A Novel Combination of High-Load Omega-3 Lysine Complex (AvailOm®) and Anthocyanins Exerts Beneficial Cardiovascular Effects
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Experimental Animals
2.3. Vascular Reactivity Studies
2.4. Evaluation of ROS Production
2.5. Statistical Analysis
3. Results
3.1. AvailOm® Evokes a Direct Vasorelaxant Action on Mice Mesenteric Arteries
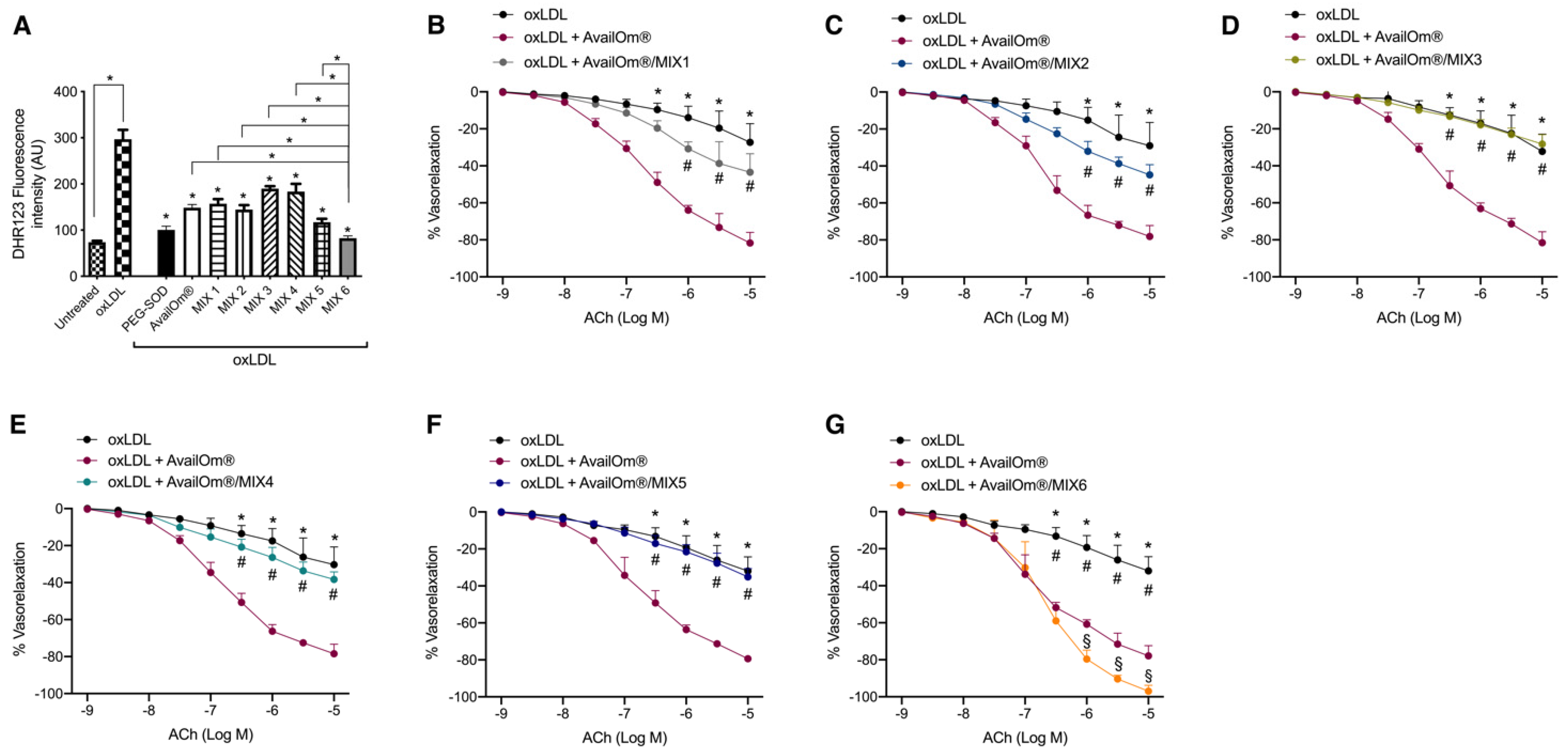
3.2. AvailOm® Prevents Vascular Oxidative Stress Damage Induced by oxLDL
3.3. AvailOm® in Combination with the Most Potent Anthocyanins, Exerts the Highest Vasorelaxant Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Jaca, A.; Durao, S.; Harbron, J. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. S. Afr. Med. J. 2020, 110, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Maehre, H.K.; Jensen, I.J.; Elvevoll, E.O.; Eilertsen, K.E. omega-3 Fatty Acids and Cardiovascular Diseases: Effects, Mechanisms and Dietary Relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lian, F.; Zhu, Y.; Xia, M.; Wang, Q.; Ling, W.; Wang, X.D. Cyanidin-3-O-beta-glucoside inhibits LPS-induced expression of inflammatory mediators through decreasing IkappaBalpha phosphorylation in THP-1 cells. Inflamm. Res. 2010, 59, 723–730. [Google Scholar] [CrossRef]
- Jensen, G.S.; Wu, X.; Patterson, K.M.; Barnes, J.; Carter, S.G.; Scherwitz, L.; Beaman, R.; Endres, J.R.; Schauss, A.G. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef] [Green Version]
- Shaughnessy, K.S.; Boswall, I.A.; Scanlan, A.P.; Gottschall-Pass, K.T.; Sweeney, M.I. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr. Res. 2009, 29, 130–138. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients 2021, 13, 2831. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Hong, S.J.; Lee, T.B.; Kwon, J.W.; Jeong, J.T.; Joo, H.J.; Park, J.H.; Ahn, C.M.; Yu, C.W.; Lim, D.S. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother. Res. 2014, 28, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Lizio, R.; Di Pietro, P.; Ciccarelli, M.; Damato, A.; Venturini, E.; Iannece, P.; Sommella, E.; Campiglia, P.; Ockermann, P.; et al. Healthberry 865® and Its Related, Specific, Single Anthocyanins Exert a Direct Vascular Action, Modulating Both Endothelial Function and Oxidative Stress. Antioxidants 2021, 10, 1191. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- McVeigh, G.E.; Brennan, G.M.; Johnston, G.D.; McDermott, B.J.; McGrath, L.T.; Henry, W.R.; Andrews, J.W.; Hayes, J.R. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1993, 36, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Goode, G.K.; Garcia, S.; Heagerty, A.M. Dietary supplementation with marine fish oil improves in vitro small artery endothelial function in hypercholesterolemic patients: A double-blind placebo-controlled study. Circulation 1997, 96, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Niazi, A.K.; McCarty, M.F.; O’Keefe, J.H.; Meier, P.; Lavie, C.J. Omega-3s and cardiovascular health. Ochsner J. 2014, 14, 399–412. [Google Scholar] [PubMed]
