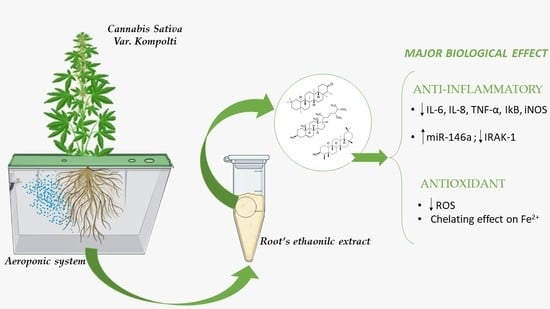
Characterization of the Biological Activity of the Ethanolic Extract from the Roots of Cannabis sativa L. Grown in Aeroponics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Cultures
2.3. Gas Chromatography (GC-MS, GC-FID)
2.4. Ethanolic Extract of Aeroponic Plant Roots (APEX)
2.5. Cell Culture
2.6. DPPH Radical Scavenging Activity
2.7. Chelating Effect on Fe2+
2.8. Cell Treatments
2.9. Cell Viability Assay
2.10. Fast Halo Assay (FHA)
2.11. Expression of Inflammatory Genes: RNA Isolation cDNA Synthesis and Quantitative Real-Time PCR
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. In Vitro Wound-Healing Assay
2.14. Statistical Analysis
3. Results
3.1. Extract Characterization
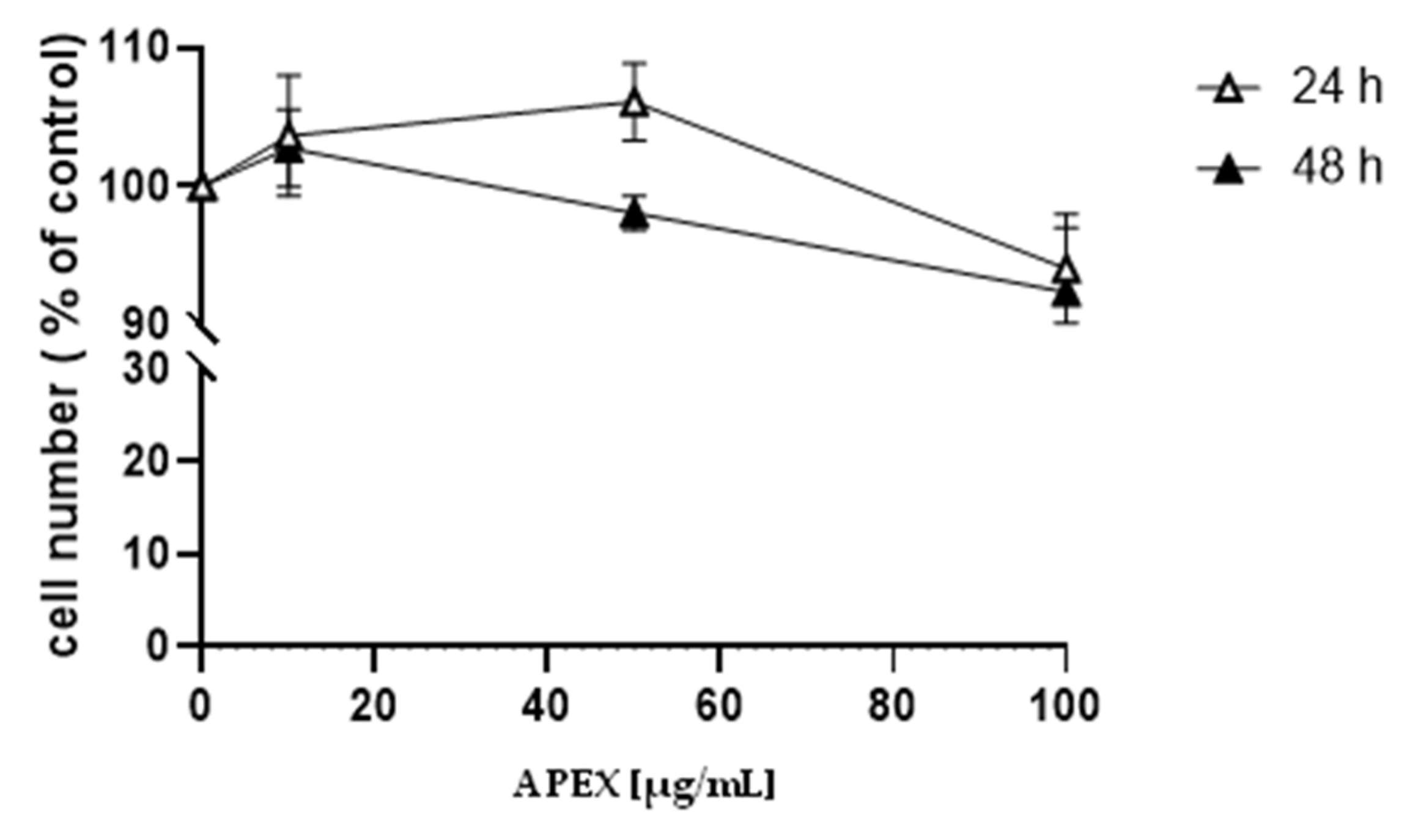
3.2. Cytotoxicity and Genotoxicity of APEX
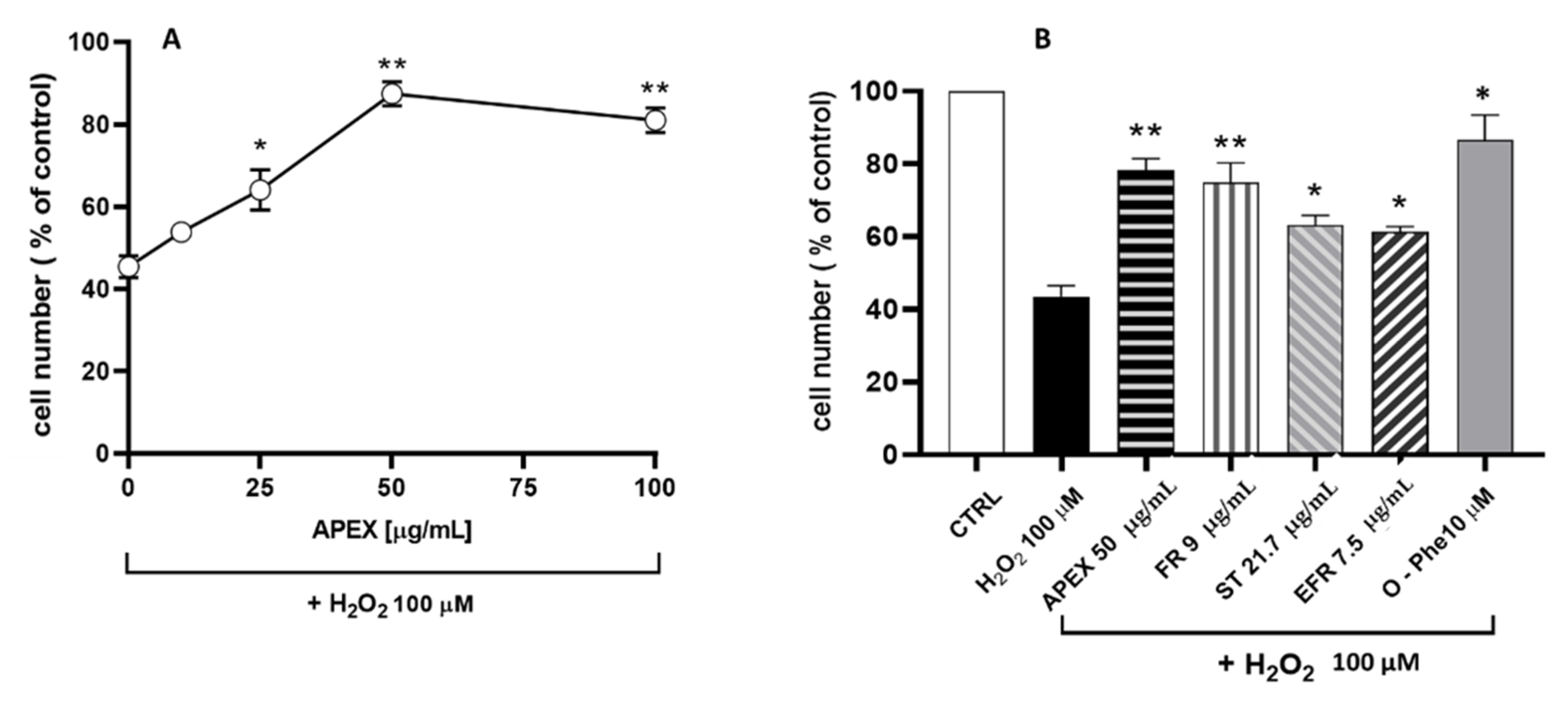
3.3. Antioxidant Activity
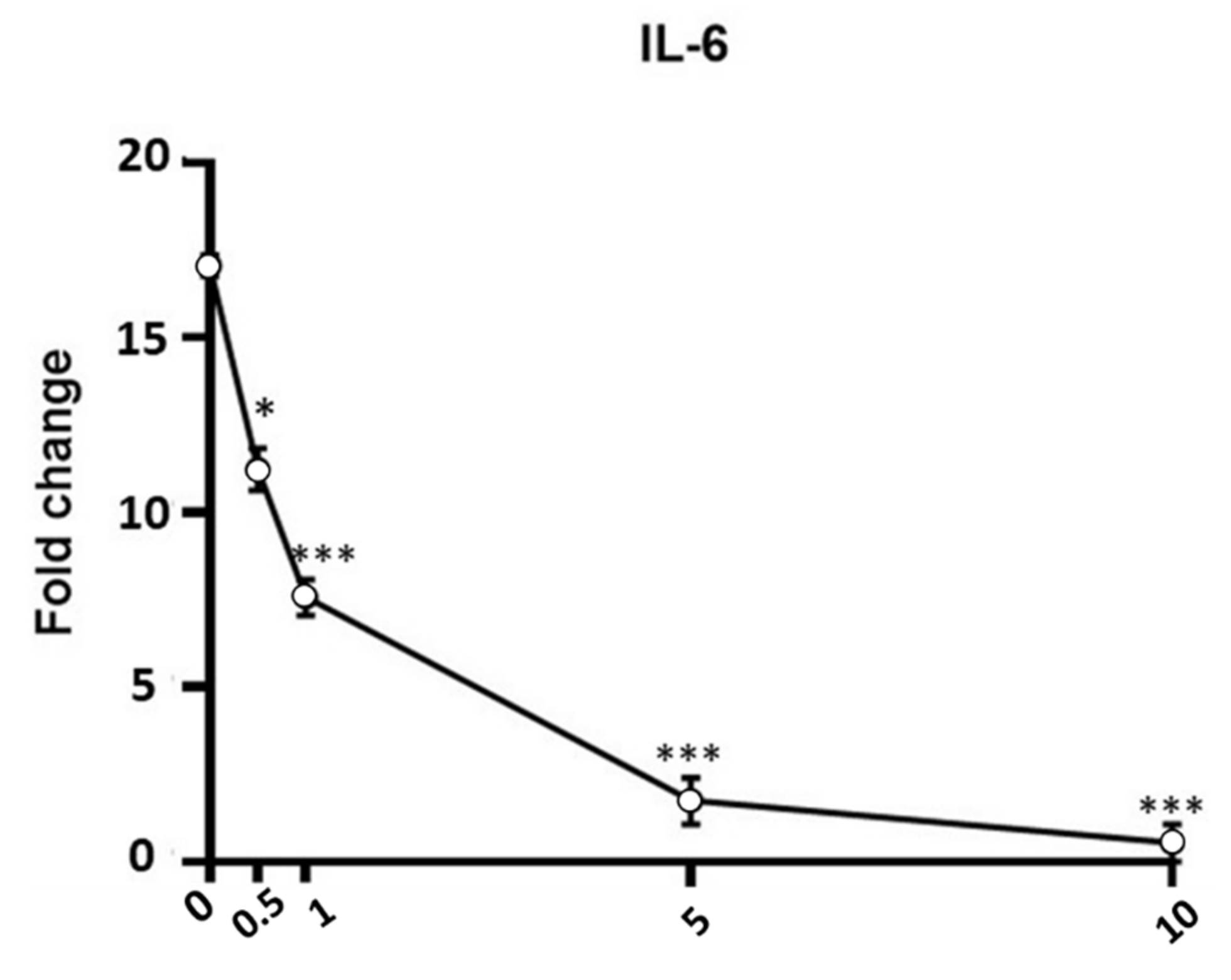
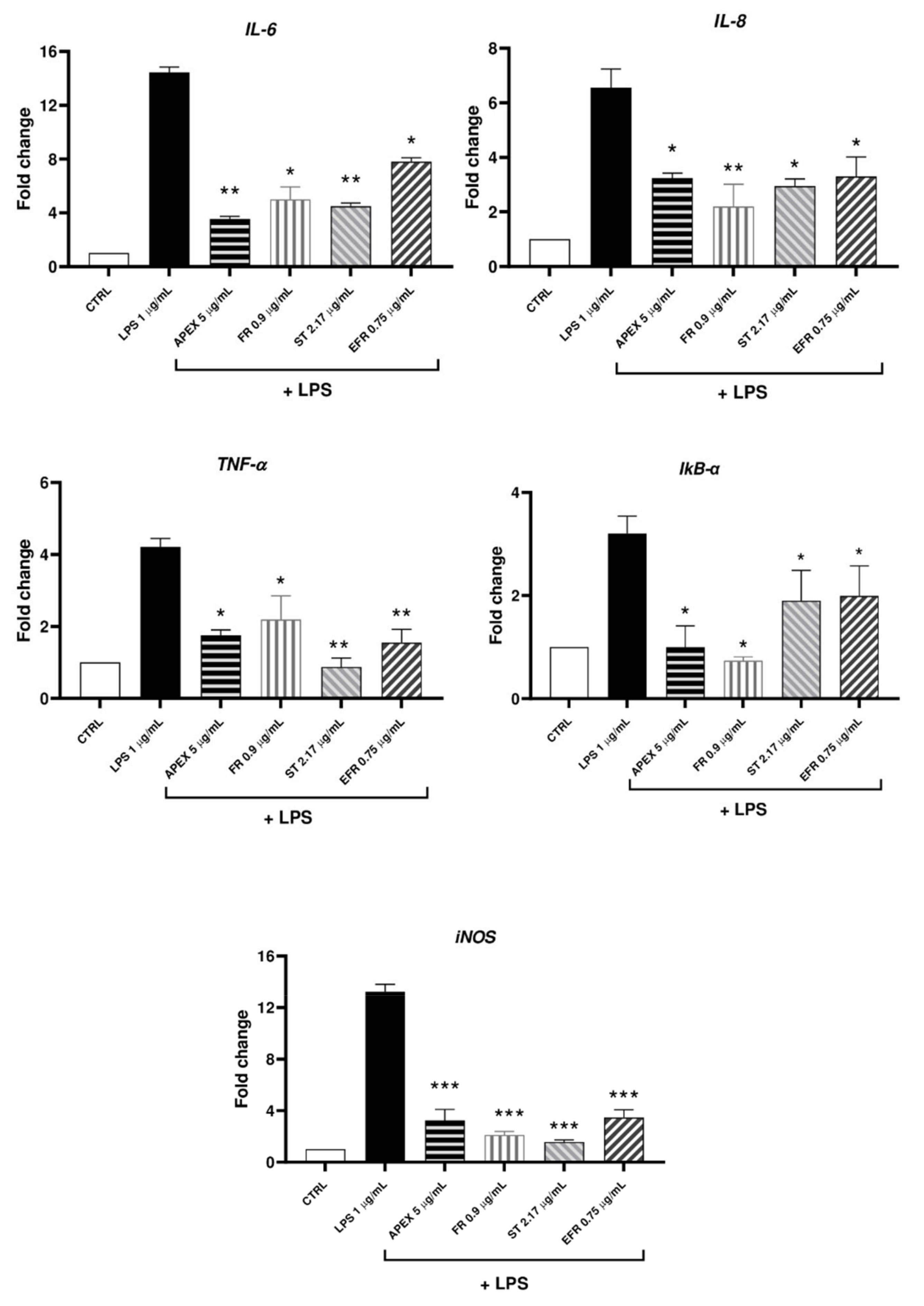
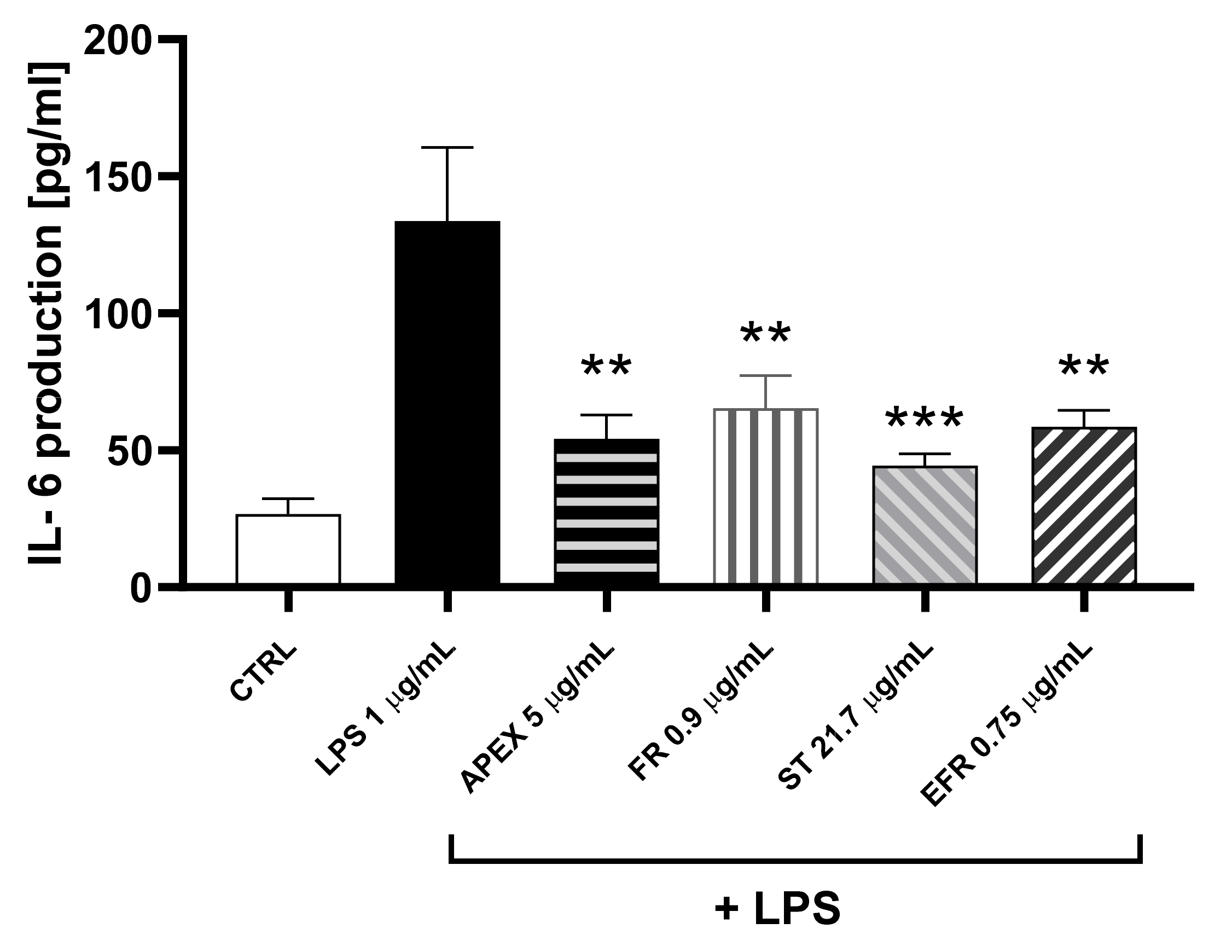
3.4. Anti-Inflammatory Activity
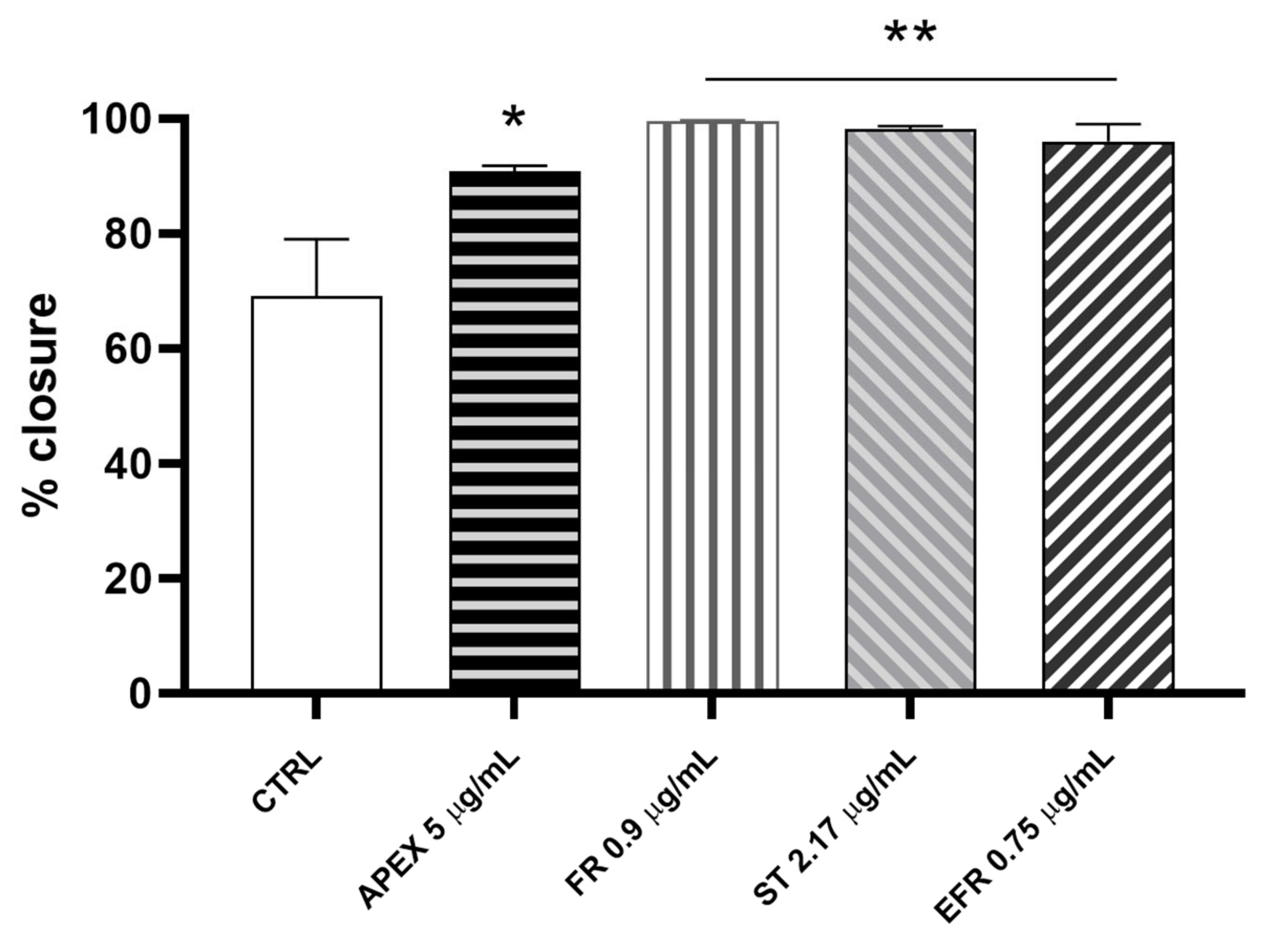
3.5. Wound-Healing Repair Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APEX | Ethanolic extracts of C. sativa aeroponic roots |
| CIBD | Chronic inflammatory bowel diseases |
| EFR | Epi-friedelanol |
| FR | Friedelin |
| SEM | Standard error of the mean |
| ST | β-Sitosterol |
References
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of Chemotypic Markers in Three Chemotype Categories of Cannabis Using Secondary Metabolites Profiled in Inflorescences, Leaves, Stem Bark, and Roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliferis, K.A.; Bernard-Perron, D. Cannabinomics: Application of Metabolomics in Cannabis (Cannabis sativa L.) Research and Development. Front. Plant Sci. 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Fraternale, D.; Donati Zeppa, S.; Verardo, G.; Gorassini, A.; Carrabs, V.; Albertini, M.C.; Sestili, P. Yield, Characterization, and Possible Exploitation of Cannabis Sativa L. Roots Grown under Aeroponics Cultivation. Molecules 2021, 26, 4889. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, D.J.; Doorenbos, N.J.; Harris, L.S.; Masoud, A.N.; Quimby, M.W.; Schiff, P.L. Chemical constituents of Cannabis sativa L. Root. J. Pharm. Sci. 1971, 60, 1891–1892. