The Inhibition of LPS-Induced Oxidative Stress and Inflammatory Responses Is Associated with the Protective Effect of (-)-Epigallocatechin-3-Gallate on Bovine Hepatocytes and Murine Liver
Abstract
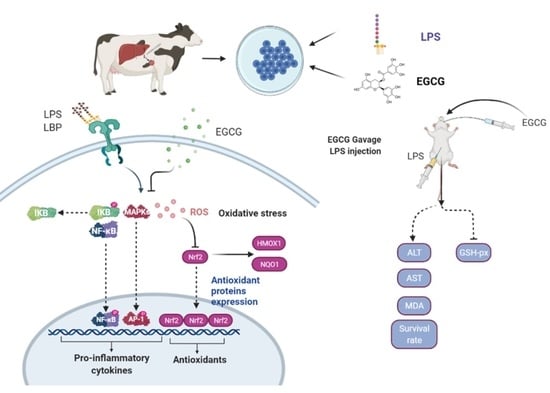
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethics
2.3. Cell Culture Conditions
2.4. In Vitro Experimental Design
2.5. Cell Viability
2.6. Apoptotic Rate Measurement by Flow Cytometry
2.7. 5-Ethynyl-2′-Deoxyuridine (EdU) Determination
2.8. Cell Migration Assay
2.9. ROS, MDA, GSH-Px, and T-AOC Detection
2.10. RNA Isolation and Quantitative Real-Time PCR
2.11. Immunoblotting
2.12. Chromatin Immunoprecipitation
2.13. Immunofluorescence
2.14. Animals
2.15. In Vivo Experimental Design
- Control group: mice were treated normally as control;
- GalN/LPS group: mice received intraperitoneal injection with GalN (700 mg/kg) and LPS (10 μg/kg) at day 11 for 6 h;
- EGCG + GalN/LPS group: mice were administrated with EGCG orally at a dose of 10, 25, or 50 mg/kg/day for 10 days, followed by GalN/LPS challenge at day 11 for 6 h.
2.16. Lethality Determination
2.17. Histology Determination
2.18. Aminotransferase Activities, Lipid Peroxidation, and GSH-Px Content
2.19. Statistical Analysis
3. Results
3.1. The Optimized EGCG Dose Was Determined by Cell Viability and TNF-α Concentration
3.2. Impaired Cell Proliferation Was Restored by Pretreatment with EGCG
3.3. LPS-Induced Activation of the NF-κB Signaling Pathway Was Reversed by EGCG Pretreatment
3.4. EGCG Suppressed the Expression of Genes Related to Proinflammation by Reducing the Binding Activity of NF-κB
3.5. The Inhibitory Effect of EGCG on MAPK Signaling Pathway Activity in Hepatocytes
3.6. The Oxidative Stress Induced by LPS Was Reversed by Supplementation with EGCG in Hepatocytes
3.7. EGCG Enhanced the Antioxidant Capacity of Hepatocytes by Activating Nrf2 Signaling
3.8. Lethality in Mice and Histological Changes in Liver Tissue
3.9. Analysis of Peripheral Aminotransferase Activity and GSH-Px and MDA Contents
4. Discussion
4.1. LPS-Impeded Proliferation Is Restored by EGCG Pretreatment
4.2. EGCG Mediated Inactivation of the NF-κB Signaling Pathway in Hepatocytes
4.3. EGCG Restricts LPS-Induced Inflammation by Silencing MAPK Signaling
4.4. Antioxidant Capacity Was Improved by EGCG Treatment in Bovine Hepatocytes
4.5. In Vivo Study Verified the Hepatoprotective Role of EGCG in Mice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| EGCG | (-)-epigallocatechin-3-gallate |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa B |
| APR | Acute-phase responses |
| Nrf2 | Nuclear factor(erythroid-derived2)-like 2 protein |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| MDA | Malondialdehyde |
| ROS | Reactive oxygen species |
| EdU | 5-Ethynyl-2′-deoxyuridine |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| RPS9 | Ribosomal protein S9 |
| UXT | Ubiquitously-expressed transcript |
References
- Amin, A.; Mahmoud-Ghoneim, D. Texture analysis of liver fibrosis microscopic images: A study on the effect of biomarkers. Acta Biochim. Biophys. Sin. 2011, 43, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, A.A.; Heeba, G.H.; Hamza, S.; Abdalla, A.; Amin, A. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/inflammation pathway. Biomed. Pharm. 2021, 134, 111102. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Cavet, M.E.; Harrington, K.L.; Vollmer, T.R.; Ward, K.W.; Zhang, J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar]
