera, E.; Segura-Uribe, J.J.; Mercadillo, R.E.; Pinto-Almazán, R.; Guerra-Araiza, C. Nutritional status influences oxidative stress and insulin resistance in preschool children. Metab. Syndr. Relat. Disord. 2021, 19, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Dadan, J.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. Bariatric surgery normalizes protein glycoxidation and nitrosative stress in morbidly obese patients. Antioxidants 2020, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, S.; Jaiswal, M.; Vivekanandan-Giri, A.; White, E.A.; Ang, L.; Raffel, D.M.; Rubenfire, M.; Pop-Busui, R. Structured lifestyle intervention in patients with the metabolic syndrome mitigates oxidative stress but fails to improve measures of cardiovascular autonomic neuropathy. J. Diabetes Complicat. 2017, 31, 1437–1443. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Adams-Huet, B.; Chen, X.; Kaur, H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E1844–E1850. [Google Scholar] [CrossRef]
- Macut, D.; Simic, T.; Lissounov, A.; Pljesa-Ercegovac, M.; Bozic, I.; Djukic, T.; Bjekic-Macut, J.; Matic, M.; Petakov, M.; Suvakov, S.; et al. Insulin resistance in non-obese women with polycystic ovary syndrome: Relation to byproducts of oxidative stress. Exp. Clin. Endocrinol. Diabetes 2011, 119, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Assaloni, R.; Da Ros, R.; Maier, A.; Quagliaro, L.; Piconi, L.; Esposito, K.; Giugliano, D. Effect of irbesartan on nitrotyrosine generation in non-hypertensive diabetic patients. Diabetologia 2004, 47, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Garley, M.; Ratajczak-Wrona, W.; Nowak, K.; Jabłońska, E.; Maślach, D.; Omeljaniuk, W.J. Profile of new vascular damage biomarkers in middle-aged men with arterial hypertension. Adv. Med. Sci. 2021, 66, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Shishehbor, M.H.; Aviles, R.J.; Brennan, M.L.; Fu, X.; Goormastic, M.; Pearce, G.L.; Gokce, N.; Keaney, J.F., Jr.; Penn, M.S.; Sprecher, D.L.; et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003, 289, 1675–1680. [Google Scholar] [CrossRef]
- Pirro, M.; Schillaci, G.; Mannarino, M.R.; Savarese, G.; Vaudo, G.; Siepi, D.; Paltriccia, R.; Mannarino, E. Effects of rosuvastatin on 3-nitrotyrosine and aortic stiffness in hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 436–441. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Currò, M.; Isola, G.; Maggio, S.; Bertuccio, M.P.; Trovato-Salinaro, A.; Matarese, G.; Alibrandi, A.; Caccamo, D.; Ientile, R. Changes in the biomarkers of oxidative/nitrosative stress and endothelial dysfunction are associated with cardiovascular risk in periodontitis patients. Curr. Issues Mol. Biol. 2021, 43, 704–715. [Google Scholar] [CrossRef]
- Quidim, A.V.L.; Bruno, T.; Leocádio, P.C.L.; Santos, I.S.; Alvarez-Leite, J.I.; Dos Reis Menta, P.L.; Lotufo, P.A.; Benseñor, I.M.; Goulart, A.C. The prognostic value of nitrotyrosine levels in coronary heart disease: Long-term evaluation in the Acute Coronary Syndrome Registry Strategy (ERICO study). Clin. Biochem. 2019, 66, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Heslop, C.L.; Frohlich, J.J.; Hill, J.S. Myeloperoxidase and C-reactive protein have combined utility for long-term prediction of cardiovascular mortality after coronary angiography. J. Am. Coll. Cardiol. 2010, 55, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Kawai, Y.; Kitayama, M.; Akao, H.; Ishida, R.; Motoyama, A.; Wakasa, M.; Saito, R.; Aoki, H.; Fujibayashi, K.; et al. Increased levels of the oxidative stress marker, nitrotyrosine in patients with provocation test-induced coronary vasospasm. J. Cardiol. 