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Minardi, M.; Mirembe, S.; Pedro, E.; Gaddi, A. Effects of a new low dose soy protein β-sitosterol association on plasma lipid levels and oxidation. Eur. J. Nutr. 2004, 43, 319–322. [Google Scholar] [CrossRef]
- Von Holtz, R.L.; Fink, C.S.; Awad, A.B. β-Sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr. Cancer 1998, 32, 8–12. [Google Scholar] [CrossRef]
- Awad, A.B.; Fink, C.S.; Williams, H.; Kim, U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur. J. Cancer Prev. 2001, 10, 507–513. [Google Scholar] [CrossRef]
- Kwon, Y. Use of saw palmetto (Serenoa repens) extract for benign prostatic hyperplasia. Food Sci. Biotechnol. 2019, 28, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Macdonald, R.; Ishani, A. β-sitosterol for the treatment of benign prostatic hyperplasia: A systematic review. BJU Int. 1999, 83, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.; Gupta, N.K.; Dixit, V.K. Development and characterization of phyto-vesicles of β-sitosterol for the treatment of androgenetic alopecia. Arch. Dermatol. Res. 2012, 304, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Prager, N.; Bickett, K.; French, N.; Marcovici, G. A Randomized, Double-Blind, Placebo-Controlled Trial to Determine the Effectiveness of Botanically Derived Inhibitors of 5- α -Reductase in the Treatment of Androgenetic Alopecia. J. Altern. Complementary Med. 2002, 8, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Vela-Navarrete, R.; Alcaraz, A.; Rodríguez-Antolín, A.; Miñana López, B.; Fernández-Gómez, J.M.; Angulo, J.C.; Castro Díaz, D.; Romero-Otero, J.; Brenes, F.J.; Carballido, J.; et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018, 122. [Google Scholar] [CrossRef] [Green Version]
- Schantz, M.M.; Bedner, M.; Long, S.E.; Molloy, J.L.; Murphy, K.E.; Porter, B.J.; Putzbach, K.; Rimmer, C.A.; Sander, L.C.; Sharpless, K.E.; et al. Development of saw palmetto (Serenoa repens) fruit and extract standard reference materials. Anal. Bioanal. Chem. 2008, 392, 427–438. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980. [Google Scholar] [CrossRef] [Green Version]