- Basini, G.; Bianco, F.; Grasselli, F. Epigallocatechin-3-gallate from green tea negatively affects swine granulosa cell function. Domest. Anim. Endocrinol. 2005, 28, 243–256. [Google Scholar] [CrossRef]
- Thielecke, F.; Boschmann, M. The potential role of green tea catechins in the prevention of the metabolic syndrome—A review. Phytochemistry 2009, 70, 11–24. [Google Scholar] [CrossRef]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 894–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Liu, G.; Tang, M.; Fang, J.; Jiang, H. Epigallocatechin Gallate Can Protect Mice From Acute Stress Induced by LPS While Stabilizing Gut Microbes and Serum Metabolites Levels. Front. Immunol. 2021, 12, 640305. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Zhao, L.; Coleman, D.N.; Gao, M.; Loor, J.J. Tea polyphenols protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative damage in vitro by activating NFE2L2/HMOX1 pathways. J. Dairy Sci. 2019, 102, 1658–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozho, G.N.; Krause, D.O.; Plaizier, J.C. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows. J. Dairy Sci. 2007, 90, 856–866. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Cheng, Y.; Fu, J.; Lu, Z.; Wang, F.; Jin, M.; Zong, X.; Wang, Y. Gut Immunity and Microbiota Dysbiosis Are Associated with Altered Bile Acid Metabolism in LPS-Challenged Piglets. Oxid. Med. Cell Longev. 2021, 2021, 6634821. [Google Scholar] [CrossRef]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Zhang, K.; Xu, T.; Jin, D.; Seyfert, H.M.; Shen, X.; Zhuang, S. Feeding a high-grain diet reduces the percentage of LPS clearance and enhances immune gene expression in goat liver. BMC Vet. Res. 2015, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Petzl, W.; Vanselow, J.; Gunther, J.; Shen, X.; Seyfert, H.M. Epigenetic mechanisms contribute to enhanced expression of immune response genes in the liver of cows after experimentally induced Escherichia coli mastitis. Vet. J. Lond. Engl. 2015, 203, 339–341. [Google Scholar] [CrossRef]
- Xu, T.; Liu, R.; Lu, X.; Wu, X.; Heneberg, P.; Mao, Y.; Jiang, Q.; Loor, J.; Yang, Z. Lycium barbarum polysaccharides alleviate LPS-induced inflammatory responses through PPARγ/MAPK/NF-κB pathway in bovine mammary epithelial cells. J. Anim. Sci. 2022, 100, 345. [Google Scholar] [CrossRef]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef]
- Dai, H.; Coleman, D.N.; Hu, L.; Martinez-Cortés, I.; Wang, M.; Parys, C.; Shen, X.; Loor, J.J. Methionine and arginine supplementation alter inflammatory and oxidative stress responses during lipopolysaccharide challenge in bovine mammary epithelial cells in vitro. J. Dairy Sci. 2020, 103, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Mølgaard, L.; Damgaard, B.M.; Bjerre-Harpøth, V.; Herskin, M.S. Effects of percutaneous needle liver biopsy on dairy cow behaviour. Res. Vet. Sci. 2012, 93, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Bertoncini, C.R.; Borecký, J.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondrial DNA damage associated with lipid peroxidation of the mitochondrial membrane induced by Fe2+-citrate. An. Acad. Bras. Cienc. 2006, 78, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, J.; Biswas, S.; Datta, A.G. Mode of action of endotoxin: Role of free radicals and antioxidants. Curr. Med. Chem. 2004, 11, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.; Fung, M.L.; Tipoe, G.L. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mei, Y.; Feng, D.; Xu, L. (-)-Epigallocatechin-3-gallate protects mice from concanavalin A-induced hepatitis through suppressing immune-mediated liver injury. Clin. Exp. Immunol. 2006, 145, 485–492. [Google Scholar] [CrossRef]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-Cancer Effects of Green Tea by Either Anti- or Pro- Oxidative Mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, H. Green tea polyphenol sensing. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Ma, N.; Wang, Y.; Shi, X.; Chang, G.; Loor, J.J.; Shen, X. Sodium Butyrate Supplementation Alleviates the Adaptive Response to Inflammation and Modulates Fatty Acid Metabolism in Lipopolysaccharide-Stimulated Bovine Hepatocytes. J. Agric. Food Chem. 2018, 66, 6281–6290. [Google Scholar] [CrossRef]
- Abaker, J.A.; Xu, T.L.; Jin, D.; Chang, G.J.; Zhang, K.; Shen, X.Z. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 2017, 100, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Peng, J.; Loor, J.J. Methionine and valine activate the mammalian target of rapamycin complex 1 pathway through heterodimeric amino acid taste receptor (TAS1R1/TAS1R3) and intracellular Ca2+ in bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 11354–11363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Xu, T.L.; Seyfert, H.M.; Shen, X.Z. Epigenetic mechanisms contribute to decrease stearoyl-CoA desaturase 1 expression in the liver of dairy cows after prolonged feeding of high-concentrate diet. J. Dairy Sci. 2018, 101, 2506–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; White, H.M. Regulation of inflammation, antioxidant production, and methyl-carbon metabolism during methionine supplementation in lipopolysaccharide-challenged neonatal bovine hepatocytes. J. Dairy Sci. 2017, 100, 8565–8577. [Google Scholar] [CrossRef] [Green Version]
- Djordjević, V.B. Free radicals in cell biology. Int. Rev. Cytol. 2004, 237, 57–89. [Google Scholar]
- Shi, H.; Guo, Y.; Liu, Y.; Shi, B.; Guo, X.; Jin, L.; Yan, S. The in vitro effect of lipopolysaccharide on proliferation, inflammatory factors and antioxidant enzyme activity in bovine mammary epithelial cells. Anim. Nutr. 2016, 2, 99–104. [Google Scholar] [CrossRef]
- Meng, Y.K.; Li, C.Y.; Li, R.Y.; He, L.Z.; Cui, H.R.; Yin, P.; Zhang, C.E.; Li, P.Y.; Sang, X.X.; Wang, Y.; et al. Cis-stilbene glucoside in Polygonum multiflorum induces immunological idiosyncratic hepatotoxicity in LPS-treated rats by suppressing PPAR-γ. Acta Pharmacol. Sin. 2017, 38, 1340–1352. [Google Scholar] [CrossRef] [Green Version]
- Quivy, V.; Van Lint, C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem. Pharm. 2004, 68, 1221–1229. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [Green Version]
- Waldron, M.R.; Nishida, T.; Nonnecke, B.J.; Overton, T.R. Effect of lipopolysaccharide on indices of peripheral and hepatic metabolism in lactating cows. J. Dairy Sci. 2003, 86, 3447–3459. [Google Scholar] [CrossRef]
- Vels, L.; Røntved, C.M.; Bjerring, M.; Ingvartsen, K.L. Cytokine and acute phase protein gene expression in repeated liver biopsies of dairy cows with a lipopolysaccharide-induced mastitis. J. Dairy Sci. 2009, 92, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Nishino, T.; Obata, Y.; Furusu, A.; Hishikawa, Y.; Koji, T.; Kohno, S. Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chem. Biol. Interact. 2012, 195, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Wachi, M.; Woo, J.T.; Kato, M.; Kasai, S.; Takahashi, F.; Lee, I.S.; Nagai, K. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef]