2014, 64, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Elfatih, A.; Anderson, N.R.; Mansoor, S.; Ahmed, S.; Horton, R.; Holland, M.R.; Gama, R. Plasma nitrotyrosine in reversible myocardial ischaemia. J. Clin. Pathol. 2005, 58, 95–96. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Liu, W.; Chen, J.; Yu, Q.; Zhang, Z.; Li, Y.; Liu, J.; Chen, X. Real time detection of 3-nitrotyrosine using smartphone-based electrochemiluminescence. Biosens. Bioelectron. 2021, 187, 113284. [Google Scholar] [CrossRef]
- Rebolledo, O.R.; Actis Dato, S.M. Postprandial hyperglycemia and hyperlipidemia-generated glycoxidative stress: Its contribution to the pathogenesis of diabetes complications. Eur. Rev. Med. Pharmacol. Sci. 2005, 9, 191–208. [Google Scholar]
- Bakillah, A.; Tedla, F.; Ayoub, I.; John, D.; Norin, A.J.; Hussain, M.M.; Brown, C. Plasma nitration of high-density and low-density lipoproteins in chronic kidney Disease Patients Receiving Kidney Transplants. Mediat. Inflamm. 2015, 2015, 352356. [Google Scholar] [CrossRef]
- Peng, L.; Li, X.; Li, Y.; Zhao, W.; Nie, S.; Yu, H.; Qi, Y.; Qin, Y.; Zhang, H. Increased concentrations of myeloperoxidase in serum and serum extracellular vesicles are associated with type 2 diabetes mellitus. Clin. Chim. Acta. 2021, 522, 70–76. [Google Scholar] [CrossRef]
- Moldoveanu, E.; Tanaseanu, C.; Tanaseanu, S.; Kosaka, T.; Manea, G.; Marta, D.S.; Popescu, L.M. Plasma markers of endothelial dysfunction in type 2 diabetics. Eur. J. Intern. Med. 2006, 17, 38–42. [Google Scholar] [CrossRef]
- Wiersma, J.J.; Meuwese, M.C.; van Miert, J.N.; Kastelein, A.; Tijssen, J.G.; Piek, J.J.; Trip, M.D. Diabetes mellitus type 2 is associated with higher levels of myeloperoxidase. Med. Sci. Monit. 2008, 14, CR406–CR410. [Google Scholar]
- Rovira-Llopis, S.; Rocha, M.; Falcon, R.; de Pablo, C.; Alvarez, A.; Jover, A.; Hernandez-Mijares, A.; Victor, V.M. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxid. Redox. Signal. 2013, 19, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, M.G.; Alanbaei, M.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Channanath, A.; Thanaraj, T.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Investigating the role of myeloperoxidase and angiopoietin-like protein 6 in obesity and diabetes. Sci. Rep. 2020, 10, 6170. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; Alssema, M.; Diamant, M.; Teerlink, T.; Dekker, J.M.; Kok, A.; Kostense, P.J.; Nijpels, G.; Heine, R.J.; Scheffer, P.G. Comparison of two consecutive fat-rich and carbohydrate-rich meals on postprandial myeloperoxidase response in women with and without type 2 diabetes mellitus. Metabolism 2008, 57, 262–267. [Google Scholar] [CrossRef]
- Hermo, R.; Mier, C.; Mazzotta, M.; Tsuji, M.; Kimura, S.; Gugliucci, A. Circulating levels of nitrated apolipoprotein A-I are increased in type 2 diabetic patients. Clin. Chem. Lab. Med. 2005, 43, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Hermo, R.; Tsuji, M.; Kimura, S. Lower serum paraoxonase-1 activity in type 2 diabetic patients correlates with nitrated apolipoprotein A-I levels. Clin. Chim. Acta 2006, 368, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-Tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of paraoxonase 1 (PON1) as an antioxidant and antiatherogenic enzyme in the cardiovascular complications of type 2 diabetes: Genotypic and phenotypic evaluation. Diabetes Res. Clin. Pract. 2020, 161, 108067. [Google Scholar] [CrossRef]