- Subash-Babu, P.; Li, D.K.; Alshatwi, A.A. In vitro cytotoxic potential of friedelin in human MCF-7 breast cancer cell: Regulate early expression of Cdkn2a and pRb1, neutralize mdm2-p53 amalgamation and functional stabilization of p53. Exp. Toxicol. Pathol. 2017, 69, 630–636. [Google Scholar] [CrossRef]
- Gonçalves Pereira, R.C.; Gontijo Evangelista, F.C.; dos Santos Júnior, V.S.; de Paula Sabino, A.; Gonçalves Maltarollo, V.; de Freitas, R.P.; Pains Duarte, L. Cytotoxic Activity of Triterpenoids from Cheiloclinium cognatum Branches against Chronic and Acute Leukemia Cell Lines. Chem. Biodivers. 2020, 17, e2000773. [Google Scholar] [CrossRef]
- Antonisamy, P.; Duraipandiyan, V.; Ignacimuthu, S. Anti-inflammatory, analgesic and antipyretic effects of friedelin isolated from Azima tetracantha Lam. in mouse and rat models. J. Pharm. Pharmacol. 2011, 63, 1070–1077. [Google Scholar] [CrossRef]
- Antonisamy, P.; Duraipandiyan, V.; Aravinthan, A.; Al-Dhabi, N.A.; Ignacimuthu, S.; Choi, K.C.; Kim, J.H. Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur. J. Pharmacol. 2015, 750, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Novrizal Abdi Sahid, M.; Nugroho, A.E.; Susidarti, R.A.; Maeyama, K. Effects of 8-Hydroxyisocapnolactone-2-3-diol and friedelin on mast cell degranulation. Asian Pac. J. Trop. Med. 2017, 10, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Antioxidant, free radical scavenging and liver protective effects of friedelin isolated from Azima tetracantha Lam. leaves. Food Chem. 2013, 139, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Aswar, U.M.; Bhaskaran, S.; Mohan, V.; Bodhankar, S.L. Estrogenic activity of friedelin rich fraction (IND-HE) separated from Cissus quadrangularis and its effect on female sexual function. Pharmacogn. Res. 2010, 2, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Zhang, Y.; Lou, D.; Wu, X.; Zhang, Y. Antihyperlipidemic and antihypertensive effect of a triterpenoid-rich extract from bamboo shavings and vasodilator effect of friedelin on phenylephrine-induced vasoconstriction in thoracic aortas of rats. Phytother. Res. 2007, 21, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Alrashood, S.T.; Alharbi, S.A.; Ignacimuthu, S. Friedelin exhibits antidiabetic effect in diabetic rats via modulation of glucose metabolism in liver and muscle. J. Ethnopharmacol. 