- Kim, S.H.; Johnson, V.J.; Shin, T.Y.; Sharma, R.P. Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-kappaB signaling pathways. Exp. Biol. Med. 2004, 229, 203–213. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Ali, H.; Shah, K.U.; Ali, H.; Shehzad, O.; Onder, A.; Kim, Y.S. Anomalin attenuates LPS-induced acute lungs injury through inhibition of AP-1 signaling. Int. Immunopharmacol. 2019, 73, 451–460. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Ooppachai, C.; Pompimon, W.; Limtrakul Dejkriengkraikul, P. O-Methylbulbocapnine and Dicentrine Suppress LPS-Induced Inflammatory Response by Blocking NF-κB and AP-1 Activation through Inhibiting MAPKs and Akt Signaling in RAW264.7 Macrophages. Biol. Pharm. Bull. 2018, 41, 1219–1227. [Google Scholar] [CrossRef] [Green Version]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.-Y.; Lee, K.-S.; Nam, K.-S. Refined Deep-Sea Water Suppresses Inflammatory Responses via the MAPK/AP-1 and NF-κB Signaling Pathway in LPS-Treated RAW 264.7 Macrophage Cells. Int. J. Mol. Sci. 2017, 18, 2282. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-Y.; Yang, Y.-C.; Li, C.-C.; Liu, K.-L.; Lii, C.-K.; Chen, H.-W. Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells. Biochem. Pharmacol. 2014, 91, 40–50. [Google Scholar] [CrossRef]
- Hu, G.; Hong, D.; Zhang, T.; Duan, H.; Wei, P.; Guo, X.; Mu, X. Cynatratoside-C from Cynanchum atratum displays anti-inflammatory effect via suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Chem.-Biol. Interact. 2018, 279, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Khodir, A.E.; Ghoneim, H.A.; Rahim, M.A.; Suddek, G.M. Montelukast attenuates lipopolysaccharide-induced cardiac injury in rats. Hum. Exp. Toxicol. 2016, 35, 388–397. [Google Scholar] [CrossRef]
- Elbling, L.; Herbacek, I.; Weiss, R.M.; Jantschitsch, C.; Micksche, M.; Gerner, C.; Pangratz, H.; Grusch, M.; Knasmüller, S.; Berger, W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Radic. Biol. Med. 2010, 49, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Piao, Y.; Nagaoka, K.; Watanabe, G.; Taya, K.; Li, C. Protective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testis. Reprod. Biol. Endocrinol. 2013, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Xu, X.; Zou, Y.; Yang, Z.; Li, S.; Cao, Z. Changes in feed intake, nutrient digestion, plasma metabolites, and oxidative stress parameters in dairy cows with subacute ruminal acidosis and its regulation with pelleted beet pulp. J. Anim. Sci. Biotechnol. 2013, 4, 31. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Liu, R.; Zhu, H.; Zhou, Y.; Pei, T.; Yang, Z. The Inhibition of LPS-Induced Oxidative Stress and Inflammatory Responses Is Associated with the Protective Effect of (-)-Epigallocatechin-3-Gallate on Bovine Hepatocytes and Murine Liver. Antioxidants 2022, 11, 914. https://doi.org/10.3390/antiox11050914
Xu T, Liu R, Zhu H, Zhou Y, Pei T, Yang Z. The Inhibition of LPS-Induced Oxidative Stress and Inflammatory Responses Is Associated with the Protective Effect of (-)-Epigallocatechin-3-Gallate on Bovine Hepatocytes and Murine Liver. Antioxidants. 2022; 11(5):914. https://doi.org/10.3390/antiox11050914
Chicago/Turabian StyleXu, Tianle, Run Liu, Hao Zhu, Yu Zhou, Tianxu Pei, and Zhangping Yang. 2022. "The Inhibition of LPS-Induced Oxidative Stress and Inflammatory Responses Is Associated with the Protective Effect of (-)-Epigallocatechin-3-Gallate on Bovine Hepatocytes and Murine Liver" Antioxidants 11, no. 5: 914. https://doi.org/10.3390/antiox11050914