- Chen, X.; Bakillah, A.; Zhou, L.; Pan, X.; Hoepfner, F.; Jacob, M.; Jiang, X.C.; Lazar, J.; Schlitt, A.; Hussain, M.M. Nitrated apolipoprotein AI/apolipoprotein AI ratio is increased in diabetic patients with coronary artery disease. Atherosclerosis 2016, 245, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Xie, S.; Li, J.; Tian, R.; Peng, Y.Y. Myeloperoxidase-mediated oxidation targets serum apolipoprotein A-I in diabetic patients and represents a potential mechanism leading to impaired anti-apoptotic activity of high density lipoprotein. Clin. Chim. Acta 2015, 441, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Pennathur, S.; Heinecke, J.W. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J. Biol. Chem. 2012, 287, 6375–6386. [Google Scholar] [CrossRef]
- Zheng, L.; Settle, M.; Brubaker, G.; Schmitt, D.; Hazen, S.L.; Smith, J.D.; Kinter, M. Localization of nitration and chlorination sites on apolipoprotein A-I catalyzed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. J. Biol. Chem. 2005, 280, 38–47. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Aulak, K.; Huang, Y.; Wagner, M.; Gerstenecker, G.; Topbas, C.; Gogonea, V.; DiDonato, A.J.; Tang, W.H.W.; Mehl, R.A.; et al. Site-specific nitration of apolipoprotein A-I at tyrosine 166 is both abundant within human atherosclerotic plaque and dysfunctional. J. Biol. Chem. 2014, 289, 10276–10292. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Heinecke, J.W. Impact of HDL oxidation by the myeloperoxidase system on sterol efflux by the ABCA1 pathway. J. Proteom. 2011, 74, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, O.M.; Briviba, K.; Klotz, L.O.; Sies, H. Oxidative modification and nitration of human low-density lipoproteins by the reaction of hypochlorous acid with nitrite. Arch. Biochem. Biophys. 1997, 343, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, A.; Eluwole, A.; Tedla, F.; Kremer, A.; Mastrogiovanni, N.; Khan, M.; Rosenberg, C.; Dreizen, P.; La Rosa, J.; Salciccioli, L.; et al. Association between nitrated lipoproteins and vascular function in type 2 diabetes. Front. Biosci. (Landmark Ed.) 2021, 26, 644–663. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.V.; Li, L.; Byun, J.; Guo, Y.; Michailidis, G.; Jaiswal, M.; Chen, Y.E.; Pop-Busui, R.; Pennathur, S. Therapeutic lifestyle changes improve HDL function by inhibiting myeloperoxidase-mediated oxidation in patients with metabolic syndrome. Diabetes Care 2018, 41, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Baydoun, D.; Ibdah, R.; Ibrahim, K. Atorvastatin reduces plasma inflammatory and oxidant biomarkers in patients with risk of atherosclerotic cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 216–225. [Google Scholar] [CrossRef]
- Sexton, T.R.; Wallace, E.L.; Macaulay, T.E.; Charnigo, R.J.; Evangelista, V.; Campbell, C.L.; Bailey, A.L.; Smyth, S.S. The effect of rosuvastatin on thromboinflammation in the setting of acute coronary syndrome. J. Thromb. Thrombolysis 2015, 39, 186–195. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Braun, S.; Schömig, A.; Kastrati, A. Impact of therapy with statins, beta-blockers and angiotensin-converting enzyme inhibitors on plasma myeloperoxidase in patients with coronary artery disease. Clin. Res. Cardiol. 2011, 100, 327–333. [Google Scholar] [CrossRef]
- Khou, V.; De La Mata, N.L.; Kelly, P.J.; Masson, P.; O’Lone, E.; Morton, R.L.; Webster, A.C. Epidemiology of cardiovascular death in kidney failure: An Australian and New Zealand cohort study using data linkage. Nephrology 2022, 27, 430–440, online ahead of print. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).