2021, 268, 113659. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.C.; Sowmya, S.; Velmurugan, D.; Sivaraman, T.; Gopalakrishnan, V.K. Assessment of dual inhibitory activity of epifriedelanol isolated from Cayratia trifolia against ovarian cancer. Bangladesh J. Pharmacol. 2016, 11, 545. [Google Scholar] [CrossRef]
- Perumal, P.; Sowmya, S.; Pratibha, P.; Vidya, B.; Anusooriya, P.; Starlin, T.; Ravi, S.; Gopalakrishnan, V. Isolation, structural characterization and in silico drug-like properties prediction of a natural compound from the ethanolic extract of cayratia trifolia (L.). Pharmacogn. Res. 2015, 7, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Kumar, S.; Gupta, J.; Arya, R.; Gupta, A. A review on chemical and biological properties of Cayratia trifolia Linn. (Vitaceae). Pharmacogn. Rev. 2011, 5, 184. [Google Scholar] [CrossRef] [Green Version]
- Van Kiem, P.; Van Minh, C.; Huong, H.T.; Nam, N.H.; Lee, J.J.; Kim, Y.H. Pentacyclic triterpenoids from Mallotus apelta. Arch. Pharmacal Res. 2004, 27, 1109–1113. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Cui, Z. New triterpenoids isolated from the root bark of Ulmus pumila L. Chem. Pharm. Bull. 2006, 54, 775–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, J.K.; Rouf, A.S.S.; Nazmul Hossain, M.; Hasan, C.M.; Rashid, M.A. Antitumor activity of epifriedelanol from Vitis trifolia. Fitoterapia 2000, 71, 577–579. [Google Scholar] [CrossRef]
- Dae, K.K.; Jong, P.L.; Jin, W.K.; Hee, W.P.; Jae, S.E. Antitumor and antiinflammatory constituents from Celtis sinensis. Arch. Pharmacal Res. 2005, 28, 39–43. [Google Scholar] [CrossRef]
- Yang, J.; Fa, J.; Li, B. Apoptosis induction of epifriedelinol on human cervical cancer cell line. Afr. J. Tradit. Complementary Altern. Med. AJTCAM 2017, 14, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.H.; Son, J.K.; Jung, B.; Zheng, M.; Kim, J.R. Epifriedelanol from the root bark of ulmus davidiana inhibits cellular senescence in human primary cells. Planta Med. 2011, 77, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Sestili, P.; Martinelli, C.; Ricci, D.; Fraternale, D.; Bucchini, A.; Giamperi, L.; Curcio, R.; Piccoli, G.; Stocchi, V. Cytoprotective effect of preparations from various parts of Punica granatum L. fruits in oxidatively injured mammalian cells in comparison with their antioxidant capacity in cell free systems. Pharmacol. Res. 2007, 56, 18–26. [Google Scholar] [CrossRef]
- Saltarelli, R.; Ceccaroli, P.; Buffalini, M.; Vallorani, L.; Casadei, L.; Zambonelli, A.; Iotti, M.; Badalyan, S.; Stocchi, V. Biochemical Characterization and Antioxidant and Antiproliferative Activities of Different Ganoderma Collections. J. Mol. Microbiol. Biotechnol. 2015, 25, 16–25. [Google Scholar] [CrossRef]
- Sestili, P.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Stocchi, V. The Fast-Halo Assay for the Detection of DNA Damage; Humana Press: Totowa, NJ, USA, 2017; pp. 75–93. [Google Scholar]
- Calcabrini, C.; Mancini, U.; De Bellis, R.; Frati, A.; Mastrogiacomo, A.R.; Annibalini, G.; Sestili, P.; Cucchiarini, L.; Stocchi, V.; Potenza, L. Protective Role of Italian Juglans regia L. nut Ethanolic Extract in Human Keratinocytes under Oxidative and Inflammatory Stress. Curr. Pharm. Biotechnol. 2018, 18, 925–934. [Google Scholar] [CrossRef]
- Coppari, S.; Colomba, M.; Fraternale, D.; Brinkmann, V.; Romeo, M.; Rocchi, M.B.L.; Di Giacomo, B.; Mari, M.; Guidi, L.; Ramakrishna, S.; et al. Antioxidant and Anti-Inflammaging Ability of Prune (Prunus Spinosa L.) Extract Result in Improved Wound Healing Efficacy. Antioxidants 2021, 10, 374. [Google Scholar] [CrossRef]
- Olivieri, F.; Lazzarini, R.; Babini, L.; Prattichizzo, F.; Rippo, M.R.; Tiano, L.; Di Nuzzo, S.; Graciotti, L.; Festa, R.; Brugè, F.; et al. Anti-inflammatory effect of ubiquinol-10 on young and senescent endothelial cells via miR-146a modulation. Free. Radic. Biol. Med. 2013, 63, 410–420. [Google Scholar] [CrossRef]