- Iwamatsu, K.; Abe, S.; Nishida, H.; Kageyama, M.; Nasuno, T.; Sakuma, M.; Toyoda, S.; Inoue, T. Which has the stronger impact on coronary artery disease, eicosapentaenoic acid or docosahexaenoic acid? Hypertens Res. 2016, 39, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Woodman, R.J.; Mori, T.A.; Burke, V.; Puddey, I.B.; Barden, A.; Watts, G.F.; Beilin, L.J. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 2003, 166, 85–93. [Google Scholar] [CrossRef]
- Jiang, S.; Li, T.; Yang, Z.; Yi, W.; Di, S.; Sun, Y.; Wang, D.; Yang, Y. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res. Rev. 2017, 38, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Xiao, H.; Liu, L.L.; Cheng, D.; Li, X.J.; Si, L.Y. FGF21 represses cerebrovascular aging via improving mitochondrial biogenesis and inhibiting p53 signaling pathway in an AMPK-dependent manner. Exp. Cell Res. 2016, 346, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Bengen, J.; Schollmeyer, P.; Wanner, C. Impairment of endothelium-dependent dilation in rabbit renal arteries by oxidized lipoprotein(a). Role of oxygen-derived radicals. Circulation 1995, 92, 1582–1589. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009, 15, 3133–3145. [Google Scholar] [CrossRef] [Green Version]
- Andriambeloson, E.; Magnier, C.; Haan-Archipoff, G.; Lobstein, A.; Anton, R.; Beretz, A.; Stoclet, J.C.; Andriantsitohaina, R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J. Nutr. 1998, 128, 2324–2333. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gomez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights from Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 967–976. [Google Scholar] [CrossRef]
- The Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietro, P.; Lizio, R.; Izzo, C.; Visco, V.; Damato, A.; Venturini, E.; De Lucia, M.; Galasso, G.; Migliarino, S.; Rasile, B.; et al. A Novel Combination of High-Load Omega-3 Lysine Complex (AvailOm®) and Anthocyanins Exerts Beneficial Cardiovascular Effects. Antioxidants 2022, 11, 896. https://doi.org/10.3390/antiox11050896
Di Pietro P, Lizio R, Izzo C, Visco V, Damato A, Venturini E, De Lucia M, Galasso G, Migliarino S, Rasile B, et al. A Novel Combination of High-Load Omega-3 Lysine Complex (AvailOm®) and Anthocyanins Exerts Beneficial Cardiovascular Effects. Antioxidants. 2022; 11(5):896. https://doi.org/10.3390/antiox11050896
Chicago/Turabian StyleDi Pietro, Paola, Rosario Lizio, Carmine Izzo, Valeria Visco, Antonio Damato, Eleonora Venturini, Massimiliano De Lucia, Gennaro Galasso, Serena Migliarino, Barbara Rasile, and et al. 2022. "A Novel Combination of High-Load Omega-3 Lysine Complex (AvailOm®) and Anthocyanins Exerts Beneficial Cardiovascular Effects" Antioxidants 11, no. 5: 896. https://doi.org/10.3390/antiox11050896
APA StyleDi Pietro, P., Lizio, R., Izzo, C., Visco, V., Damato, A., Venturini, E., De Lucia, M., Galasso, G., Migliarino, S., Rasile, B., Ciccarelli, M., Vecchione, C., & Carrizzo, A. (2022). A Novel Combination of High-Load Omega-3 Lysine Complex (AvailOm®) and Anthocyanins Exerts Beneficial Cardiovascular Effects. Antioxidants, 11(5), 896. https://doi.org/10.3390/antiox11050896