- Quinn, E.M.; Wang, J.H.; O’Callaghan, G.; Redmond, H.P. MicroRNA-146a Is Upregulated by and Negatively Regulates TLR2 Signaling. PLoS ONE 2013, 8, e62232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paine, M.F.; Roe, A.L. “Green Medicine”: The Past, Present, and Future of Botanicals. Clin. Pharmacol. Ther. 2018, 104, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Diamantini, G.; Bedini, A.; Cerioni, L.; Tommasini, I.; Tarzia, G.; Cantoni, O. Plant-derived phenolic compounds prevent the DNA single-strand breakage and cytotoxicity induced by tert-butylhydroperoxide via an iron-chelating mechanism. Biochem. J. 2002, 364, 121–128. [Google Scholar] [CrossRef]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis Roots: A Traditional Therapy with Future Potential for Treating Inflammation and Pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef]
- Sestili, P.; Stocchi, V. Repositioning Chromones for Early Anti-inflammatory Treatment of COVID-19. Front. Pharmacol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Gong, H.; Li, Y.; Jie, K.; Ding, C.; Shao, Q.; Liu, F.; Zhan, Y.; Nie, C.; Zhu, W.; et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Exp. Lung Res. 2013, 39, 275–282. [Google Scholar] [CrossRef]
- Fraternale, D.; Rudov, A.; Prattichizzo, F.; Olivieri, F.; Ricci, D.; Giacomini, E.; Carloni, S.; Azzolini, C.; Gordillo, B.; Jara-Palacios, M.J.; et al. Chemical composition and “in vitro” anti-inflammatory activity of Vitis vinifera L. (var. Sangiovese) tendrils extract. J. Funct. Foods 2016, 20, 291–302. [Google Scholar] [CrossRef]
- Kishimoto, T.; Kang, S. IL-6 Revisited: From Rheumatoid Arthritis to CAR T Cell Therapy and COVID-19. Annu. Rev. Immunol. 2022, 40, 40. [Google Scholar] [CrossRef]
- Wohlrab, J. Topical preparations and their use in dermatology. JDDG J. Dtsch. Dermatol. Ges. 2016, 14, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower–Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and Natural History of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.F.; Ettich, J.; Weitz, H.T.; Krusche, M.; Floss, D.M.; Scheller, J.; Moll, J.M. Exclusive inhibition of IL-6 trans-signaling by soluble gp130FlyRFc. Cytokine X 2021, 3, 100058. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; MacSharry, J.; Darby, T.; Fanning, A.; Shanahan, F.; Houston, A.; Brint, E. Differential expression of key regulators of Toll-like receptors in ulcerative colitis and Crohn’s disease: A role for Tollip and peroxisome proliferator-activated receptor gamma? Clin. Exp. Immunol. 2016, 183, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Liu, S.; Huang, A.; Zhou, M.; Sun, B.; Cao, H.; Shan, J.; Sun, B.; Lin, J. Revealing the Mechanism of Friedelin in the Treatment of Ulcerative Colitis Based on Network Pharmacology and Experimental Verification. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Huang, H.; Lou, Z.; Zheng, S.; Wu, J.; Yao, Q.; Chen, R.; Kou, L.; Chen, D. Intra-articular drug delivery systems for osteoarthritis therapy: Shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 2022, 29, 767–791. [Google Scholar] [CrossRef]
- Chen, L.; DiPietro, L.A. Toll-Like Receptor Function in Acute Wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Peng, J.; Dai, Z.; Liao, Y.; Liu, Q.; Li, J.; Jiang, Y. Articular Cartilage Fragmentation Improves Chondrocyte Migration by Upregulating Membrane Type 1 Matrix Metalloprotease. CARTILAGE 2021, 13, 1054S–1063S. [Google Scholar] [CrossRef]
- Furukawa, S.; Ikeda, Y.; Yagi, S.; Miyake, T.; Shiraishi, K.; Tange, K.; Hashimoto, Y.; Mori, K.; Ninomiya, T.; Suzuki, S.; et al. Association Between Peripheral Blood Monocyte Count and Mucosal Healing in Japanese Patients With Ulcerative Colitis. Clin. Transl. Gastroenterol. 2021, 12, e00429. [Google Scholar] [CrossRef]
- Liao, P.-C.; Lai, M.-H.; Hsu, K.-P.; Kuo, Y.-H.; Chen, J.; Tsai, M.-C.; Li, C.-X.; Yin, X.-J.; Jeyashoke, N.; Chao, L.K.-P. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]




| Gene | Primer | Sequence | Accession Number |
|---|---|---|---|
| IL-6 | Forward | AGGGCTCTTCGGCAAATGTA | NG_011640.1 |
| IL-6 | Reverse | GAAGGAATGCCCATTAACAACAA | NG_011640.1 |
| IRAK-1 | Forward | CAGACAGGGAAGGGAAACATTTT | NG_008387.1 |
| IRAK-1 | Reverse | CATGAAACCTGACTTGCTTCTGAA | NG_008387.1 |
| TNF- α | Forward | CTGCTGCACTTTGGAGTGAT | NG_007462.1 |
| TNF-α | Reverse | TCTCAGCTCCACGCCATT | NG_007462.1 |
| IL-8 | Forward | GCAGAGGGTTGTGGAGAAGT | NG_029889.1 |
| IL-8 | Reverse | GCTTGAAGTTTCACTGGCATC | NG_029889.1 |
| IkB-α | Forward | GGTGCTGATGTCAATGCTCA | NG_007571.1 |
| IkB-α | Reverse | ACACCAGGTCAGGATTTTGC | NG_007571.1 |
| iNOS | Forward | CCCTTCAATGGCTGGTACATGG | NG_011470.1 |
| iNOS | Reverse | ATGTTGATCTCAACGACAGCC | NG_011470.1 |
| TBP | Forward | TGCACAGGAGCCAAGAGTGA | NG_008165.1 |
| TBP | Reverse | CACATCACAGCTCCCCACCA | NG_008165.1 |
| Compound | Amount (µg/100 mg Dry Roots) a |
|---|---|
| Campesterol | 17.2 ± 0.4 |
| Stigmasterol | 20.2 ± 0.2 |
| β-Sitosterol | 68.7 ± 1.1 |
| Epi-Friedelanol | 23.9 ± 0.1 |
| Friedelin | 28.8 ± 1.2 |
| Total | 158.8 ± 1.9 |
| Antioxidant Activity (EC50 Values, μg/mL) | ||||
|---|---|---|---|---|
| APEX | Friedelin | β-Sitosterol | Epi-Friedelanol | |
| DPPH scavenging activity a | 420.1 ± 2.1 | 832.4 ± 1.7 | 920.2 ± 3.4 | 875.1 ± 1.7 |
| Chelating activity a | 385.5 ± 3.0 | 883.5 ± 7.2 | 858.8 ± 6.2 | 547.6 ± 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrini, F.; Donati Zeppa, S.; Fraternale, D.; Carrabs, V.; Annibalini, G.; Verardo, G.; Gorassini, A.; Albertini, M.C.; Ismail, T.; Fimognari, C.; et al. Characterization of the Biological Activity of the Ethanolic Extract from the Roots of Cannabis sativa L. Grown in Aeroponics. Antioxidants 2022, 11, 860. https://doi.org/10.3390/antiox11050860
Ferrini F, Donati Zeppa S, Fraternale D, Carrabs V, Annibalini G, Verardo G, Gorassini A, Albertini MC, Ismail T, Fimognari C, et al. Characterization of the Biological Activity of the Ethanolic Extract from the Roots of Cannabis sativa L. Grown in Aeroponics. Antioxidants. 2022; 11(5):860. https://doi.org/10.3390/antiox11050860
Chicago/Turabian StyleFerrini, Fabio, Sabrina Donati Zeppa, Daniele Fraternale, Vittoria Carrabs, Giosuè Annibalini, Giancarlo Verardo, Andrea Gorassini, Maria Cristina Albertini, Tariq Ismail, Carmela Fimognari, and et al. 2022. "Characterization of the Biological Activity of the Ethanolic Extract from the Roots of Cannabis sativa L. Grown in Aeroponics" Antioxidants 11, no. 5: 860. https://doi.org/10.3390/antiox11050860
APA StyleFerrini, F., Donati Zeppa, S., Fraternale, D., Carrabs, V., Annibalini, G., Verardo, G., Gorassini, A., Albertini, M. C., Ismail, T., Fimognari, C., & Sestili, P. (2022). Characterization of the Biological Activity of the Ethanolic Extract from the Roots of Cannabis sativa L. Grown in Aeroponics. Antioxidants, 11(5), 860. https://doi.org/10.3390/antiox11050860